Lipidomics Revealed Aberrant Lipid Metabolism Caused by Inflammation in Cardiac Tissue in the Early Stage of Systemic Lupus Erythematosus in a Murine Model
Abstract
:1. Introduction
2. Results
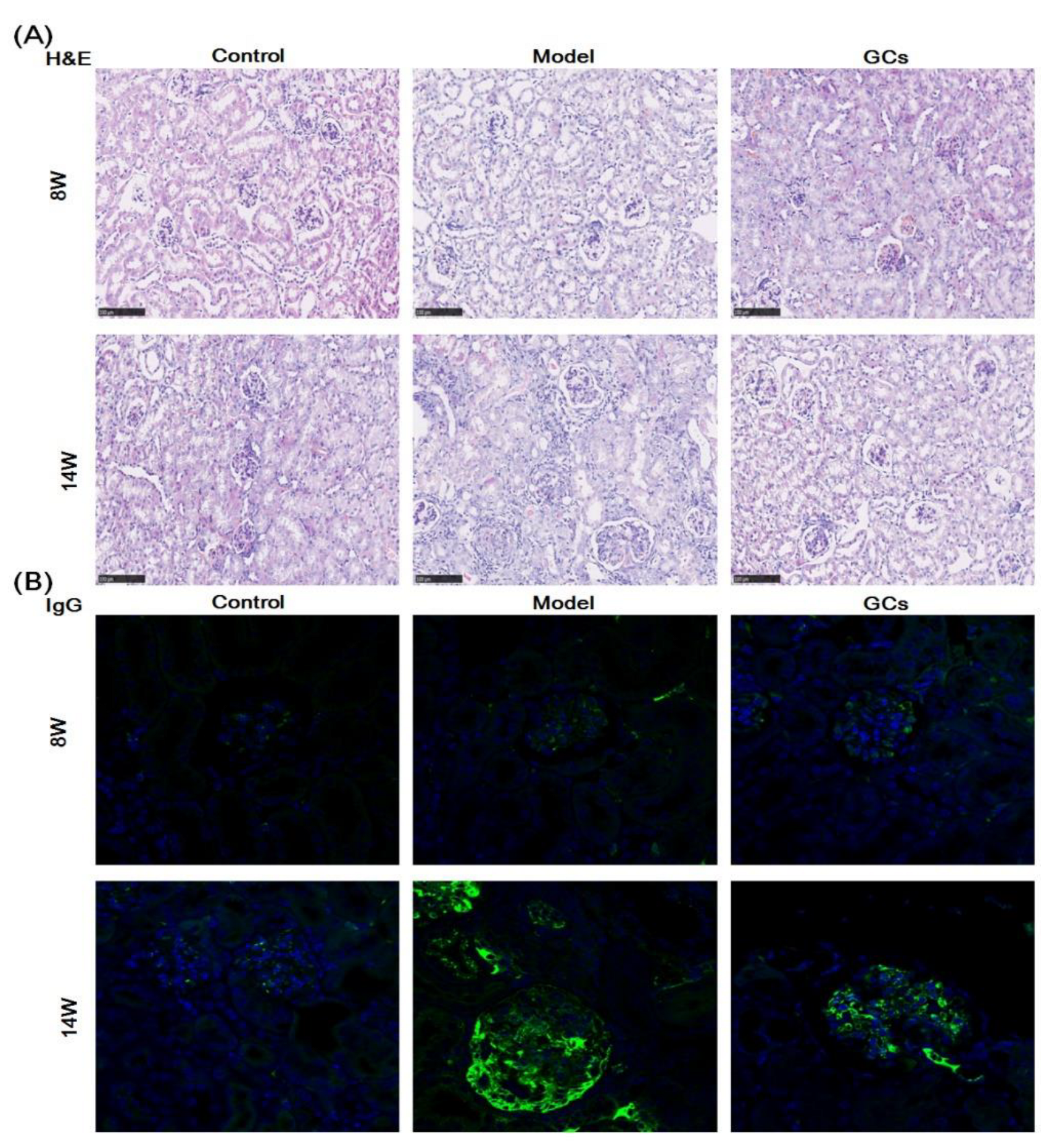
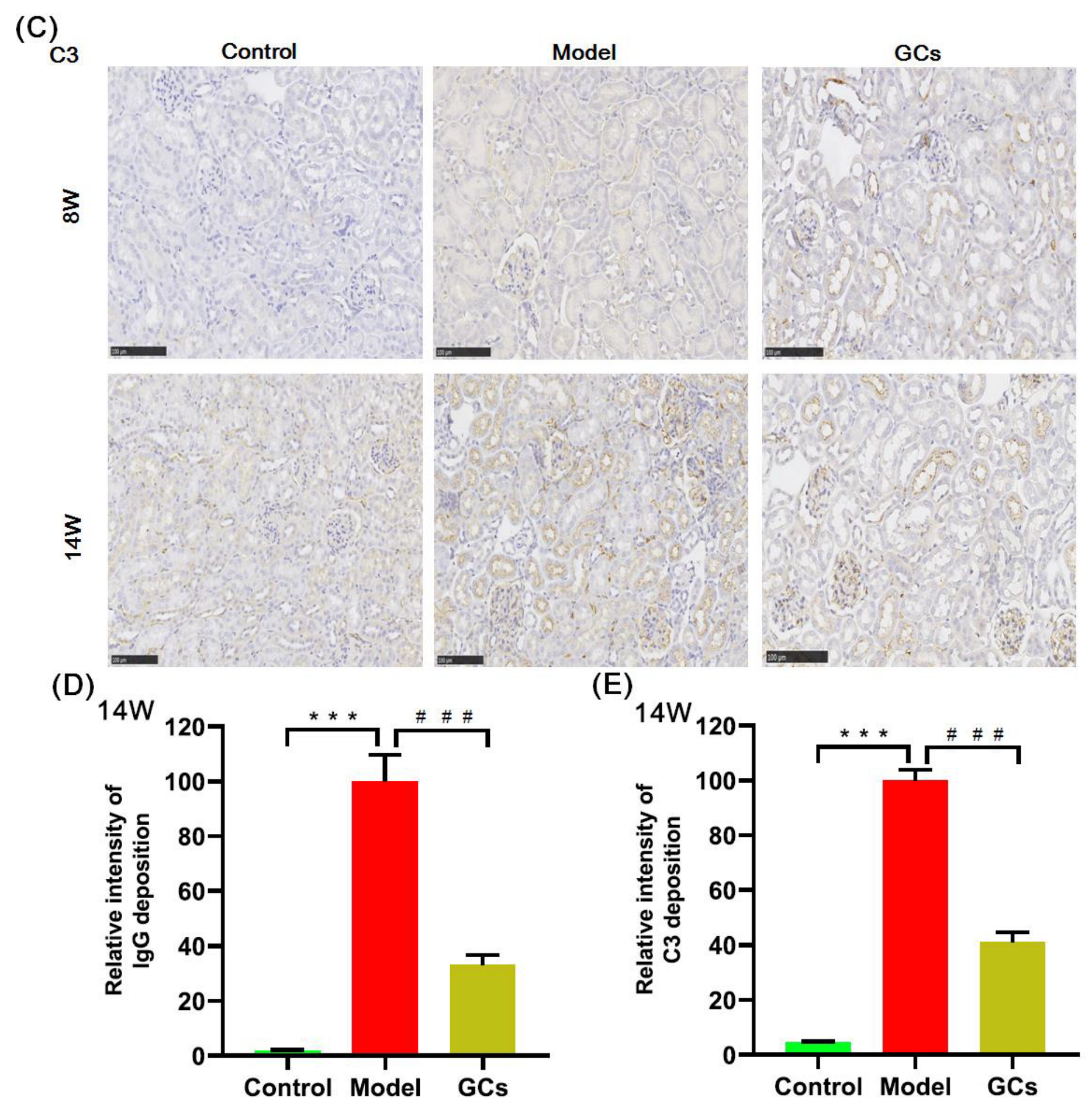
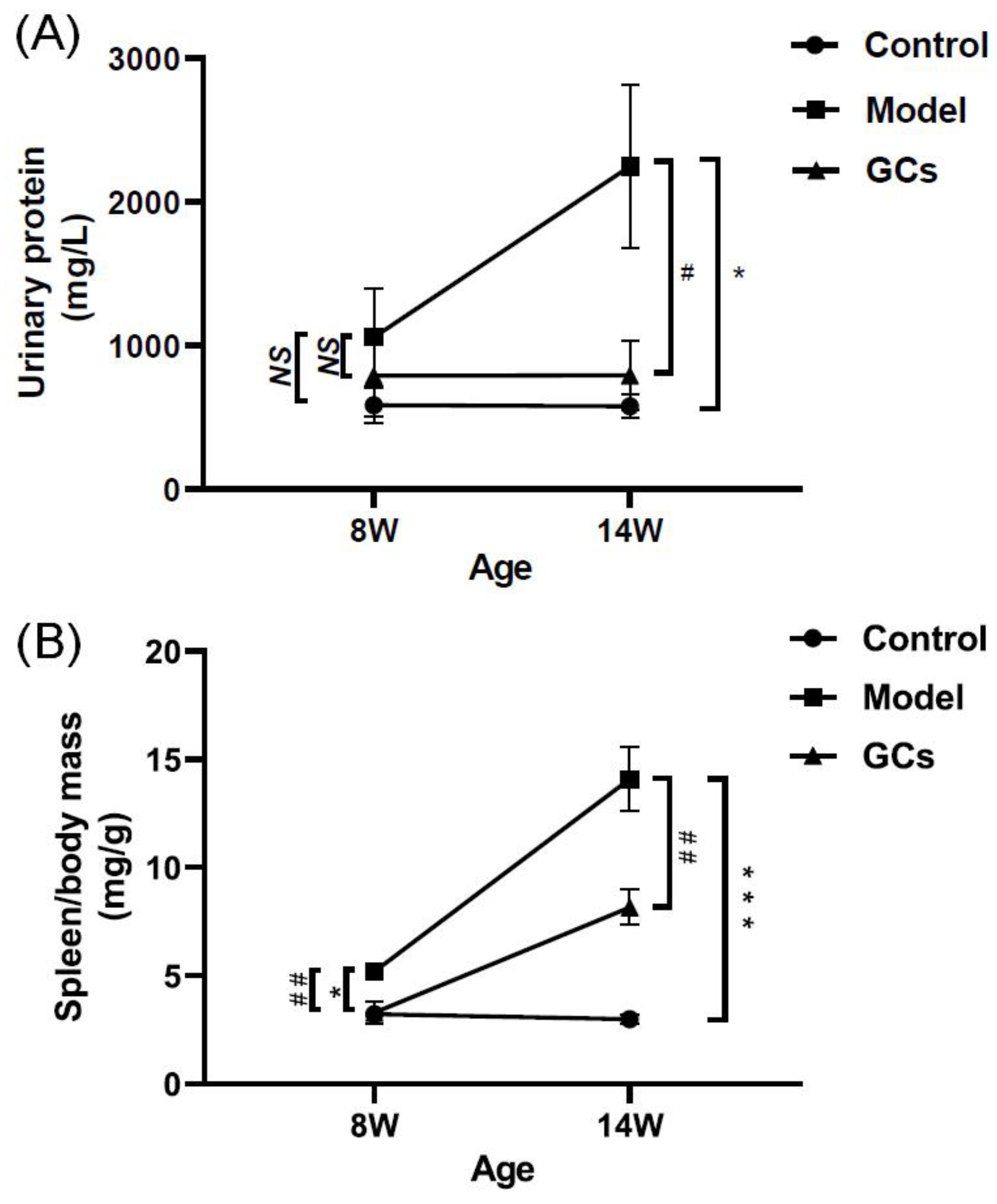
2.1. SLE Onset and Severity, and/or Outcome of MRL/lpr Mice
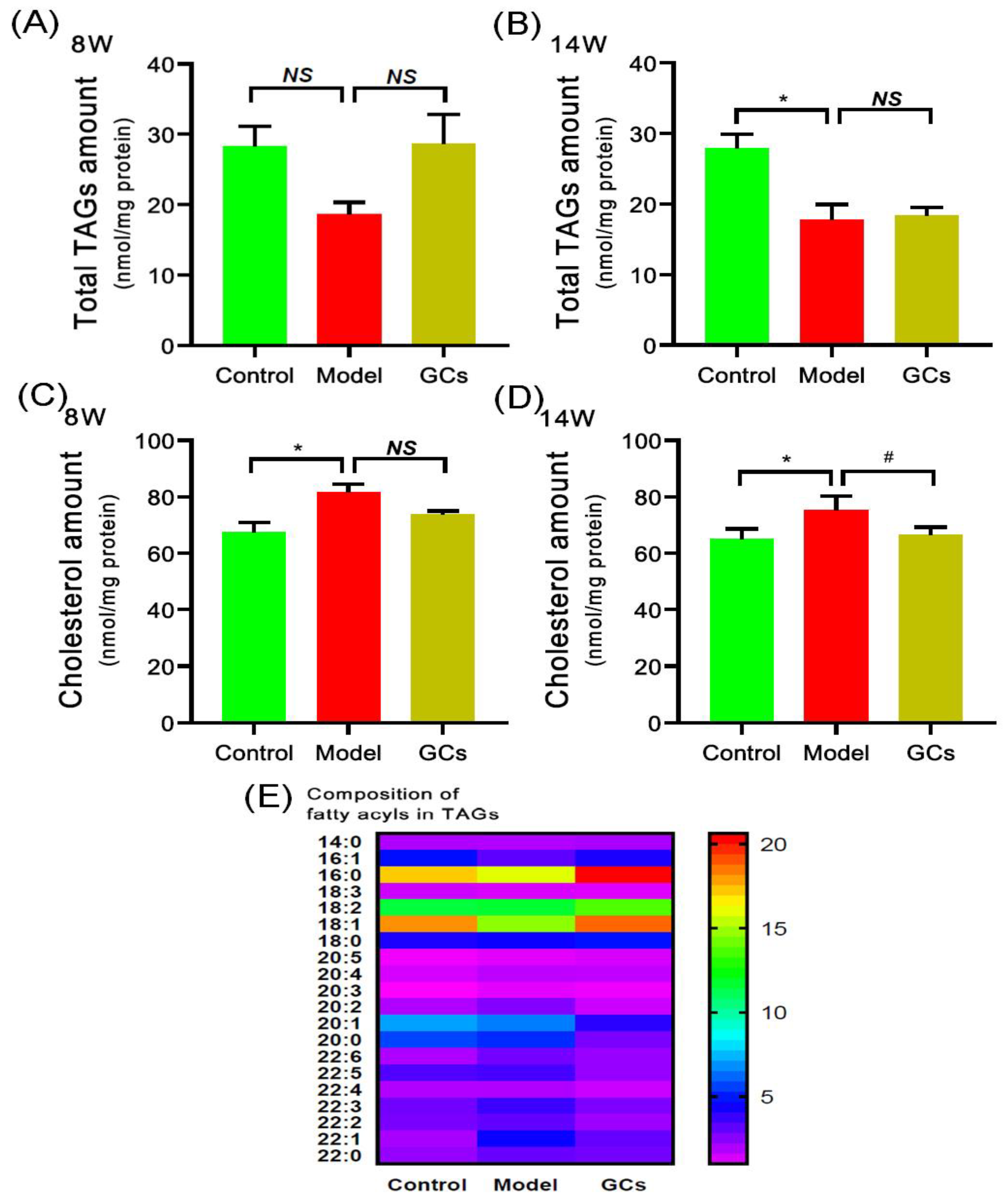
2.2. The Alterations of TAGs and Cholesterol Masses in Cardiac Tissues of MRL/lpr Mice
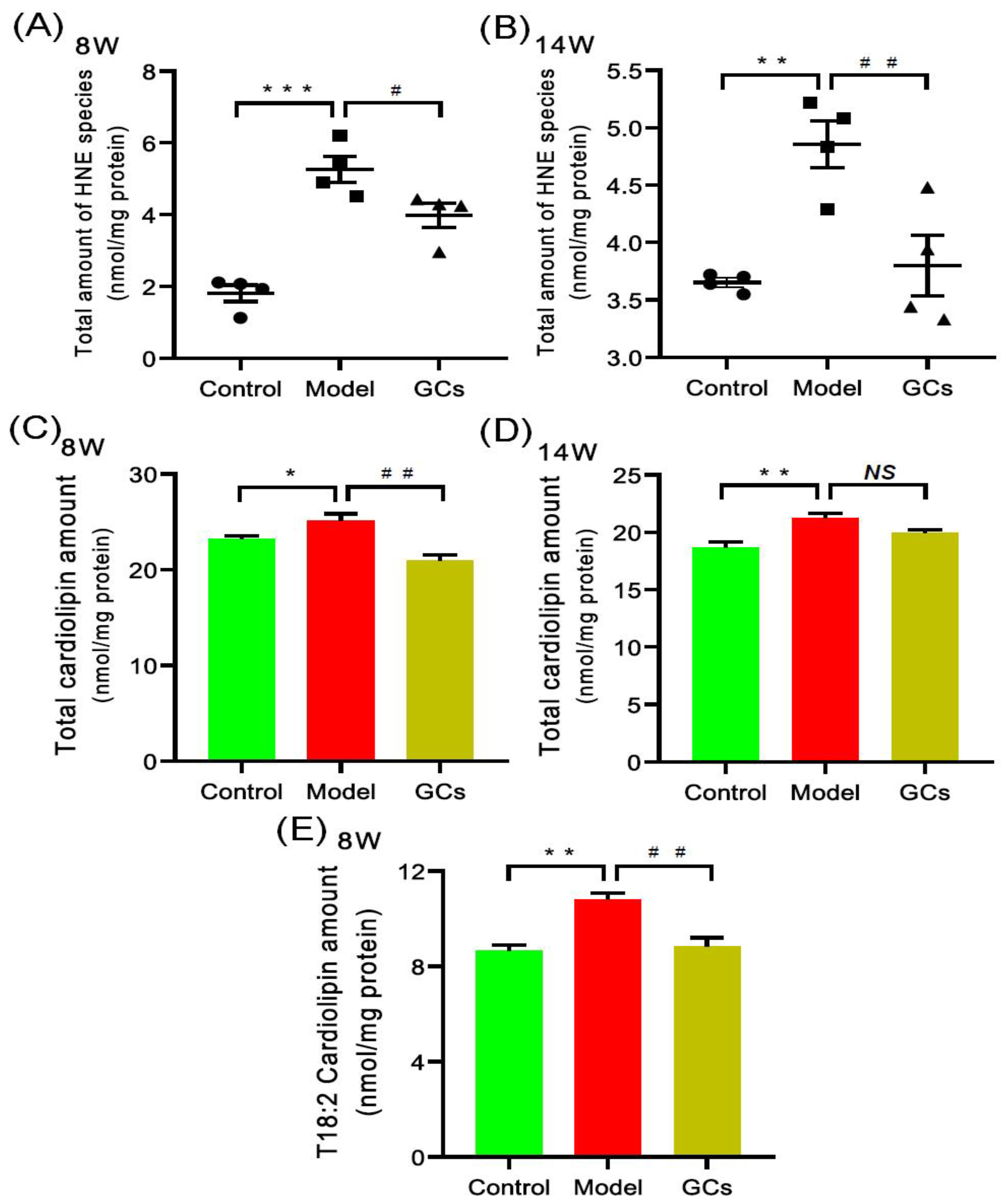
2.3. Lipidomics Analysis Revealed Increased Levels of Hydroxyalkenals and Cardiolipin Species in Heart Tissues from MRL/lpr Mice
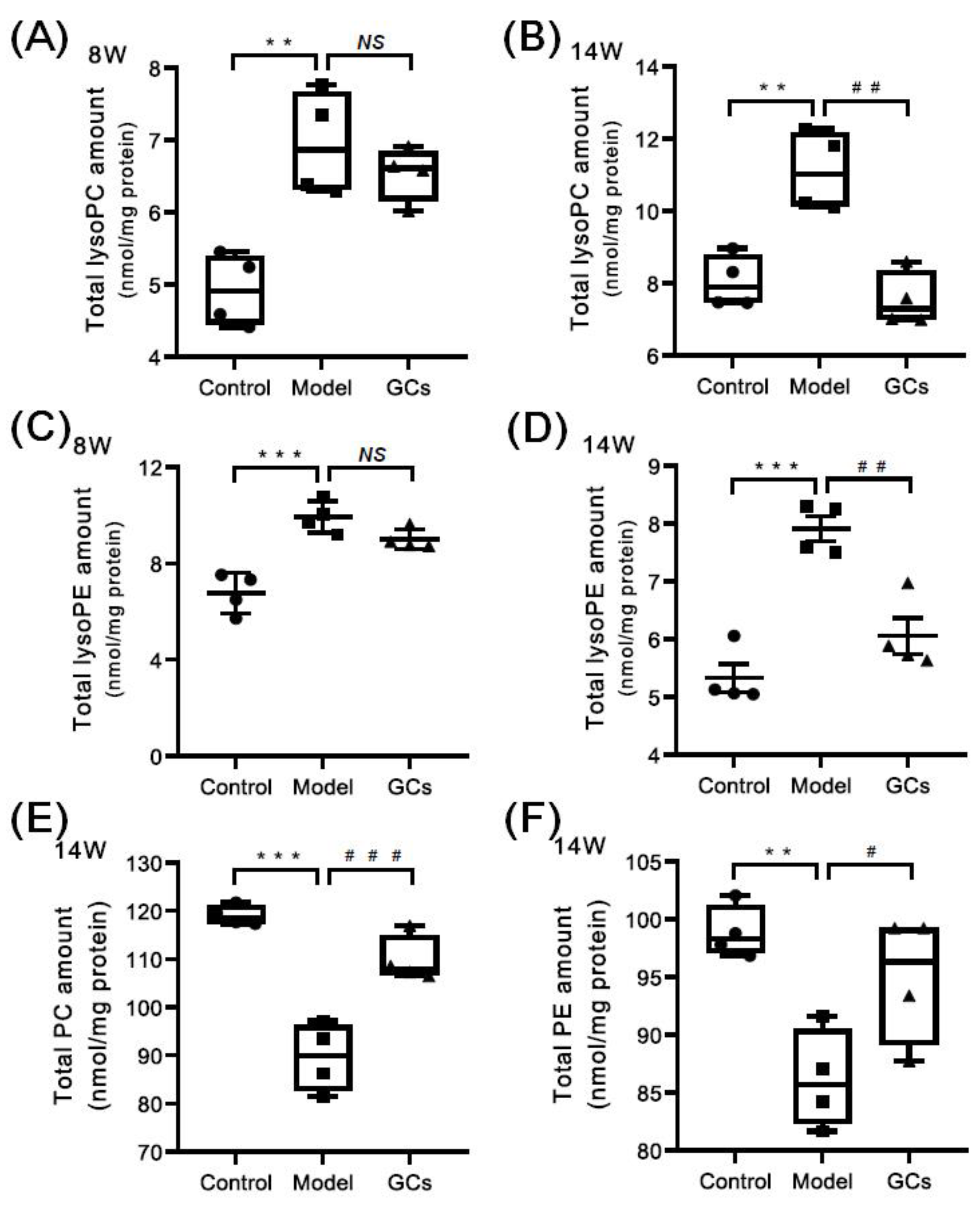
2.4. Lipidomics Analysis Revealed the Aberrant Metabolism of Phospholipids in Heart Tissues from MRL/lpr Mice
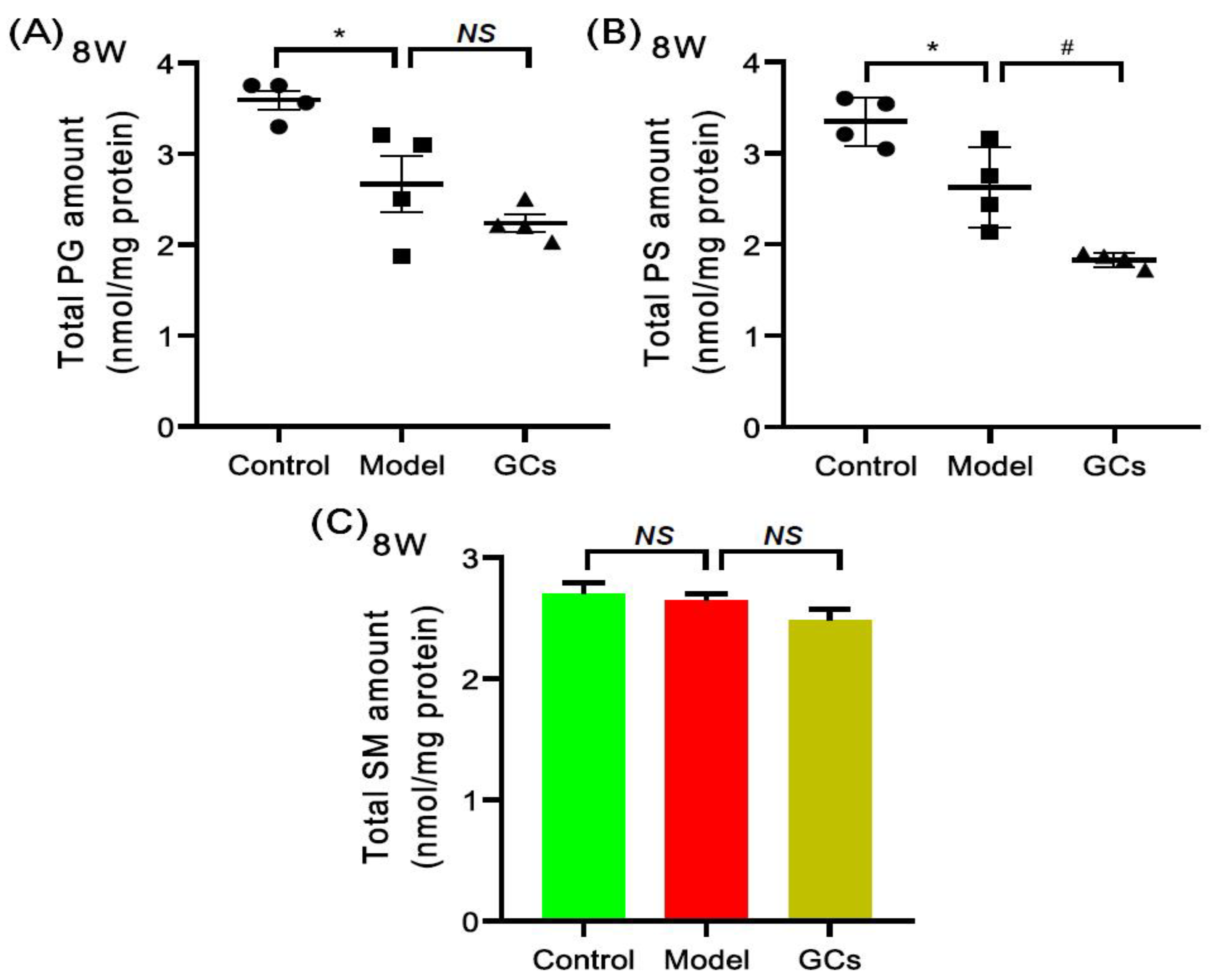
2.5. Lipidomics Analysis Also Revealed the Alterations of Other Classes of Phospholipids and SM Species in Heart Tissues from MRL/lpr Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
4.3. Assessment of SLE Onset and/or Outcome
4.3.1. Urine Protein and Spleen Index Measurement
4.3.2. Renal Histopathological Evaluation
4.4. Preparation of Lipid Extracts from Heart Samples
4.5. Lipid Analysis and Data Processing
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Lipson, A.; Alexopoulos, N.; Hartlage, G.R.; Arepalli, C.; Oeser, A.; Bian, A.; Gebretsadik, T.; Shintani, A.; Stillman, A.E.; Stein, C.M.; et al. Epicardial adipose tissue is increased in patients with systemic lupus erythematosus. Atherosclerosis 2012, 223, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfeld, S.R.; Kasturi, S.; Costenbader, K.H. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: A systematic review. Semin. Arthritis Rheum. 2013, 43, 77–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Herz, A.; Ensworth, S.; Shojania, K.; Esdaile, J.M. Cardiovascular risk factor screening in systemic lupus erythematosus. J. Rheumatol. 2003, 30, 493–496. [Google Scholar] [PubMed]
- Taylor, E.B.; Ryan, M.J. Immunosuppression with mycophenolate mofetil attenuates hypertension in an experimental model of autoimmune disease. J. Am. Heart Assoc. 2017, 6, e005394. [Google Scholar] [CrossRef]
- de Groot, P.G.; de Laat, B. Mechanisms of thrombosis in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract. Res. Clin. Rheumatol. 2017, 31, 334–341. [Google Scholar] [CrossRef]
- Lipsker, D. The Importance of thrombosis in patients with lupus erythematosus. Dermatology 2006, 212, 214–215. [Google Scholar] [CrossRef]
- Luo, S.; Hu, D.; Wang, M.; Zipfel, P.F.; Hu, Y. Complement in Hemolysis- and Thrombosis- Related Diseases. Front. Immunol. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Andrianova, I.A.; Ponomareva, A.A.; Mordakhanova, E.R.; Le Minh, G.; Daminova, A.G.; Nevzorova, T.A.; Rauova, L.; Litvinov, R.I.; Weisel, J.W. In systemic lupus erythematosus anti-dsDNA antibodies can promote thrombosis through direct platelet activation. J. Autoimmun. 2020, 107, 102355. [Google Scholar] [CrossRef]
- Tektonidou, M.G. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J. Autoimmun. 2022, 128, 102813. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Adams, M.J. Thrombosis in systemic lupus erythematosus: Role of impaired fibrinolysis. Semin. Thromb. Hemost. 2013, 39, 434–440. [Google Scholar] [CrossRef]
