Plasma Levels and Renal Handling of Amino Acids Contribute to Determination of Risk of Mortality or Feed of Ventilation in Patients with COVID-19
Abstract
:1. Introduction
2. Results
2.1. General Study Population
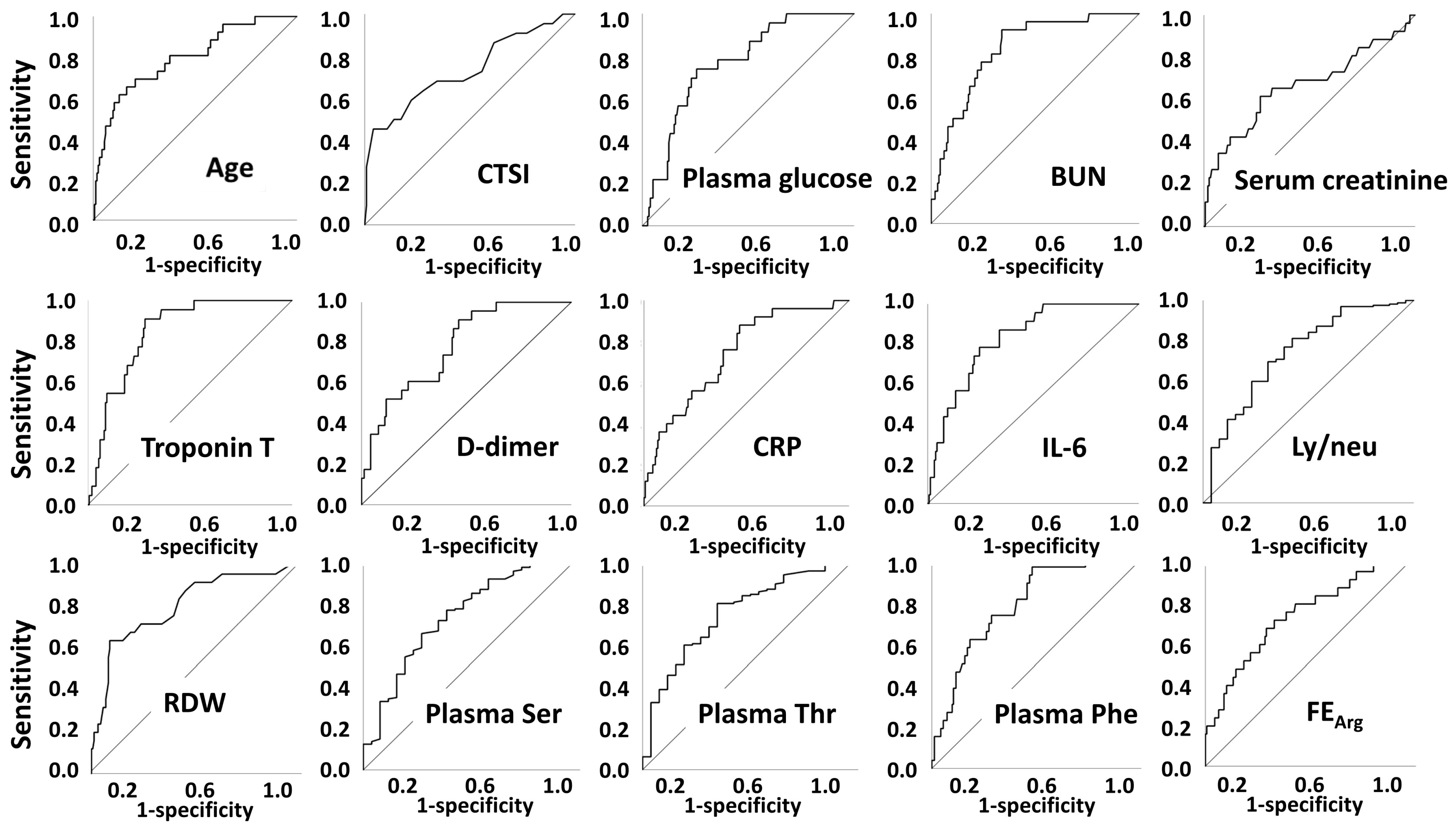
2.2. In-Hospital Mortality
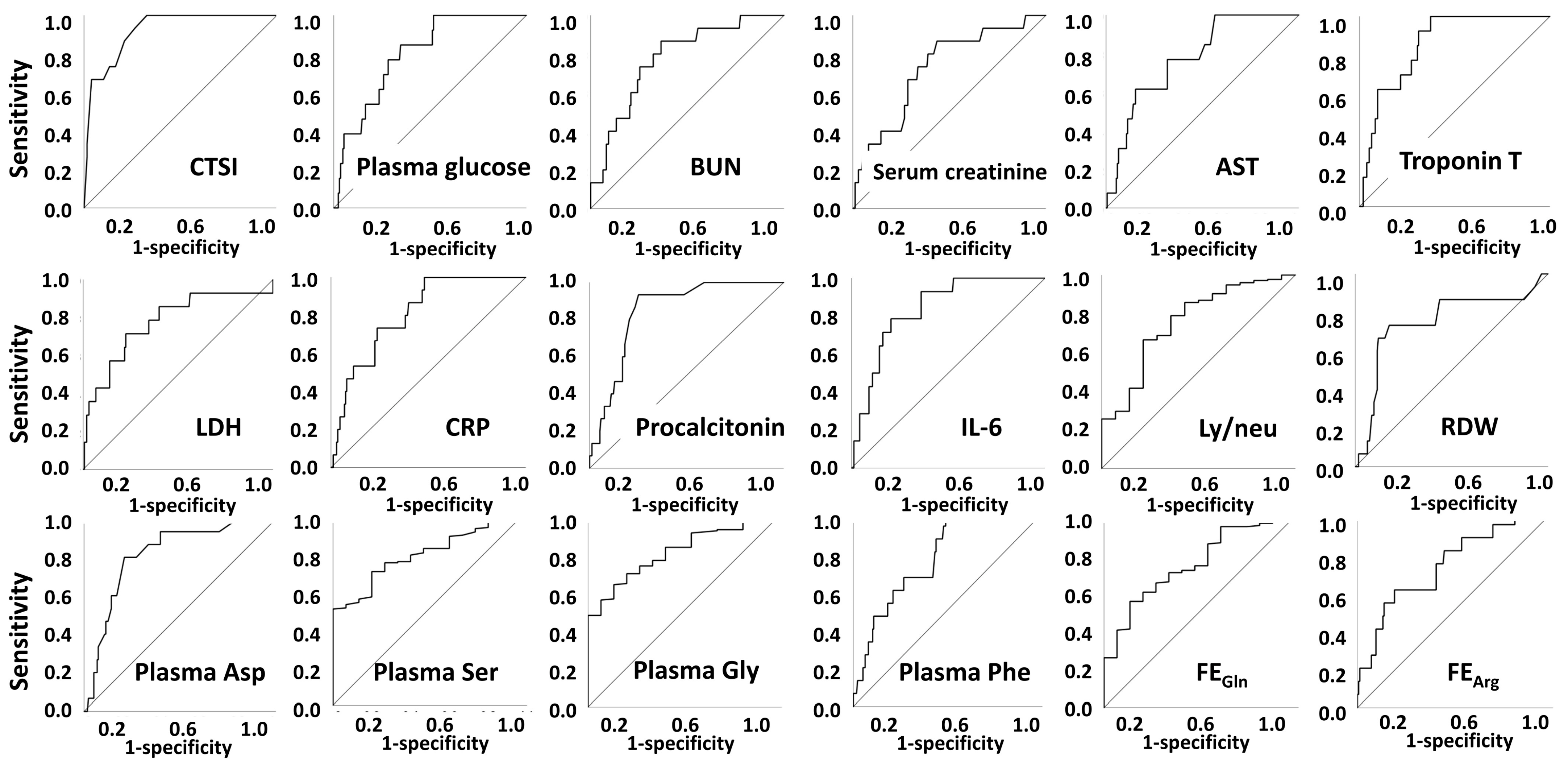
2.3. Mechanical Ventilation
3. Discussion
4. Patients and Methods
4.1. Patients and Parameters
4.2. Amino Acid Analysis from Plasma and Urine Samples
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M.; Altersberger, M.; Binder, C.; Hengstenberg, C.; Binder, T. The COVID-19 Burden for Health Care Professionals: Results of a Global Survey. Eur. J. Intern. Med. 2021, 83, 96–98. [Google Scholar] [CrossRef]
- Di Fusco, M.; Shea, K.M.; Lin, J.; Nguyen, J.L.; Angulo, F.J.; Benigno, M.; Malhotra, D.; Emir, B.; Sung, A.H.; Hammond, J.L.; et al. Health Outcomes and Economic Burden of Hospitalized COVID-19 Patients in the United States. J. Med. Econ. 2021, 24, 308–317. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of COVID-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Aghagoli, G.; Gallo Marin, B.; Soliman, L.B.; Sellke, F.W. Cardiac Involvement in COVID-19 Patients: Risk Factors, Predictors, and Complications: A Review. J. Card. Surg. 2020, 35, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Gorna, R.; MacDermott, N.; Rayner, C.; O’Hara, M.; Evans, S.; Agyen, L.; Nutland, W.; Rogers, N.; Hastie, C. Long COVID Guidelines Need to Reflect Lived Experience. Lancet 2021, 397, 455–457. [Google Scholar] [CrossRef]
- Berenguer, J.; Ryan, P.; Rodríguez-Baño, J.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R.; Aznar Muñoz, E.; Gil Divasson, P.; et al. Characteristics and Predictors of Death among 4035 Consecutively Hospitalized Patients with COVID-19 in Spain. Clin. Microbiol. Infect. 2020, 26, 1525–1536. [Google Scholar] [CrossRef]
- Luca, N.; Federico, R.; Arianna, G.; Dario, P.; Davide, C.; Giovanni, S.; Giulio, G.; Michele, S.; Filippo, M.R.; Ferdinando, L.L.; et al. At the Peak of COVID-19 Age and Disease Severity but Not Comorbidities Are Predictors of Mortality: COVID-19 Burden in Bergamo, Italy. Panminerva Med. 2021, 63, 51–61. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe Obesity Is Associated with Higher In-Hospital Mortality in a Cohort of Patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting Blood Glucose at Admission Is an Independent Predictor for 28-Day Mortality in Patients with COVID-19 without Previous Diagnosis of Diabetes: A Multi-Centre Retrospective Study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Damen, J.A.A.; Debray, T.P.A.; De Vos, M.; et al. Prediction Models for Diagnosis and Prognosis of COVID-19: Systematic Review and Critical Appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical Course and Predictors of 60-Day Mortality in 239 Critically Ill Patients with COVID-19: A Multicenter Retrospective Study from Wuhan, China. Crit. Care 2020, 24, 394. [Google Scholar] [CrossRef]
- Chilimuri, S.; Sun, H.; Alemam, A.; Mantri, N.; Shehi, E.; Tejada, J.; Yugay, A.; Nayudu, S.K. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West. J. Emerg. Med. 2020, 21, 779–784. [Google Scholar] [CrossRef]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early Predictors of Clinical Outcomes of COVID-19 Outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT Score in COVID-19 Patients: Correlation with Disease Severity and Short-Term Prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Gayam, V.; Chobufo, M.D.; Merghani, M.A.; Lamichhane, S.; Garlapati, P.R.; Adler, M.K. Clinical Characteristics and Predictors of Mortality in African-Americans with COVID-19 from an Inner-City Community Teaching Hospital in New York. J. Med. Virol. 2021, 93, 812–819. [Google Scholar] [CrossRef]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate Dehydrogenase Levels Predict Coronavirus Disease 2019 (COVID-19) Severity and Mortality: A Pooled Analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Henry, B.M.; De Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, Biochemical and Immune Biomarker Abnormalities Associated with Severe Illness and Mortality in Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-Lymphocyte Ratio as an Independent Risk Factor for Mortality in Hospitalized Patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Braun, F.; Lütgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Nörz, D.; Heinrich, F.; Meißner, K.; Wichmann, D.; et al. SARS-CoV-2 Renal Tropism Associates with Acute Kidney Injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef]
- Meijers, B.; Hilbrands, L.B. The Clinical Characteristics of Coronavirus-Associated Nephropathy. Nephrol. Dial. Transplant. 2020, 35, 1279–1281. [Google Scholar] [CrossRef]
- Vijayan, A.; Humphreys, B.D. SARS-CoV-2 in the Kidney: Bystander or Culprit? Nat. Rev. Nephrol. 2020, 16, 703–704. [Google Scholar] [CrossRef]
