Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening
Abstract
:1. Introduction
2. Results
2.1. Measurement of Color
2.2. Metabolic Profiling of Fruit at Different Stages of Ripening
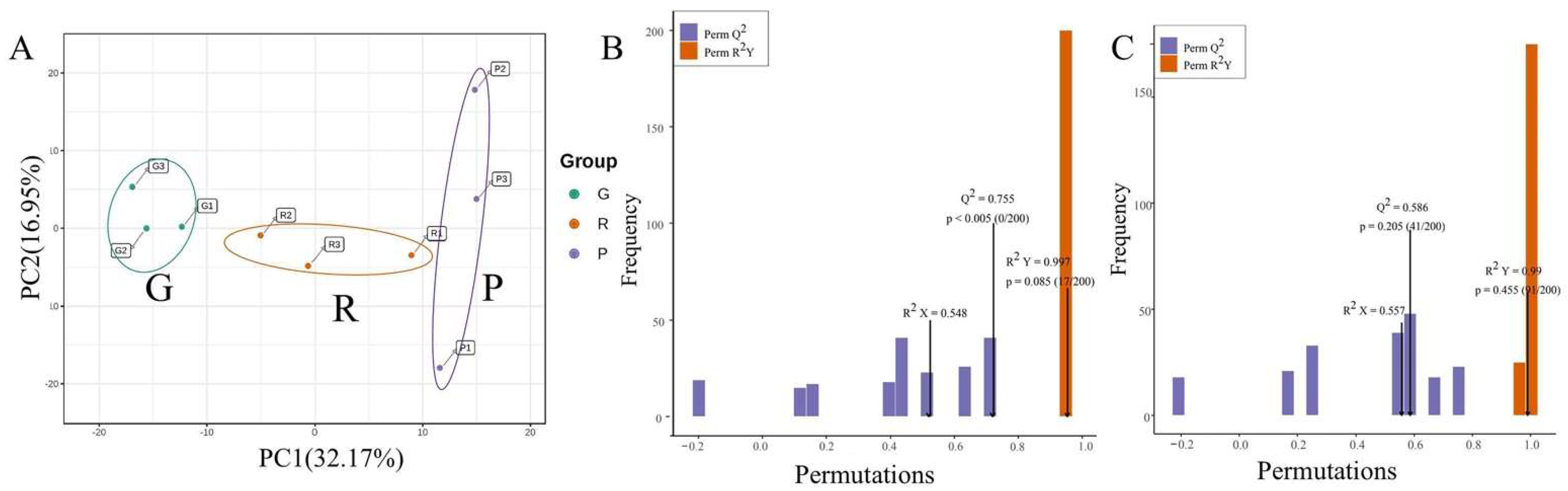
2.3. PCA and OPLS-DA Analysis of Differentially Accumulated Metabolites (DAMs)
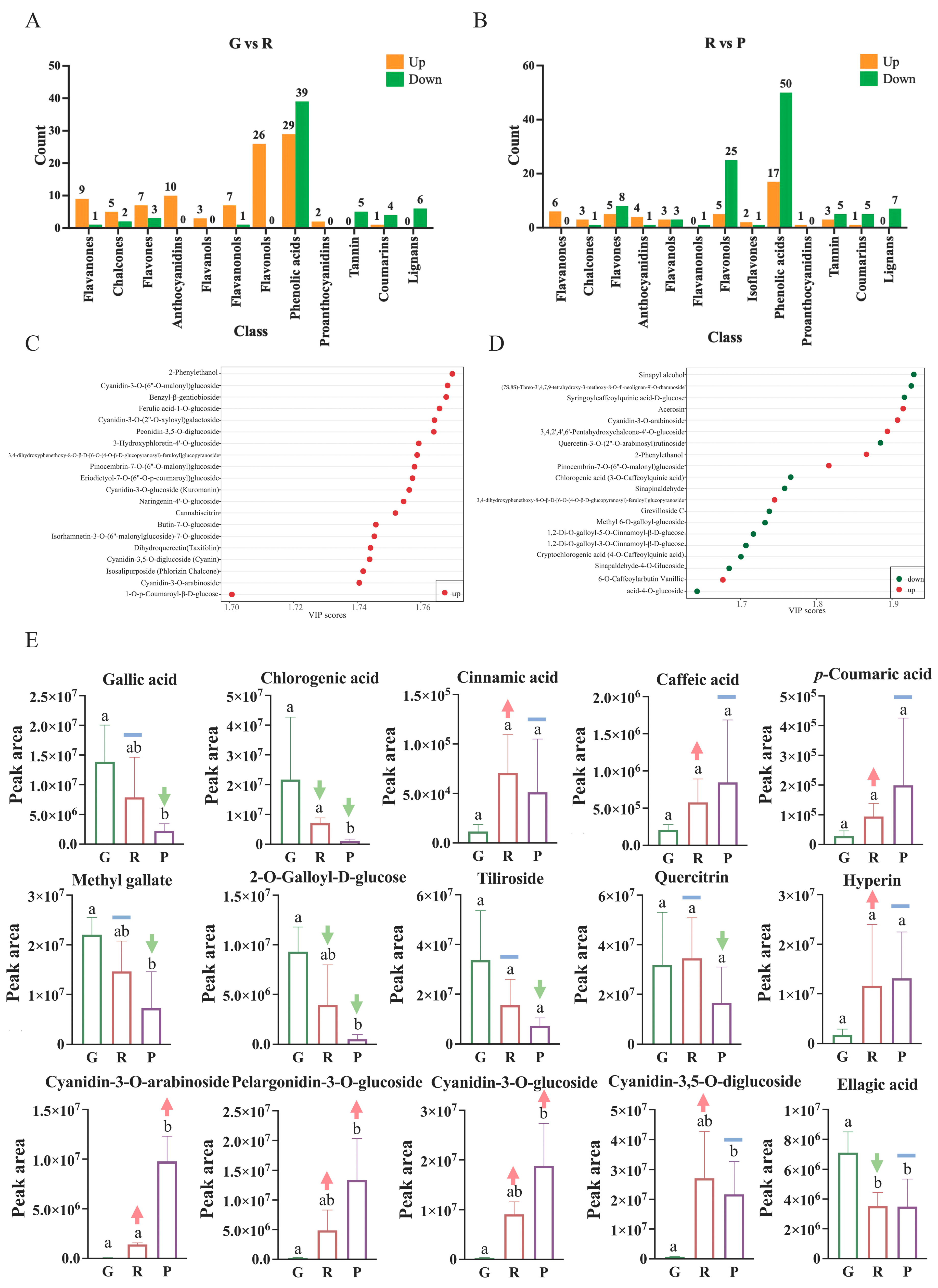
2.4. Differential Metabolic Profiling during Fruit Growth and Development
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Rose Hip Color Measurements
4.3. Sample Preparation and Extraction
4.4. Conditions for Chromatography-Mass Spectrometry
4.5. Qualitative and Quantitative Analysis of Metabolites
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT—Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Xu, J.W.; Vidyarthi, S.K.; Bai, W.B.; Pan, Z.L. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Nađpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef]
- Czyzowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Li, X.; Cao, W.; Shen, Y.; Li, N.; Dong, X.P.; Wang, K.J.; Cheng, Y.X. Antioxidant compounds from Rosa laevigata fruits. Food Chem. 2012, 130, 575–580. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.D.; Muller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The Effect of Ripening Stages on the Accumulation of Carotenoids, Polyphenols and Vitamin C in Rosehip Species/Cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Hou, Z.; Yang, H.; Zhao, Y.; Xu, L.; Zhao, L.; Wang, Y.; Liao, X. Chemical characterization and comparison of two chestnut rose cultivars from different regions. Food Chem. 2020, 323, 126806. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.C.; Robertson, K.R. Flora of China; Science Press: Beijing, China, 2003; Volume 9. [Google Scholar]
- Ren, J.; Wang, J.D.; Chai, Q.Y.; Yang, G.; Wei, G. Study on antithrombotic effects of Rosa xanthina Lindl. fruit and its mechanism. J. Shanxi Med. Univ. 2017, 48, 539–542. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Parijadi, A.A.R.; Putri, S.P.; Ridwani, S.; Dwivany, F.M.; Fukusaki, E. Metabolic profiling of Garcinia mangostana (mangosteen) based on ripening stages. J. Biosci. Bioeng. 