Algal Lipids as Modulators of Skin Disease: A Critical Review
Abstract
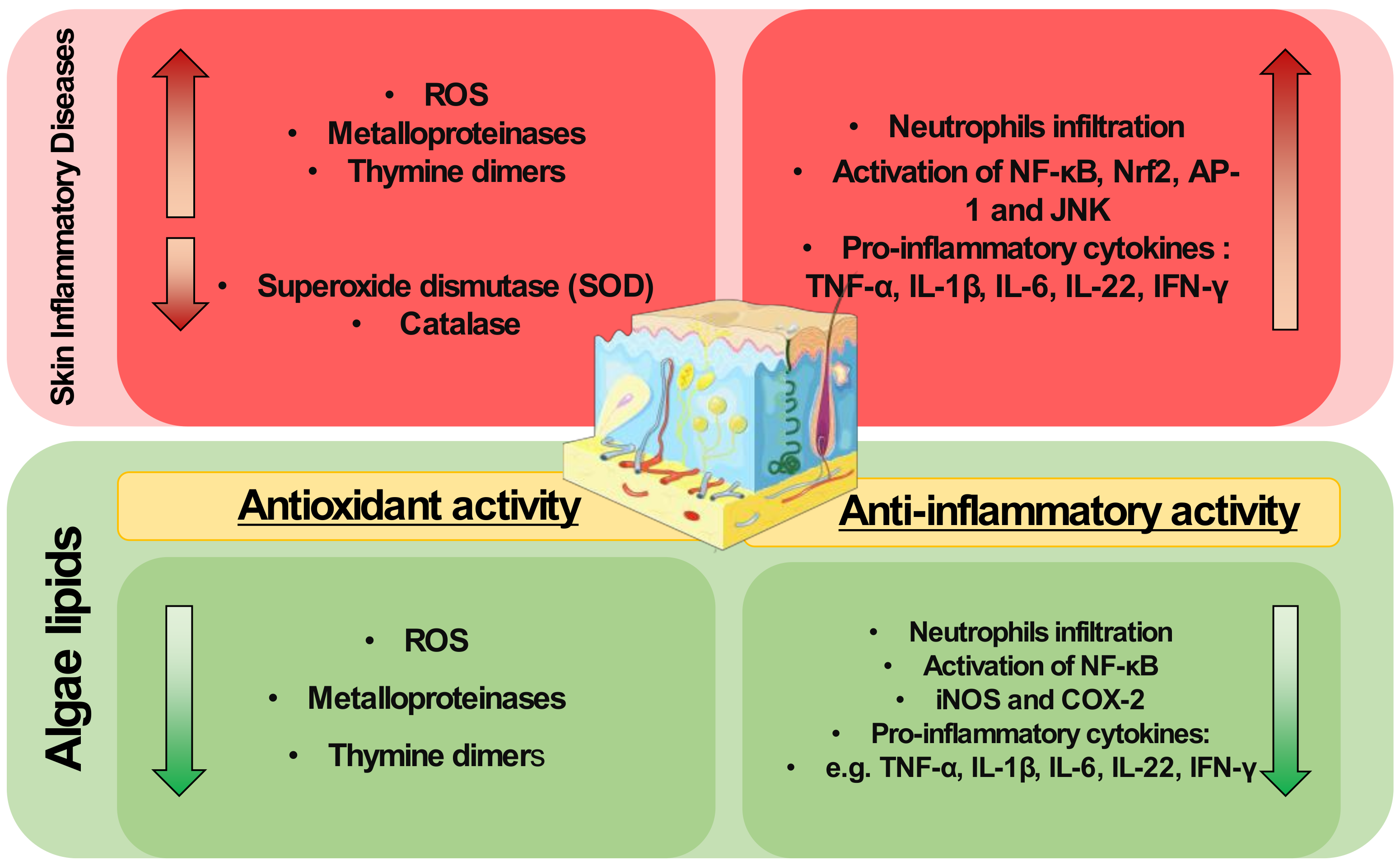
:1. Introduction
2. Inflammatory Skin Diseases
3. Algae Lipids with Antioxidant Activity
4. Algae Lipids with Anti-Inflammatory Activity
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Benson, H.A.E.; Watkinson, A.C. Transdermal and Topical Drug Delivery: Principles and Practice; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-45029-1. [Google Scholar]
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The Fibroblast-Derived Paracrine Factor Neuregulin-1 Has a Novel Role in Regulating the Constitutive Color and Melanocyte Function in Human Skin. J. Cell Sci. 2010, 123, 3102–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of Oxidative Stress and the Antioxidant Network in Cutaneous Carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Duchnik, E. Oxidative Stress and Skin Diseases: Possible Role of Physical Activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Pastore, S. Pathobiology of Chronic Inflammatory Skin Diseases: Interplay Between Keratinocytes and Immune Cells as a Target for Anti-Inflammatory Drugs. Curr. Drug Metab. 2010, 11, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Rashigni, M.; Harris, J.E. Vitiligo Pathogenesis and Emerging Treatments. Dermatol. Clin. 2017, 35, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez Carrera, Y.I.; Al Hammadi, A.; Huang, Y.H.; Llamado, L.J.; Mahgoub, E.; Tallman, A.M. Epidemiology, Diagnosis, and Treatment of Atopic Dermatitis in the Developing Countries of Asia, Africa, Latin America, and the Middle East: A Review. Dermatol. Ther. 2019, 9, 685–705. [Google Scholar] [CrossRef] [Green Version]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine Algae as Attractive Source to Skin Care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and Mechanism of Skin Depigmenting and Lightening Agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guesmi, A.; Boumaiza, M.; Boudabous, A. Microbiological Quality and Safety of Commercialized Thalassotherapy Products Based on Marine Mud and Algae Extracts in Tunisia. Arch. Microbiol. 2020, 202, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Choo, W.T.; Teoh, M.L.; Phang, S.M.; Convey, P.; Yap, W.H.; Goh, B.H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an Attractive Source for Cosmetics to Counter Environmental Stress. Sci. Total. Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- European Commission. The EU Blue Economy Report 2020; European Commission: Brussels, Belgium, 2020; pp. 2–165. [Google Scholar]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; 215AD; United Nations General Assembly: New York, NY, USA, 2015. [Google Scholar]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory Cytokines Regulate Epidermal Stem Cells in Wound Epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Dreno, B.; Gollnick, H.P.M.; Kang, S.; Thiboutot, D.; Bettoli, V.; Torres, V.; Leyden, J. The Global Alliance to Improve Outcomes in Acne Understanding Innate Immunity and Inflammation in Acne: Implications for Management. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovatiya, R.; Silverberg, J.I. Pathophysiology of Atopic Dermatitis and Psoriasis: Implications for Management in Children. Children 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, K.A.; Balogh, E.A.; Kaplan, S.G.; Feldman, S.R. Skin Disease in Children: Effects on Quality of Life, Stigmatization, Bullying, and Suicide Risk in Pediatric Acne, Atopic Dermatitis, and Psoriasis Patients. Children 2021, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; van de Kerkhof, P.; Czarnecka-Operacz, M. Psoriasis and Atopic Dermatitis. Dermatol. Ther. 2017, 7, 31–41. [Google Scholar] [CrossRef]