- Hartung, R.; Humbsch, A.; Stein, G. Influence of an Extracellular Volume Expansion (ECVE) on Renal Amino Acid- and Sodium Handling in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). Amino Acids 1997, 13, 311–322. [Google Scholar] [CrossRef]
- Fleck, C.; Gräfe, K.; Kart, I. Renal Handling of Amino Acids in 5/6-Nephrectomized Rats: Stimulation of Renal Amino Acid Reabsorption after Treatment with Triiodothyronine or Dexamethasone under Amino Acid Load. Amino Acids 1999, 16, 149–164. [Google Scholar] [CrossRef]
- Oroszi, B.; Juhász, A.; Nagy, C.; Horváth, J.K.; Komlós, K.E.; Túri, G.; McKee, M.; Ádány, R. Characteristics of the Third COVID-19 Pandemic Wave with Special Focus on Socioeconomic Inequalities in Morbidity, Mortality and the Uptake of COVID-19 Vaccination in Hungary. J. Pers. Med. 2022, 12, 388. [Google Scholar] [CrossRef]
- Sarkar, S.; Kannan, S.; Khanna, P.; Singh, A.K. Role of Platelet-to-Lymphocyte Count Ratio (PLR), as a Prognostic Indicator in COVID-19: A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 94, 211–221. [Google Scholar] [CrossRef]
- Zhu, Z.; Mao, Y.; Chen, G. Predictive Value of HbA1c for In-Hospital Adverse Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Prim. Care Diabetes 2021, 15, 910–917. [Google Scholar] [CrossRef]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 Ng/ML 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef] [PubMed]
- Földi, M.; Farkas, N.; Kiss, S.; Zádori, N.; Váncsa, S.; Szakó, L.; Dembrovszky, F.; Solymár, M.; Bartalis, E.; Szakács, Z.; et al. Obesity Is a Risk Factor for Developing Critical Condition in COVID-19 Patients: A Systematic Review and Meta-Analysis. Obes. Rev. 2020, 21, e13095. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, Y.; Ye, L.; Sun, H.; Du, S.; Huang, H.; Zeng, F.; Chen, X.; Deng, G. Clinical Significance of Plasma D-Dimer in COVID-19 Mortality. Front. Med. 2021, 8, 638097. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Cheruiyot, I.; Vikse, J.; Mutua, V.; Kipkorir, V.; Benoit, J.; Plebani, M.; Bragazzi, N.; Lippi, G. Lymphopenia and Neutrophilia at Admission Predicts Severity and Mortality in Patients with COVID-19: A Meta-Analysis. Acta Biomed. 