2018, 125, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Uggla, M.; Gustavsson, K.E.; Olsson, M.E.; Nybom, H. Changes in colour and sugar content in rose hips (Rosa dumalis L. and R. rubiginosa L.) during ripening. J. Hortic. Sci. Biotech. 2005, 80, 204–208. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J. Agr. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Gao, X.; Björk, L.; Trajkovski, V.; Uggla, M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000, 80, 2021–2027. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerod, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.H.; Gao, Z.F.; Jiao, X.; Shi, J.F.; Wang, R.F. Widely targeted metabolomic analysis of active compounds at different maturity stages of ‘Hupingzao’ jujube. J. Food Compos. Anal. 2020, 88, 103417. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Cunja, V.; Mikulic-Petkovsek, M.; Weber, N.; Jakopic, J.; Zupan, A.; Veberic, R.; Stampar, F.; Schmitzer, V. Fresh from the Ornamental Garden: Hips of Selected Rose Cultivars Rich in Phytonutrients. J. Food Sci. 2016, 81, C369–C379. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Guglielmi, F.; Meoni, M.; Dolara, P. Effect of natural phenolic acids on DNA oxidation in vitro. Food Chem. Toxicol. 2001, 39, 1205–1210. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic Acid: A Potential Anti-Cancer Agent. Chin. J. Integr. Med. 2021. [Google Scholar] [CrossRef]
- Santana-Galvez, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Brumme, H.; Bruns, E.; Mehring, B.; Proll, T.; Wiegand, J. Phenological growth stages of roses (Rosa sp.): Codification and description according to the BBCH scale. Ann. Appl. Biol. 2009, 154, 231–238. [Google Scholar] [CrossRef]
- Xu, J.; Yan, J.; Li, W.; Wang, Q.; Wang, C.; Guo, J.; Geng, D.; Guan, Q.; Ma, F. Integrative Analyses of Widely Targeted Metabolic Profiling and Transcriptome Data Reveals Molecular Insight into Metabolomic Variations during Apple (Malus domestica) Fruit Development and Ripening. Int. J. Mol. Sci. 2020, 21, 4797. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Duan, Y.; Zhao, Y.; Zhang, D.; Zang, L.; Ya, H. Widely Targeted Metabolomics Analysis of Different Parts of Salsola collina Pall. Molecules 2021, 26, 1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Li, R.S.; Ren, L.; Gao, X.L.; Zhang, Y.G.; Ma, Z.M.; Ma, D.F.; Luo, Y.H. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]