- Palatinus, A.; Adams, M. Thrombosis in systemic lupus erythematosus. Semin. Thromb. Hemost. 2009, 35, 621–629. [Google Scholar] [CrossRef]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid metabolite biomarkers in cardiovascular disease: Discovery and biomechanism translation from human studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef]
- Reichlin, M.; Fesmire, J.; Quintero-Del-Rio, A.I.; Wolfson-Reichlin, M. Autoantibodies to lipoprotein lipase and dyslipidemia in systemic lupus erythematosus. Arthritis Care Res. 2002, 46, 2957–2963. [Google Scholar] [CrossRef]
- Borba, E.; Bonfa, E.; Vinagre, C.G.C.; Ramires, J.A.F.; Maranhão, R. Chylomicron metabolism is markedly altered in systemic lupus erythematosus. Arthritis Care Res. 2000, 43, 1033–1040. [Google Scholar] [CrossRef]
- Frostegård, J.; Svenungsson, E.; Wu, R.; Gunnarsson, I.; Lundberg, I.E.; Klareskog, L.; Hörkkö, S.; Witztum, J.L. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Care Res. 2005, 52, 192–200. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, J.; Yang, S.; Li, H.; Wang, C.; Fang, X.; Fan, Y.; Zhang, J.; Han, X.; Wen, C. Oxidative stress leads to reduction of plasmalogen serving as a novel biomarker for systemic lupus erythematosus. Free Radic. Biol. Med. 2016, 101, 475–481. [Google Scholar] [CrossRef]
- Lu, L.; Hu, C.; Zhao, Y.; He, L.; Zhou, J.; Li, H.; Du, Y.; Wang, Y.; Wen, C.; Han, X.; et al. Shotgun lipidomics revealed altered profiles of serum lipids in systemic lupus erythematosus closely associated with disease activity. Biomolecules 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Zhang, J.; Hong, S.; Li, H.; Lu, L.; Xie, G.; Luo, W.; Du, Y.; Xie, Z.; Han, X.; et