2020, 91, e2020008. [Google Scholar] [CrossRef]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, T.C.; Cadirci, E.; Bayrak, B.; Celik, S.; Atila, N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The Serum Amino Acid Profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O.; et al. Amino Acid Metabolism Is Significantly Altered at the Time of Admission in Hospital for Severe COVID-19 Patients: Findings from Longitudinal Targeted Metabolomics Analysis. Microbiol. Spectr. 2021, 9, e00338-21. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-Dimer Levels on Admission to Predict in-Hospital Mortality in Patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Rad, S.; Rostami, T.; Rostami, M.; Mousavi, S.A.; Mirhoseini, S.A.; Kiumarsi, A. Hematologic Predictors of Mortality in Hospitalized Patients with COVID-19: A Comparative Study. Hematology 2020, 25, 383–388. [Google Scholar] [CrossRef]
- Rizo-Téllez, S.A.; Méndez-García, L.A.; Flores-Rebollo, C.; Alba-Flores, F.; Alcántara-Suárez, R.; Manjarrez-Reyna, A.N.; Baltazar-López, N.; Hernández-Guzmán, V.A.; León-Pedroza, J.I.; Zapata-Arenas, R.; et al. The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict in-Hospital Mortality in Mexican Patients with Severe Sars-Cov-2 Infection (COVID-19). Microorganisms 2020, 8, 1560. [Google Scholar] [CrossRef]
- Imam, Z.; Odish, F.; Gill, I.; O’Connor, D.; Armstrong, J.; Vanood, A.; Ibironke, O.; Hanna, A.; Ranski, A.; Halalau, A. Older Age and Comorbidity Are Independent Mortality Predictors in a Large Cohort of 1305 COVID-19 Patients in Michigan, United States. J. Intern. Med. 2020, 288, 469–476. [Google Scholar] [CrossRef]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARSCoV-2: A Prospective Cohort Study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.S.; Lee, K.; Park, J.; Yun, S.; Lee, Y.S.; Lee, D.S. Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals. J. Korean Med. Sci. 2020, 35, e328. [Google Scholar] [CrossRef]
- Gómez, N.F.P.; Lobo, I.M.; Cremades, I.G.; Tejerina, A.F.; Rueda, F.R.; von Teleki, A.W.; Campos, F.M.A.; de Benito, A.S.M. Potential Biomarkers Predictors of Mortality in COVID-19 Patients in the Emergency Department. Rev. Esp. Quimioter. 2020, 33, 267–273. [Google Scholar] [CrossRef]
- Tian, W.; Jiang, W.; Yao, J.; Nicholson, C.J.; Li, R.H.; Sigurslid, H.H.; Wooster, L.; Rotter, J.I.; Guo, X.; Malhotra, R. Predictors of Mortality in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Med. Virol. 2020, 92, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Mesas, A.E.