- Kantor, R.; Silverberg, J.I. Environmental Risk Factors and Their Role in the Management of Atopic Dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Marzano, A.V.; Ortega-Loayza, A.G.; Heath, M.; Morse, D.; Genovese, G.; Cugno, M. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front. Immunol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handy, D.E.; Loscalzo, J. Redox Regulation of Mitochondrial Function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl Radical and Its Scavengers in Health and Disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [Green Version]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T Cells and Reactive Oxygen Species. J. Biomed. Sci. 2015, 22, 85. [Google Scholar] [CrossRef] [Green Version]
- Yarosz, E.L.; Chang, C.-H. The Role of Reactive Oxygen Species in Regulating T Cell-Mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Hsu, Y.-J.; Lu, C.-Y.; Tsai, M.-C.; Hung, W.-C.; Chen, P.-C.; Wang, J.-C.; Hsu, L.-A.; Yeh, Y.-H.; Chu, P. Pharmacological Activation of Aldehyde Dehydrogenase 2 Protects Against Heatstroke-Induced Acute Lung Injury by Modulating Oxidative Stress and Endothelial Dysfunction. Front. Immunol. 2021, 12, 740562. [Google Scholar] [CrossRef] [PubMed]
- Christie, P.E.; Henderson, W.R. Lipid Inflammatory Mediators: Leukotrienes, Prostaglandins, Platelet-Activating Factor. Clin. Allergy Immunol. 2002, 16, 233–254. [Google Scholar]
- Wójcik, P.; Žarković, N.; Gęgotek, A.; Skrzydlewska, E. Involvement of Metabolic Lipid Mediators in the Regulation of Apoptosis. Biomolecules 2020, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Höhn, A.; Jung, T.; Grune, T. Pathophysiological Importance of Aggregated Damaged Proteins. Free Radic. Biol. Med. 2014, 71, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pall, M.L.; Levine, S. Nrf2, a Master Regulator of Detoxification and Also Antioxidant, Anti-Inflammatory and Other Cytoprotective Mechanisms, Is Raised by Health Promoting Factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Dallio, M.; Della Corte, C.M.; Gravina, A.G.; Viscardi, G.; Loguercio, C.; Ciardiello, F.; Federico, A. Carcinogenesis as a Result of Multiple Inflammatory and Oxidative Hits: A Comprehensive Review from Tumor Microenvironment to Gut Microbiota. Neoplasia 2018, 20, 721. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Waris, G.; Ahsan, H. Reactive Oxygen Species: Role in the Development of Cancer and Various Chronic Conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Derakhshani, A.; Rostami, Z.; Taefehshokr, S.; Safarpour, H.; Astamal, R.V.; Taefehshokr, N.; Alizadeh, N.; Argentiero, A.; Silvestris, N.; Baradaran, B. Oncogenic Signaling Pathways in Cancer: An Overview. Preprints 2020, 2020030201. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-L.; Chou, H.-M.; Chou, H.-C.; Lu, H.-F.; Chu, Y.-L.; Shang, H.-S.; Chung, J.-G. Casticin Impairs Cell Migration and Invasion of Mouse Melanoma B16F10 Cells via PI3K/AKT and NF-ΚB Signaling Pathways. Environ. Toxicol. 2017, 32, 2097–2112. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 12, 488. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.P.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.F.M.; Clark, R.A. CD8+ T Cells in the Lesional Skin of Atopic Dermatitis and Psoriasis Patients Are an Important Source of IFN-γ, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, A.P.; Murali, M.; Kroshinsky, D.; Duncan, L.M.; Nazarian, R.M. Immunologic Overlap of Helper T-Cell Subtypes 17 and 22 in Erythrodermic Psoriasis and Atopic Dermatitis. JAMA Dermatol. 2015, 151, 753. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.-U.; Wollenberg, A. Cellular and Molecular Immunologic Mechanisms in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.I.; Oh, E.H.; Song, H.J.; Choi, W.J.; Baek, J.O.; Lee, J.R.; Roh, J.Y. Oxidative Damage to Macromolecules in Atopic Dermatitis Patients. Korean J. Dermatol. 2015, 53, 456–461. [Google Scholar]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-Mediated Changes to the Phospholipid Profile of UVB-Irradiated Keratinocytes from Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of Reactive Oxygen Species and Antioxidants in Atopic Dermatitis. J. Clin. Diagnostic Res. 2013, 7, 2683. [Google Scholar] [CrossRef]
- Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. Int. J. Mol. Sci. 2019, 20, 4249. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Skrzydlewska, E. Changes in Proteome of Fibroblasts Isolated from Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 5363. [Google Scholar] [CrossRef]
- Pavel, A.B.; Zhou, L.; Diaz, A.; Ungar, B.; Dan, J.; He, H.; Estrada, Y.D.; Xu, H.; Fernandes, M.; Renert-Yuval, Y. The Proteomic Skin Profile of Moderate-to-Severe Atopic Dermatitis Patients Shows an Inflammatory Signature. J. Am. Acad. Dermatol. 2020, 82, 690–699. [Google Scholar] [CrossRef]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Diana, A.B.M.; Milton-Laskibar, I.; Fernández-Quintela, A.; Silván, J.M.; Rai, D.K.; Choudhary, A.; Peñas, E.; de Luis, D.A.; Martínez-Villaluenga, C. Characterization and in vitro Evaluation of Seaweed Species as Potential Functional Ingredients to Ameliorate Metabolic Syndrome. J. Funct. Foods 2018, 46, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.A.; Abo-elfadl, M.T.; Abd El Baky, H.H.; Murad, S.A. Chemical and Biological Characterization of Lipid Profile from Hydroclathrus clathraus. Egypt. J. Chem. 2021, 64, 5477–5484. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Ríos Pinto, L.F.; Carvalho, P.O.; Coelho, M.B.; Eberlin, M.N.; Maciel Filho, R.; Fregolente, L.V. Biomass and Lipid Characterization of Microalgae Genera Botryococcus, Chlorella, and Desmodesmus Aiming High-Value Fatty Acid Production. Biomass Conv. Bioref. 2021, 11, 1675–1689. [Google Scholar] [CrossRef]
- Coniglio, D.; Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Lipidomics of the Edible Brown Alga Wakame (Undaria pinnatifida) by Liquid Chromatography Coupled to Electrospray Ionization and Tandem Mass Spectrometry. Molecules 2021, 26, 4480. [Google Scholar] [CrossRef]
- Carriot, N.; Paix, B.; Greff, S.; Viguier, B.; Briand, J.-F.; Culioli, G. Integration of LC/MS-Based Molecular Networking and Classical Phytochemical Approach Allows in-Depth Annotation of the Metabolome of Non-Model Organisms-The Case Study of the Brown Seaweed Taonia atomaria. Talanta 2021, 225, 121925. [Google Scholar] [CrossRef]
- Calvano, C.D.; Coniglio, D.; D’Alesio, P.E.; Losito, I.; Cataldi, T.R.I. The Occurrence of Inositolphosphoceramides in Spirulina Microalgae. Electrophoresis 2020, 41, 1760–1767. [Google Scholar] [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and Cardiovascular Disease: Are Marine Phospholipids the Answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [Green Version]
- Chouinard-Watkins, R.; Lacombe, R.J.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine Is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, 1801224. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Boulho, R.; Kucma, J.-P.; Bourgougnon, N.; Bedoux, G. Radical Scavenging Activity of Lipids from Seaweeds Isolated by Solid-Liquid Extraction and Supercritical Fluids. OCL 2018, 25, D505. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Edachery, B.; Nooruddin, T.; Lee, S.-Y.; Kim, J.-W. An Evidence of C16 Fatty Acid Methyl Esters Extracted from Microalga for Effective Antimicrobial and Antioxidant Property. Microb. Pathog. 2018, 115, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Han, X.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, C.S.; et al. Photoprotective Effect of Carpomitra costata Extract against Ultraviolet B-Induced Oxidative Damage in Human Keratinocytes. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 11–28. [Google Scholar] [CrossRef]