al. Oxidative stress-induced aberrant lipid metabolism is an important causal factor for dysfunction of immunocytes from patients with systemic lupus erythematosus. Free Radic. Biol. Med. 2020, 163, 210–219. [Google Scholar] [CrossRef]
- Toller-Kawahisa, J.E.; Canicoba, N.C.; Venancio, V.P.; Kawahisa, R.; Antunes, L.M.G.; Cunha, T.M.; Marzocchi-Machado, C.M. Systemic lupus erythematosus onset in lupus-prone B6.MRL/lpr mice is influenced by weight gain and is preceded by an increase in neutrophil oxidative burst activity. Free Radic. Biol. Med. 2015, 86, 362–373. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Zhang, X.; Wang, X. Erythropoietin treatment ameliorates lupus nephritis of MRL/lpr mice. Inflammation 2018, 41, 1888–1899. [Google Scholar] [CrossRef]
- Hu, C.; Du, Y.; Xu, X.; Li, H.; Duan, Q.; Xie, Z.; Wen, C.; Han, X. Lipidomics revealed aberrant metabolism of lipids including FAHFAs in renal tissue in the progression of lupus nephritis in a murine model. Metabolites 2021, 11, 142. [Google Scholar] [CrossRef]
- Shoshan, Y.; Mevorach, D. Accelerated autoimmune disease in MRL/MpJ-Faslprbut not in MRL/MpJ following immunization with high load of syngeneic late apoptotic cells. Autoimmunity 2004, 37, 103–109. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Han, X. Shotgun lipidomics in substantiating lipid peroxidation in redox biology: Methods and applications. Redox Biol. 2017, 12, 946–955. [Google Scholar] [CrossRef]