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Cabrera, M.A.S.; de Andrade, S.M.; Sequí-Dominguez, I.; Martínez-Vizcaíno, V. Predictors of In-Hospital COVID-19 Mortality: A Comprehensive Systematic Review and Meta-Analysis Exploring Differences by Age, Sex and Health Conditions. PLoS ONE 2020, 15, e0241742. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, H.X.; Hu, K.; Wu, X.J.; Zhang, Y.T.; Wang, M.M.; Wang, T.; Zheng, Z.S.; Li, X.C.; Zeng, S.L. Abnormal Immunity of Non-Survivors with COVID-19: Predictors for Mortality. Infect. Dis. Poverty 2020, 9, 108. [Google Scholar] [CrossRef]
- Li, X.; Ge, P.; Zhu, J.; Li, H.; Graham, J.; Singer, A.; Richman, P.S.; Duong, T.Q. Deep Learning Prediction of Likelihood of ICU Admission and Mortality in COVID-19 Patients Using Clinical Variables. PeerJ 2020, 8, e10337. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Patti, G.; Hayden, E.; Azzolina, D.; Pirisi, M.; Acquaviva, A.; Aimaretti, G.; Aluffi Valletti, P.; Angilletta, R.; Arioli, R.; et al. Fatality Rate and Predictors of Mortality in an Italian Cohort of Hospitalized COVID-19 Patients. Sci. Rep. 2020, 10, 20731. [Google Scholar] [CrossRef]
- Kissling, S.; Rotman, S.; Gerber, C.; Halfon, M.; Lamoth, F.; Comte, D.; Lhopitallier, L.; Sadallah, S.; Fakhouri, F. Collapsing Glomerulopathy in a COVID-19 Patient. Kidney Int. 2020, 98, 228–231. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-Associated Acute Kidney Injury: Consensus Report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Wendel Garcia, P.D.; Fumeaux, T.; Guerci, P.; Heuberger, D.M.; Montomoli, J.; Roche-Campo, F.; Schuepbach, R.A.; Hilty, M.P. Prognostic Factors Associated with Mortality Risk and Disease Progression in 639 Critically Ill Patients with COVID-19 in Europe: Initial Report of the International RISC-19-ICU Prospective Observational Cohort. EClinicalMedicine 2020, 25, 100449. [Google Scholar] [CrossRef]
- Figliozzi, S.; Masci, P.G.; Ahmadi, N.; Tondi, L.; Koutli, E.; Aimo, A.; Stamatelopoulos, K.; Dimopoulos, M.A.; Caforio, A.L.P.; Georgiopoulos, G. Predictors of Adverse Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2020, 50, e13362. [Google Scholar] [CrossRef]
- Liu, R.; Ma, Q.; Han, H.; Su, H.; Liu, F.; Wu, K.; Wang, W.; Zhu, C. The Value of Urine Biochemical Parameters in the Prediction of the Severity of Coronavirus Disease 2019. Clin. Chem. Lab. Med. 2020, 58, 1121–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wei, Y.; Chen, M.; Wan, Q.; Chen, X. Clinical Analysis of Risk Factors for Severe COVID-19 Patients with Type 2 Diabetes. J. Diabetes Complicat. 2020, 34, 107666. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic Changes in Amino Acid Concentration Profiles in Patients with Sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef] [Green Version]