- Lee, J.-J.; An, S.; Kim, K.B.; Heo, J.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S.; Bae, S. Extract of Ettlia sp. YC001 Exerts Photoprotective Effects against UVB Irradiation in Normal Human Dermal Fibroblasts. J. Microbiol. Biotechnol. 2016, 26, 775–783. [Google Scholar] [CrossRef]
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro Anti-Inflammatory and Anti-Proliferative Activity of Sulfolipids from the Red Alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, K.B.; Heo, J.; Cho, D.-H.; Kim, H.-S.; Han, S.H.; Ahn, K.J.; An, I.-S.; An, S.; Bae, S. Protective Effect of Arthrospira platensis Extracts against Ultraviolet B-Induced Cellular Senescence through Inhibition of DNA Damage and Matrix Metalloproteinase-1 Expression in Human Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 173, 196–203. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Sun, Z.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The Protective Effects of Fucosterol against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Kim, M.-S.; Oh, G.-H.; Kim, M.-J.; Hwang, J.-K. Fucosterol Inhibits Matrix Metalloproteinase Expression and Promotes Type-1 Procollagen Production in UVB-Induced HaCaT Cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef]
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of Mitochondrial Oxidative Damage as a Therapeutic Strategy in Diabetes. Diabetes 2004, 53, S110–S118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.M.; Murphy, M.P. How Mitochondrial Damage Affects Cell Function. J. Biomed. Sci. 2002, 9, 475–487. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for Polar Lipids, Antioxidant, and Anti-Inflammatory Activities of Gloeothece sp. Lipid Extracts Pursuing New Phytochemicals from Cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar Lipidomic Profile Shows Chlorococcum amblystomatis as a Promising Source of Value-Added Lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the Polar Lipid Profile of the Microalgae Chlorella vulgaris Grown under Heterotrophic and Autotrophic Conditions. Algal Res. 2020, 53, 102128. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid Composition and Some Bioactivities of 3 Newly Isolated Microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Pham, T.H.; Nguyen, V.T.A.; Do, T.T.T.; Do, A.D.; Dam, D.T.; Tran, T.T.V.; Pham, Q.L.; Le, T.T. Lipidomics and Anti-Inflammation Activity of Brown Algae, Lobophora sp., in Vietnam. J. Chem. 2020, 2020, 8829054. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids Isolated from the Cultivated Red Alga Chondrus crispus Inhibit Nitric Oxide Production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar Lipids from the Marine Macroalga Palmaria palmata Inhibit Lipopolysaccharide-Induced Nitric Oxide Production in RAW264.7 Macrophage Cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; Oleary, S.J.B. Monogalactosyldiacylglycerols, Potent Nitric Oxide Inhibitors from the Marine Microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; McGinn, P.J. Mono- and Digalactosyldiacylglycerols: Potent Nitric Oxide Inhibitors from the Marine Microalga Nannochloropsis granulata. J. Appl. Phycol. 2013, 25, 349–357. [Google Scholar] [CrossRef]
- Novichkova, E.; Chumin, K.; Eretz-Kdosha, N.; Boussiba, S.; Gopas, J.; Cohen, G.; Khozin-Goldberg, I. DGLA from the Microalga Lobosphaera incsa P127 Modulates Inflammatory Response, Inhibits INOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients 2020, 12, 2892. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New Diacylglyceryltrimethylhomoserines from the Marine Microalga Nannochloropsis granulata and Their Nitric Oxide Inhibitory Activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; Osborne, J.A.; Melanson, R.; O’Leary, S.J.B. Nitric Oxide Inhibitory Activity of Monogalactosylmonoacylglycerols from a Freshwater Microalgae Chlorella sorokiniana. Nat. Prod. Res. 2013, 27, 1028–1031. [Google Scholar] [CrossRef]