- Doherty, E.; Oaks, Z.; Perl, A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid. Redox Sign. 2014, 21, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Gergely, P.; Niland, B.; Gonchoroff, N.; Pullmann, R.; Phillips, P.E.; Perl, A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J. Immunol. 2002, 169, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef] [Green Version]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- McMahon, M.; Grossman, J.; FitzGerald, J.; Dahlin-Lee, E.; Wallace, D.J.; Thong, B.; Badsha, H.; Kalunian, K.; Charles, C.; Navab, M.; et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res. 2006, 54, 2541–2549. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Feldman, C.H.; Chen, S.K.; Guan, H.; Fischer, M.A.; Everett, B.M.; Costenbader, K.H. Comparative risks of cardiovascular disease in patients with systemic lupus erythematosus, diabetes mellitus, and in general medicaid recipients. Arthritis Care Res. 2020, 72, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Zhang, J.; Pu, D.; Hu, N.; Luo, J.; An, Q.; He, L. Patients with systemic lupus erythematosus face a high risk of cardiovascular disease: A systematic review and Meta-analysis. Int. Immunopharmacol. 2021, 94, 107466. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Han, X. Cardiolipin remodeling in diabetic heart. Chem. Phys. Lipids 2014, 179, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sparagna, G.C.; Chicco, A.J.; Murphy, R.C.; Bristow, M.R.; Johnson, C.A.; Rees, M.L.; Maxey, M.L.; McCune, S.A.; Moore, R.L. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 2007, 48, 1559–1570. [Google Scholar] [CrossRef] [Green Version]
- Saini-Chohan, H.K.; Holmes, M.G.; Chicco, A.J.; Taylor, W.A.; Moore, R.; McCune, S.A.; Hickson-Bick, D.L.; Hatch, G.M.; Sparagna, G.C. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid Res. 2009, 50, 1600–1608. [Google Scholar] [CrossRef] [Green Version]