- Coleman, D.N.; Lopreiato, V.; Alharthi, A.; Loor, J.J. Amino Acids and the Regulation of Oxidative Stress and Immune Function in Dairy Cattle. J. Anim. Sci. 2020, 98, S175–S193. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, W.S.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Chen, L.; Sun, J.; Chen, L.; Kou, Q. Metabonomics Analysis of Sepsis and Non-Infected SIRS Patients Based on Mass Spectrometry. Int. J. Clin. Exp. Med. 2019, 12, 5023–5032. [Google Scholar]
- Hamanaka, R.B.; Mutlu, G.M. The Role of Metabolic Reprogramming and de Novo Amino Acid Synthesis in Collagen Protein Production by Myofibroblasts: Implications for Organ Fibrosis and Cancer. Amino Acids 2021, 53, 1851–1862. [Google Scholar] [CrossRef]
- Robison, S.W.; Li, J.D.; Viera, L.; Blackburn, J.P.; Patel, R.P.; Blalock, J.E.; Gaggar, A.; Xu, X. A Mechanism for Matrikine Regulation in Acute Inflammatory Lung Injury. JCI Insight 2021, 6, e140750. [Google Scholar] [CrossRef]
- Rozga, M.; Cheng, F.W.; Moloney, L.; Handu, D. From The Academy Evidence Analysis Center Effects of Micronutrients or Conditional Amino Acids on COVID-19-Related Outcomes: An Evidence Analysis Center Scoping Review. J. Acad. Nutr. Diet. 2021, 121, 1354–1363. [Google Scholar] [CrossRef]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Seyed Alinaghi, S.A.; Jahanfar, S. Predictors of Mortality in Patients with COVID-19—A Systematic Review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef] [PubMed]
- Fürst, P.; Pollack, L.; Graser, T.A.; Godel, H.; Stehle, P. Appraisal of Four Pre-Column Derivatization Methods for the High-Performance Liquid Chromatographic Determination of Free Amino Acids in Biological Materials. J. Chromatogr. A 1990, 499, 557–569. [Google Scholar] [CrossRef]
- Tang, Z.H.; Liu, J.; Zeng, F.; Li, Z.; Yu, X.; Zhou, L. Comparison of Prediction Model for Cardiovascular Autonomic Dysfunction Using Artificial Neural Network and Logistic Regression Analysis. PLoS ONE 2013, 8, e70571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logistic Regression Variable Selection Methods—IBM Documentation. Available online: https://www.ibm.com/docs/en/spss-statistics/28.0.0?topic=regression-logistic-variable-selection-methods (accessed on 1 May 2022).