- Suh, S.S.; Hong, J.M.; Kim, E.J.; Jung, S.W.; Kim, S.M.; Kim, J.E.; Kim, I.C.; Kim, S. Anti-Inflammation and Anti-Cancer Activity of Ethanol Extract of Antarctic Freshwater Microalga, Micractinium sp. Int. J. Med Sci. 2018, 15, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Abu-Serie, M.M.; Habashy, N.H.; Attia, W.E. In vitro Evaluation of the Synergistic Antioxidant and Anti-Inflammatory Activities of the Combined Extracts from Malaysian Ganoderma lucidum and Egyptian Chlorella vulgaris. BMC Complement. Altern. Med. 2018, 18, 154. [Google Scholar] [CrossRef]
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-Inflammatory Effects of Phaeodactylum tricornutum Extracts on Human Blood Mononuclear Cells and Murine Macrophages. J. Appl. Phycol. 2018, 30, 2837–2846. [Google Scholar] [CrossRef]
- Suh, S.S.; Hong, J.M.; Kim, E.J.; Jung, S.W.; Chae, H.; Kim, J.E.; Kim, J.H.; Kim, I.C.; Kim, S. Antarctic Freshwater Microalga, Chloromonas reticulata, Suppresses Inflammation and Carcinogenesis. Int. J. Med. Sci. 2019, 16, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauder, D.N. The Role of Epidermal Cytokines in Inflammatory Skin Diseases. J. Investig. Dermatol. 1990, 95, S27–S28. [Google Scholar] [CrossRef]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.; Yi, Y.-S.; Lee, J.; Park, S.H.; Kim, S.; Hossain, M.A.; Jang, S.; Choi, Y.I.; Park, K.J.; Kim, D.S.; et al. Anti-Apoptotic and Anti-Inflammatory Activities of Edible Fresh Water Algae Prasiola japonica in UVB-Irradiated Skin Keratinocytes. Am. J. Chin. Med. 2019, 47, 1853–1868. [Google Scholar] [CrossRef]
- Lakshmegowda, S.B.; Rajesh, S.K.; Kandikattu, H.K.; Nallamuthu, I.; Khanum, F. In vitro and in vivo Studies on Hexane Fraction of Nitzschia palea, a Freshwater Diatom for Oxidative Damage Protective and Anti-Inflammatory Response. Rev. Bras. Farmacogn. 2020, 30, 189–201. [Google Scholar] [CrossRef]
- Sibi, G.; Rabina, S. Inhibition of Pro-Inflammatory Mediators and Cytokines by Chlorella vulgaris Extracts. Pharmacogn. Res. 2016, 8, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, W.S.; Choi, Y.J.; Kim, H.J.; Nam, B.H.; Hong, S.H.; Lee, G.A.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. Anti-Inflammatory Effect of Microalgal Extracts from Tetraselmis suecica. Food Sci. Biotechnol. 2010, 19, 1519–1528. [Google Scholar] [CrossRef]
- Takahashi, S.; Sakamaki, M.; Ferdousi, F.; Yoshida, M.; Demura, M.; Watanabe, M.M.; Isoda, H. Ethanol Extract of Aurantiochytrium mangrovei 18W-13a Strain Possesses Anti-Inflammatory Effects on Murine Macrophage RAW264 Cells. Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.Y.; Sim, J.H.; Lee, J.Y.; Kang, D.H.; Lee, H.Y. Increased Anti-Inflammatory Effects on LPS-Induced Microglia Cells by Spirulina maxima Extract from Ultrasonic Process. Appl. Sci. 2019, 9, 2144. [Google Scholar] [CrossRef] [Green Version]
- Mosxou, D.; Letsiou, S. Exploring the Protective Effects of Phaeodactylum tricornutum Extract on LPS-Treated Fibroblasts. Cosmetics 2021, 8, 76. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, J. Laminaria ochroleuca Extract Reduces Skin Inflammation. J. Eur. Acad. Dermatol. Venerol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef]
- Serrano, G.; Almudéver, P.; Serrano, J.-M.; Milara, J.; Torrens, A.; Expósito, I.; Cortijo, J. Phosphatidylcholine Liposomes as Carriers to Improve Topical Ascorbic Acid Treatment of Skin Disorders. Clin. Cosmet. Investig. Dermatol. 2015, 8, 591–599. [Google Scholar]