- Mejia, E.M.; Nguyen, H.; Hatch, G.M. Mammalian cardiolipin biosynthesis. Chem. Phys. Lipids 2014, 179, 11–16. [Google Scholar] [CrossRef]
- Ye, J.; Keller, J. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Rhee, J.; Lin, J.; Wu, Z.; Yoon, J.; Zhang, C.-Y.; Krauss, S.; Mootha, V.K.; Lowell, B.B.; Spiegelman, B.M. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol. Cell 2001, 8, 971–982. [Google Scholar] [CrossRef]
- Luoma, A.M.; Kuo, F.; Cakici, O.; Crowther, M.N.; Denninger, A.R.; Avila, R.L.; Brites, P.; Kirschner, D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015, 84, 296–310. [Google Scholar] [CrossRef]
- Elkind, M.S. Inflammatory mechanisms of stroke. Stroke 2010, 41, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Ghesquiere, S.A.I.; Hofker, M.H.; De Winther, M.P.J. The role of phospholipases in lipid modification and atherosclerosis. Cardiovasc. Toxicol. 2005, 5, 161–182. [Google Scholar] [CrossRef]
- Olofsson, K.E.; Andersson, L.; Nilsson, J.; Björkbacka, H. Nanomolar concentrations of lysophosphatidylcholine recruit monocytes and induce pro-inflammatory cytokine production in macrophages. Biochem. Biophys. Res. Commun. 2008, 370, 348–352. [Google Scholar] [CrossRef]
- Murugesan, G.; Sandhya Rani, M.R.; Gerber, C.E.; Mukhopadhyay, C.; Ransohoff, R.M.; Chisolm, G.M.; Kottke-Marchant, K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J. Mol. Cell. Cardiol. 2003, 35, 1375–1384. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Shuijuan, Z.; Wang, Y.; Fan, Y.; Li, H.; Wang, C.; Zhang, J.; Zhang, S.; Changfeng, H.; Wen, C. Lipidomics revealed idiopathic pulmonary fibrosis-induced hepatic lipid disorders corrected with treatment of baicalin in a murine model. AAPS J. 2015, 17, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, H.; Wei, F.; Zhao, Q.; Su, Q.; Lei, Y.; Yin, M.; Tian, X.; Liu, Z.; Yu, B.; et al. α-mangostin attenuates pristane-induced lupus nephritis by regulating Th17 differentiation. Int. J. Rheum. Dis. 2020, 23, 74–83. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Li, W.; Lian, W.; Huang, J.; Lai, B.; Li, L.; Huang, Z. Additive therapeutic effects of mesenchymal stem cells and IL-37 for systemic lupus erythematosus. J. Am. Soc. Nephrol. 2019, 31, 54–65. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Yang, K.; Zhongdan, Z.; Abendschein, D.R.; Gross, R.W. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry 2007, 46, 6417–6428. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Cheng, H.; Gross, R.W.; Han, X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal. Chem. 2009, 81, 4356–4368. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Yang, K.; Cheng, H.; Fikes, K.N.; Gross, R.W. Shotgun lipidomics of phosphoethanolamine-containing lipids in biological samples after one-step in situ derivatization. J. Lipid Res. 2005, 46, 1548–1560. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fang, H.; Han, X. Shotgun lipidomics analysis of 4-hydroxyalkenal species directly from lipid extracts after one-step in situ derivatization. Anal. Chem. 2012, 84, 4580–4586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gross, R.W. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2004, 24, 367–412. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, K.; Gross, R.W. Microfluidics-based electrospray ionization enhances the intrasource separation of lipid classes and extends identification of individual molecular species through multi-dimensional mass spectrometry: Development of an automated high-throughput platform for shotgun lipidomics. Rapid Commun. Mass Spectrom. 2008, 22, 2115–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lu, L.; Tian, X.; Wang, K.; Xie, G.; Li, H.; Wen, C.; Hu, C. Lipidomics Revealed Aberrant Lipid Metabolism Caused by Inflammation in Cardiac Tissue in the Early Stage of Systemic Lupus Erythematosus in a Murine Model. Metabolites 2022, 12, 415. https://doi.org/10.3390/metabo12050415
Zhang J, Lu L, Tian X, Wang K, Xie G, Li H, Wen C, Hu C. Lipidomics Revealed Aberrant Lipid Metabolism Caused by Inflammation in Cardiac Tissue in the Early Stage of Systemic Lupus Erythematosus in a Murine Model. Metabolites. 2022; 12(5):415. https://doi.org/10.3390/metabo12050415
Chicago/Turabian StyleZhang, Jida, Lu Lu, Xiaoyu Tian, Kaili Wang, Guanqun Xie, Haichang Li, Chengping Wen, and Changfeng Hu. 2022. "Lipidomics Revealed Aberrant Lipid Metabolism Caused by Inflammation in Cardiac Tissue in the Early Stage of Systemic Lupus Erythematosus in a Murine Model" Metabolites 12, no. 5: 415. https://doi.org/10.3390/metabo12050415
APA StyleZhang, J., Lu, L., Tian, X., Wang, K., Xie, G., Li, H., Wen, C., & Hu, C. (2022). Lipidomics Revealed Aberrant Lipid Metabolism Caused by Inflammation in Cardiac Tissue in the Early Stage of Systemic Lupus Erythematosus in a Murine Model. Metabolites, 12(5), 415. https://doi.org/10.3390/metabo12050415