- Peng, C.Y.J.; Lee, K.L.; Ingersoll, G.M. An Introduction to Logistic Regression Analysis and Reporting. J. Educ. Res. 2002, 96, 3–14. [Google Scholar] [CrossRef]
| Survivors | Non-Survivors | |||
|---|---|---|---|---|
| Parameter | Unit | Median (q1–q3) | Median (q1–q3) | p |
| Number of patients | n | 163 | 26 | |
| Age | year | 58.5 (45.4–67.2) | 76.2 (63.9–83.5) | <0.001 |
| Diabetes (yes/no) | n/n (%/%) | 35/130 (21.2/78.8) | 11/15 (42.3/57.7) | 0.019 |
| CV disease (yes/no) | n/n (%/%) | 28/137 (17.0/83.0) | 10/16 (38.5/61.5) | 0.011 |
| Hypertension (yes/no) | n/n (%/%) | 79/86 (47.9/52.1) | 60/6 (76.9/23.1) | 0.006 |
| Cancer (yes/no) | n/n (%/%) | 8/157 (4.8/95.2) | 5/21 (19.2/80.8) | 0.019 |
| Renal replacement therapy | n [%] | 1 [0.6] | 3 [11.5] | 0.008 |
| Mechanical ventilation | n [%] | 2 [1.2] | 13 [50.0] | <0.001 |
| CTSI | 9 (6–13) | 16 (8–21) | 0.001 | |
| Plasma glucose | mmol/L | 6.34 (5.60–7.80) | 8.91 (7.30–9.80) | <0.001 |
| BUN | mmol/L | 4.4 (3.4–6.1) | 9.3 (6.4–15.9) | <0.001 |
| Serum creatinine | µmol/L | 80 (68–95) | 97 (74–169) | 0.008 |
| Troponin T | µg/L | 6.8 (4.0–12.3) | 25.8 (13.6–38.5) | <0.001 |
| D-dimer | µg/L | 680 (411–1118) | 1697 (832–4316) | <0.001 |
| hsCRP | mg/L | 36.3 (9.9–98.7) | 109.5 (61.5–180.7) | <0.001 |
| IL-6 | pg/mL | 21.8 (9.8–48.4) | 96.9 (53.5–225.7) | <0.001 |
| RDW | %CV | 12.8 (12.2–13.5) | 14.6 (13.1–15.1) | <0.001 |
| Ly/neu | 0.263 (0.163–0.427) | 0.130 (0.078–0.214) | <0.001 | |
| Plasma Ser | µmol/L | 67.0 (59.6–76.4) | 56.6 (47.9–65.0) | <0.001 |
| Plasma Thr | µmol/L | 75.7 (65.7–91.0) | 61.4 (50.0–72.0) | <0.001 |
| Plasma Phe | µmol/L | 77.0 (65.0–96.0) | 103.6 (84.0–123.8) | <0.001 |
| FE Arg | % | 3.069 (1.750–5.120) | 5.614 (3.487–10.112) | <0.001 |
| Parameter | B | p |
|---|---|---|
| Model 1 | ||
| Age | 0.148 | <0.001 |
| CTSI | 0.294 | <0.001 |
| Plasma Thr | −0.077 | 0.009 |
| Plasma Phe | 0.036 | 0.004 |
| Model 2 | ||
| Age | 0.148 | <0.001 |
| CTSI | 0.296 | <0.001 |
| Plasma Thr | −0.077 | 0.009 |
| Plasma Phe | 0.036 | 0.004 |
| No Mechanical Ventilation | Mechanical Ventilation | |||
|---|---|---|---|---|
| Parameter | Unit | Median (q1–q3) | Median (q1–q3) | p |
| Number of patients | n | 176 | 15 | |
| Diabetes (yes/no) | n/n (%/%) | 38/138 (21.6/78.4) | 8/7 (53.3/46.7) | 0.006 |
| Hypertension (yes/no) | n/n (%/%) | 87/89 (49.4/50.6) | 12/3 (80.0/20.0) | 0.030 |
| CTSI | 9 (6–13) | 20 (16–22) | <0.001 | |
| Plasma glucose | mmol/L | 6.38 (5.69–8.44) | 9.05 (7.72–11.85) | <0.001 |
| BUN | mmol/L | 4.53 (3.49–6.6) | 7.58 (6.22–13.43) | <0.001 |
| Serum creatinine | µmol/L | 80 (68–98) | 96 (87–156) | 0.004 |
| Troponin T | µg/L | 7.23 (4.06–14.68) | 26.58 (13.56–42.74) | <0.001 |
| LDH | U/L | 535 (409–714) | 834 (631–1433) | <0.000 |
| hsCRP | mg/L | 38.7 (10.0–99.5) | 155.6 (82.2–192.3) | <0.001 |
| Procalcitonin | ng/mL | 0.06 (0.04–0.11) | 0.17 (0.13–0.47) | <0.001 |
| IL-6 | pg/mL | 24.0 (10.2–56.6) | 109.2 (70.4–233.9) | <0.001 |