- De Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; De La Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the Microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and Their Activity as TNF-α Inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A Mixture of Phytosterols from Dunaliella tertiolecta Affects Proliferation of Peripheral Blood Mononuclear Cells and Cytokine Production in Sheep. Vet. Immunol. Immunopathol. 2012, 150, 27–35. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo Anti-Inflammatory Action of the Galactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Pliego, L.; Martínez-Carrillo, B.; Reséndiz-Albor, A.; Arciniega-Martínez, I.; Escoto-Herrera, J.; Rosales-Gómez, C.; Valdés-Ramos, R. Effect of Supplementation with n-3 Fatty Acids Extracted from Microalgae on Inflammation Biomarkers from Two Different Strains of Mice. J. Lipids 2018, 2018, 4765358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila-Román, J.; Talero, E.; Alcaide, A.; de Los Reyes, C.; Zubía, E.; García-Mauriño, S.; Motilva, V. Preventive Effect of the Microalga Chlamydomonas debaryana on the Acute Phase of Experimental Colitis in Rats. Br. J. Nutr. 2014, 112, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.-Y.; Gyawali, Y.P.; Ahn, S.-H.; Khan, M.N.A.; Kong, I.-S.; Hong, Y.-K. A Methoxylated Fatty Acid Isolated from the Brown Seaweed Ishige okamurae Inhibits Bacterial Phospholipase A2. Phytother. Res. 2008, 22, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Pebriani, S.A.; Ilhami, B.T.K.; Frediansyah, A.; Sunarwidhi, A.L.; Widyastuti, S.; Sunarpi, H. Macroalgae Sargassum Cristaefolium Extract Inhibits Proinflammatory Cytokine Expression in BALB/C Mice. Scientifica 2020, 2020, 9769454. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Talero, E.; Terencio, M.; González-Rodríguez, M.; Rabasco, A.; de los Reyes, C.; Motilva, V.; Ávila-Román, J. Topical Application of Glycolipids from Isochrysis galbana Prevents Epidermal Hyperplasia in Mice. Mar. Drugs 2017, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Balbás, G.; Sánchez-Regaña, M.; Millet, U. Study on the Use of Omega-3 Fatty Acids as a Therapeutic Supplement in Treatment of Psoriasis. Clin. Cosmet. Investig. Dermatol. 2011, 4, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khnykin, D.; Miner, J.H.; Jahnsen, F. Role of Fatty Acid Transporters in Epidermis: Implications for Health and Disease. Dermato-Endocrinology 2011, 3, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Dahli, L.; Atrahimovich, D.; Vaya, J.; Khatib, S. Lyso-DGTS Lipid Isolated from Microalgae Enhances PON1 Activities in vitro and in vivo, Increases PON1 Penetration into Macrophages and Decreases Cellular Lipid Accumulation. BioFactors 2018, 44, 299–310. [Google Scholar] [CrossRef] [PubMed]
| Studies | Mechanism | Assay | Identified Lipids | Algae Species | Ref. |
|---|---|---|---|---|---|
| in chemico | Free radical scavenging | ABTS, DPPH, hydroxyl radical, superoxide anion | Polar lipids, neutral lipids, FAME | Macroalgae: Bifurcaria bifurcata, Codium tomentosum, Fucus vesiculosus, Gracilaria gracilis Grateloupia turuturu, Palmaria palmata, Porphyra dioica Sargassum muticum, Solieria chordalis, Ulva rigida Microalgae: Chlorella vulgaris, Chlorococcum amblystomatis, Nannochloropsis oceanica, Phaeodactylum tricornutum, Scenedesmus intermedius Scenedesmus obliquus, Spirulina sp., Tetraselmis chui | [69,70,82,83] |
| in vitro | Detoxify intracellular ROS | Increased the expression of Nrf2 in irradiated HaCat cells Upregulate target antioxidant enzymes Cu/Zn SOD, CAT, and HO-1 | Crude ethanolic extract | Macroalga: Carpomitra costata | [84] |