| L* | a* | b* | C | h | |
|---|---|---|---|---|---|
| Green hips | 63.60 ± 2.6 c | −8.81 ± 2.8 a | 28.51 ± 3.7 c | 29.92 ± 4.10 b | 106.95 ± 4.34 a |
| Red hips | 39.82 ± 5.21 b | 26.40 ± 6.91 c | 9.25 ± 6.08 b | 28.67 ± 6.75 b | 19.21 ± 11.99 b |
| Purple hips | 31.13 ± 3.9 a | 6.16 ± 2.0 b | 1.43 ± 3.1 a | 6.77 ± 2.78 a | 10.44 ± 17.30 b |
| Class I | Class II | Number of Metabolites |
|---|---|---|
| Flavonoids | Flavanones | 19 |
| Chalcones | 10 | |
| Flavones | 48 | |
| Anthocyanidins | 17 | |
| Flavanols | 22 | |
| Flavanonols | 11 | |
| Flavonols | 83 | |
| Isoflavones | 9 | |
| Phenolic acids | Phenolic acids | 220 |
| Tannins | Proanthocyanidins | 9 |
| Tannins | 41 | |
| Lignans and Coumarins | Coumarins | 17 |
| Lignans | 25 | |
| Total | 531 | |
| Conditions | Parameters | |
|---|---|---|
| Column | Agilent SB-C18 (1.8 µm, 2.1 mm × 100 mm) | |
| Mobile phase | Mobile phase A (pure water with 0.1% formic acid) Mobile phase B (acetonitrile with 0.1% formic acid) | |
| Gradient program | 0 min | 95:5 v/v (Mobile phase A: Mobile phase B) |
| 0–9 min | a linear gradient to 5:95 v/v | |
| 9–10 min | 5:95 v/v | |
| 10–11.1 min | adjust to 95:5 v/v | |
| 11.1–14 min | 95:5 v/v | |
| Flow rate | 0.35 mL/min | |
| Column temperature | 40 °C | |
| Injection volume | 4 μL | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Yang, Y.; Zhou, M.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening. Metabolites 2022, 12, 438. https://doi.org/10.3390/metabo12050438
Sun Y, Yang Y, Zhou M, Luo L, Pan H, Zhang Q, Yu C. Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening. Metabolites. 2022; 12(5):438. https://doi.org/10.3390/metabo12050438
Chicago/Turabian StyleSun, Yanlin, Yu Yang, Meichun Zhou, Le Luo, Huitang Pan, Qixiang Zhang, and Chao Yu. 2022. "Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening" Metabolites 12, no. 5: 438. https://doi.org/10.3390/metabo12050438
APA StyleSun, Y., Yang, Y., Zhou, M., Luo, L., Pan, H., Zhang, Q., & Yu, C. (2022). Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening. Metabolites, 12(5), 438. https://doi.org/10.3390/metabo12050438