| RDW | %CV | 12.9 (12.3–13.6) | 14.6 (13.6–14.9) | 0.001 |
| Ly/neu | 0.254 (0.149–0.424) | 0.114 (0.079–0.243) | 0.008 | |
| Plasma Asp | µmol/L | 5.0 (4.0–6.2) | 8.0 (7.0–9.3) | <0.001 |
| Plasma Ser | µmol/L | 66.97 (58.9–76.2) | 55.2 (49.5–60.9) | <0.001 |
| Plasma Gly | µmol/L | 117.2 (97.9–142.2) | 93.0 (83.1–100.5) | <0.001 |
| Plasma Phe | µmol/L | 79.0 (66.3–98.0) | 103.2 (84.0–126.4) | <0.001 |
| Urinary total AA/creatinine | µmol/mmol | 0.252 (0.187–0.349) | 0.184 (0.142–0.204) | <0.001 |
| FE Gln | % | 0.550 (0.372–0.742) | 0.386 (0.248–0.460) | 0.002 |
| FE Gly | % | 2.586 (1.819–3.980) | 1.763 (1.350–2.000) | 0.002 |
| FE Arg | % | 3.177 (1.776–5.216) | 7.163 (3.594–9.072) | 0.003 |
| Parameter | B | p |
|---|---|---|
| Models 1 and 2 | ||
| CTSI | 0.261 | 0.004 |
| Plasma Gly | −0.071 | 0.028 |
| FE Gly | −1.200 | 0.053 |
| FE Arg | 0.141 | 0.101 |
| Model 3 | ||
| CTSI | 3.496 | 0.090 |
| Plasma glucose | −3.635 | 0.148 |
| Troponin T | 0.218 | 0.128 |
| IL-6 | −0.023 | 0.073 |
| Ly neu | 40.809 | 0.123 |
| Plasma Ser | −0.682 | 0.143 |
| Plasma Gly | −0.405 | 0.108 |
| Plasma Phe | 0.156 | 0.111 |
| FE Gly | −10.758 | 0.090 |
| FE Arg | 0.917 | 0.124 |
| LDH | −0.003 | 0.178 |
| Serum creatinine | −0.210 | 0.108 |
| Model 4 | ||
| CTSI | 0.856 | 0.005 |
| Plasma glucose | −0.470 | 0.067 |
| BUN | −0.243 | 0.053 |
| Troponin T | 0.049 | 0.038 |
| IL-6 | −0.006 | 0.090 |
| Plasma Gly | −0.160 | 0.027 |
| Plasma Phe | 0.055 | 0.086 |
| FE Gly | −2.390 | 0.019 |
| LDH | −0.002 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bánfai, G.; Kanizsai, P.; Csontos, C.; Kun, S.; Lakatos, Á.; Lajtai, A.; Lelovics, V.; Szukits, S.; Bogner, P.; Miseta, A.; et al. Plasma Levels and Renal Handling of Amino Acids Contribute to Determination of Risk of Mortality or Feed of Ventilation in Patients with COVID-19. Metabolites 2022, 12, 486. https://doi.org/10.3390/metabo12060486
Bánfai G, Kanizsai P, Csontos C, Kun S, Lakatos Á, Lajtai A, Lelovics V, Szukits S, Bogner P, Miseta A, et al. Plasma Levels and Renal Handling of Amino Acids Contribute to Determination of Risk of Mortality or Feed of Ventilation in Patients with COVID-19. Metabolites. 2022; 12(6):486. https://doi.org/10.3390/metabo12060486
Chicago/Turabian StyleBánfai, Gábor, Péter Kanizsai, Csaba Csontos, Szilárd Kun, Ágnes Lakatos, Anikó Lajtai, Vanessza Lelovics, Sándor Szukits, Péter Bogner, Attila Miseta, and et al. 2022. "Plasma Levels and Renal Handling of Amino Acids Contribute to Determination of Risk of Mortality or Feed of Ventilation in Patients with COVID-19" Metabolites 12, no. 6: 486. https://doi.org/10.3390/metabo12060486
APA StyleBánfai, G., Kanizsai, P., Csontos, C., Kun, S., Lakatos, Á., Lajtai, A., Lelovics, V., Szukits, S., Bogner, P., Miseta, A., Wittmann, I., & Molnár, G. A. (2022). Plasma Levels and Renal Handling of Amino Acids Contribute to Determination of Risk of Mortality or Feed of Ventilation in Patients with COVID-19. Metabolites, 12(6), 486. https://doi.org/10.3390/metabo12060486