| Free radical scavenging | Superoxide generation on peritoneal leukocytes | Sulfoquinovosylacylglycerols | Microalgae: Porphyridium cruentum | [86] | |
| Inhibition of ROS | Photoprotective against UVB in NHDF | Crude ethyl acetate extract | Microalga: Ettlia sp. YC001 | [85] | |
| Enzyme/protein expression | Downregulation of expression of MMPs | Crude ethanolic extract | Microalga: Arthrospira platensis | [87] | |
| Enzyme/protein expression | Downregulation of expression of MMPs, IL-6 and TGF-1 in human dermal fibroblast Modulate MAPK in irradiated HaCat cells | Fucosterol | Macroalga: Sargassum fusiforme | [88,89] |
| Studies | Action | Model | Identified Lipids | Algae Species | Ref. |
|---|---|---|---|---|---|
| In chemico | COX-2 inhibition | COX-2 kit assay | Polar lipids | Macroalgae: Codium tomentosum, Fucus vesiculosus Gracilaria gracilis, Palmaria palmata, Porphyra dioica, Ulva rigida, Microalgae: Chlorella vulgaris, Chlorococcum amblystomatis, Gloeothece sp., Skeletonema sp., Tetraselmis sp. mutants | [69,92,93,94,95] |
| In vitro | NO inhibition | Raw 264.7 | Polar and non-polar lipids; PC, PG, DGDG, DGTS, MGDG, MGMG, SQDG classes; Free and ethyl esterified DGLA | Macroalgae: Chondrus crispus, Lobophora sp.Palmaria palmata, Microalgae: Chlorella sorokiniana Lobosphaera incisa, Nannochloropsis granulata, Tetraselmis chui, | [96,97,98,99,100,101,102,103] |
| Decrease in PGE2 Downregulation of COX-2 | Raw 264.7; White blood cells; Epidermal cells | Crude ethanolic extracts; lipid extracts rich in PC; free and ethyl esterified DGLA | Macroalgae: Laminaria ochroleuca Microalgae: Chlorella vulgaris, Chloromonas reticulata, Lobosphaera incisa Micractinium sp., Phaeodactylum tricornutum, | [101,104,105,106,107,119] | |
| Downregulation of mRNA expression of pro-inflammatory cytokines Downregulation of cytokines levels: TNF-α, IL-6, IL-1α, and IL-1β | THP-1; PBMC; Epidermal cells; HaCaT cells | Crude ethanolic extracts; lipid extracts; lipid extracts rich in MGDG, DGDG and SQDG; Lipid extracts rich in PC; LPC(16:0); oxylipins; ergosterol and 7-dehydroporiferasterol; free and ethyl esterified DGLA | Macroalgae: Chondrus crispus, Laminaria ochroleuca, Palmaria palmata, Porphyra dioica, Prasiola japonica Microalgae: Aurantiochytrium mangrovei, Chlamydomonas debaryana, Chlorella vulgaris, Chloromonas reticulata, Cylindrotheca closterium, Dunaliella tertiolecta, Micratinium sp., Nannochloropsis gaditana, Nitzschia palea, Phaeodactylum tricornutum, Lobosphaera incisa Spirulina maxima, Pavlova lutheri, Tetraselmis suecica, | [84,101,104,105,106,107,110,111,112,113,114,115,116,118,119,121,122] | |
| Inhibition of pro-inflammatory signaling pathways mediated by TLR and NF-κB | THP-1 | Lipid extracts rich in MGDG, DGDG, and SQDG | Macroalgae: Chondrus crispus, Palmaria palmata, Porphyra dioica Microalgae: Pavlova lutheri | [110] | |
| In vivo | Attenuation of ear oedema | PLA2 kit assay; Mice with ear oedema; DNFB-induced in naive C57BL/6 mice | MMHDA; Lipid extracts rich in PC; MGDG, DGDG, and SQDG fractions | Macroalgae: Ishige okamurae, Laminaria ochroleuca Microalgae: ETS-05 cyanobacterium. | [119,123,127] |
| Neutrophil gathering in the wound region | Wounded zebrafish model | Glycolipids rich in γ-linolenic acid | Microlagae: Spirulina platensis | [124] | |
| Inhibition of pro-inflammatory cytokines production: TNF-α, IL-6, IL-8, IFN- γ, IL-1β, IL-17 | db/db and CD1 mice model of diabetes mellitus; TNBS-induced colitis rats; BALB/c mice skin; TPA-induced hyperplasia murine model | Crude ethanolic extract; omega-3 fatty acids; oxylipins; MGDG cream | Macroalgae: Sargassum cristaefolium Microalgae: Chlamydomonas debaryana, Isochrysis galbana | [125,126,128,129] | |
| Downregulation of iNOS and COX-2, and decrease in NO and PGE2 production | TNBS-induced colitis rat; BALB/c mice skin; TPA-induced hyperplasia murine model | Crude ethanolic extract; oxylipins; MGDG cream | Macroalgae: Sargassum cristaefolium Microalgae: Chlamydomonas debaryana, Isochrysis galbana | [126,128,129] |
| Lipid Class | Lipid Species (C:N) | Molecular Species (sn-1/sn-2) | Algae Species | Reference |
|---|---|---|---|---|
| Betaine lipids | DGTS (34:5) | DGTS (20:5/14:0) | Nannochloropsis granulata | [102] |
| DGTS (36:5) | DGTS (20:5/16:0) | |||
| DGTS (36:6) | DGTS (20:5/16:1) | |||
| DGTS (38:7) | DGTS (20:5/18:2) | |||
| DGTS (40:9) | DGTS (20:5/20:4) | |||
| DGTS (40:10) | DGTS (20:5/20:5) | |||
| MGTS (20:5) | MGTS (20:5) | Nannochloropsis sp. | [134] | |
| Glycolipids | MGDG (34:3) | MGDG (16:0/18:3) | ETS-05 cyanobacterium | [123] |
| MGDG (34:4) | MGDG (18:4/16:0) | Chondrus crispus | [97] | |
| MGDG (34:5) | MGDG (20:5/14:0) | Nannochloropsis granulata | [100] | |
| MGDG (34:7) | MGDG (18:3/16:4) | Tetraselmis chui | [99] | |
| MGDG (34:8) | MGDG (18:4/16:4) | |||
| MGDG (36:4) | MGDG (20:4/16:0) | Chondrus crispus | [97] | |
| MGDG (36:5) | MGDG (20:5/16:0) | Chondrus crispus, Nannochloropsis granulata | [97,100] | |
| MGDG (36:6) | MGDG (20:5/16:1) | Nannochloropsis granulata | [100] | |
| MGDG (38:7) | MGDG (20:5/18:2) | Porphyridium cruentum | [86] | |
| MGDG (40:8) | MGDG (20:4/20:4) | Chondrus crispus | [97] | |
| MGDG (40:9) | MGDG (20:5/20:4) | Chondrus crispus, Porphyridium cruentum | [86,97] | |
| MGDG (40:10) | MGDG (20:5/20:5) | Chondrus crispus, Nannochloropsis granulata | [97,100] | |
| MGMG (16:2) | MGMG (16:2) | Chlorella sorokiniana | [103] | |
| MGMG (16:3) | MGMG (16:3) | |||
| DGDG (34:4) | DGDG (16:0/18:4) | ETS-05 cyanobacterium | [123] | |
| DGDG (34:5) | DGDG (20:5/14:0) | Nannochloropsis granulata | [100] | |
| DGDG (36:4) | DGDG (20:4/16:0) | Chondrus crispus, Porphyridium cruentum | [86,97] | |
| DGDG (36:5) | DGDG (20:5/16:0) | Chondrus crispus, Nannochloropsis granulata | [97,100] | |
| DGDG (36:6) | DGDG (20:5/16:1) | Nannochloropsis granulata | [100] | |
| DGDG (38:7) | DGDG (20:5/18:2) | Porphyridium cruentum | [86] | |
| DGDG (40:10) | DGDG (20:5/20:5) | Nannochloropsis granulata | [100] | |
| DGDG (40:9) | DGDG (20:5/20:4) | Porphyridium cruentum | [86] | |
| SQDG (34:3) | SQDG (18:3/16:0) | ETS-05 cyanobacterium | [123] | |
| SQDG (34:5) | SQDG (20:5/14:0) | Palmaria palmata | [98] | |
| SQDG (36:5) | SQDG (20:5/16:0) | |||
| Phospholipids | PC (40:10) | PC (20:5/20:5) | Palmaria palmata | [98] |
| LPC (16:0) | LPC (16:0) | Cylindrotheca closterium | [118] | |
| PG (34:2) | PG (16:0/18:2) | ETS-05 cyanobacterium | [123] | |
| PG (36:6) | PG (20:5/trans-16:1) | Palmaria palmata | [98] | |
| PG (20:5/16:1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, T.; Lopes, D.; Łuczaj, W.; Neves, B.; Pinto, B.; Maurício, T.; Domingues, P.; Skrzydlewska, E.; Domingues, M.R. Algal Lipids as Modulators of Skin Disease: A Critical Review. Metabolites 2022, 12, 96. https://doi.org/10.3390/metabo12020096
Conde T, Lopes D, Łuczaj W, Neves B, Pinto B, Maurício T, Domingues P, Skrzydlewska E, Domingues MR. Algal Lipids as Modulators of Skin Disease: A Critical Review. Metabolites. 2022; 12(2):96. https://doi.org/10.3390/metabo12020096
Chicago/Turabian StyleConde, Tiago, Diana Lopes, Wojciech Łuczaj, Bruno Neves, Bruno Pinto, Tatiana Maurício, Pedro Domingues, Elżbieta Skrzydlewska, and M. Rosário Domingues. 2022. "Algal Lipids as Modulators of Skin Disease: A Critical Review" Metabolites 12, no. 2: 96. https://doi.org/10.3390/metabo12020096
APA StyleConde, T., Lopes, D., Łuczaj, W., Neves, B., Pinto, B., Maurício, T., Domingues, P., Skrzydlewska, E., & Domingues, M. R. (2022). Algal Lipids as Modulators of Skin Disease: A Critical Review. Metabolites, 12(2), 96. https://doi.org/10.3390/metabo12020096











