Intravenous Arginine Administration Attenuates the Inflammatory Response and Improves Metabolic Profiles in Diet-Induced Obese Mice after Sleeve Gastrectomy
Abstract
:1. Introduction
2. Results
2.1. Body Weights (BWs), Epididymal Fat Weights, and Blood Glucose Changes during the Intraperitoneal Glucose Tolerance Test (IPGTT)
2.2. Body Weight Change and Epidydymal Weights after the Gastrectomy
2.3. Plasma Levels of Biochemical Parameters
2.4. Plasma Nitric Oxide (NO) and Adipokine Levels
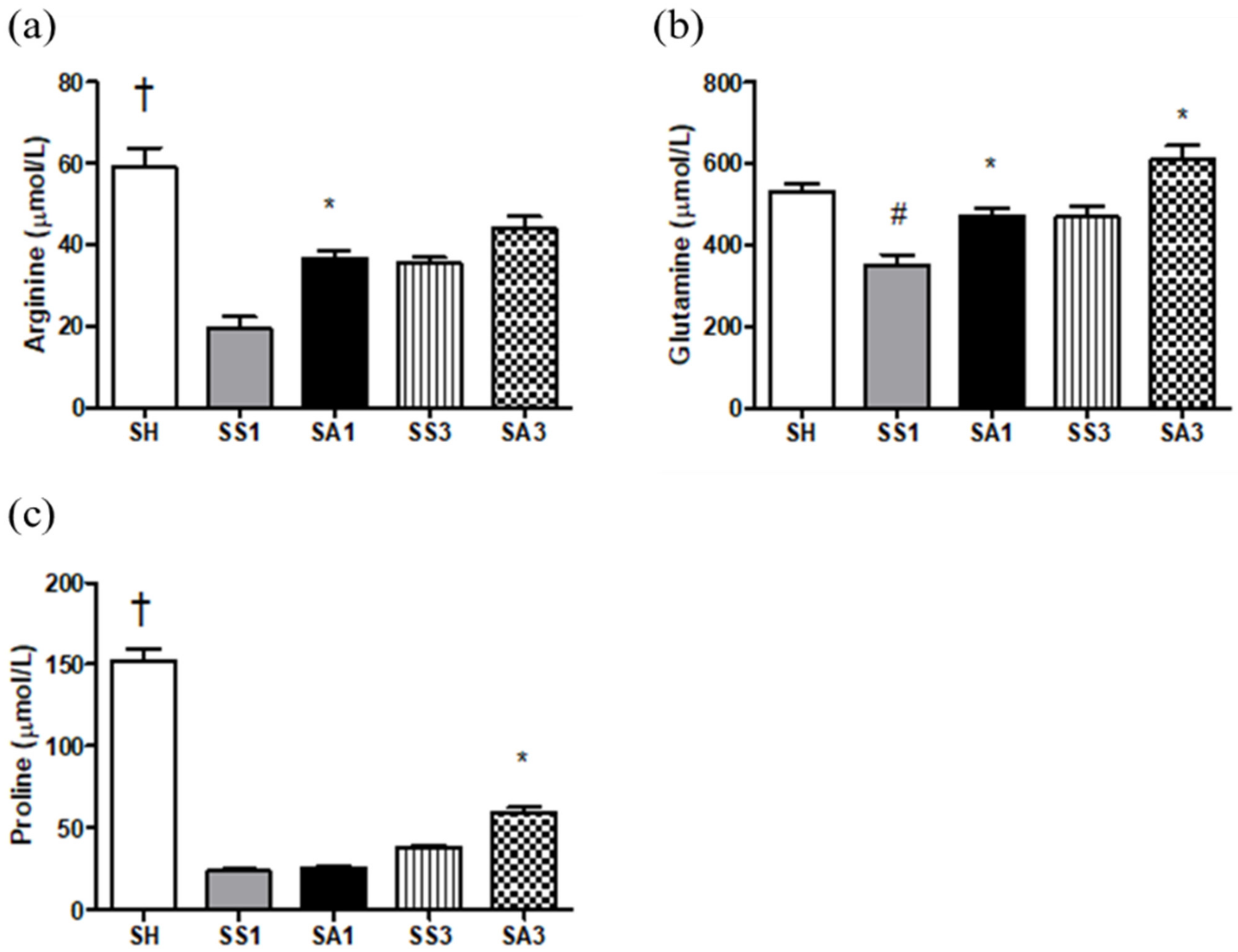
2.5. Plasma Amino Acid Concentrations
2.6. Inflammatory Cytokine Levels in Peritoneal Lavage Fluid (PLF)
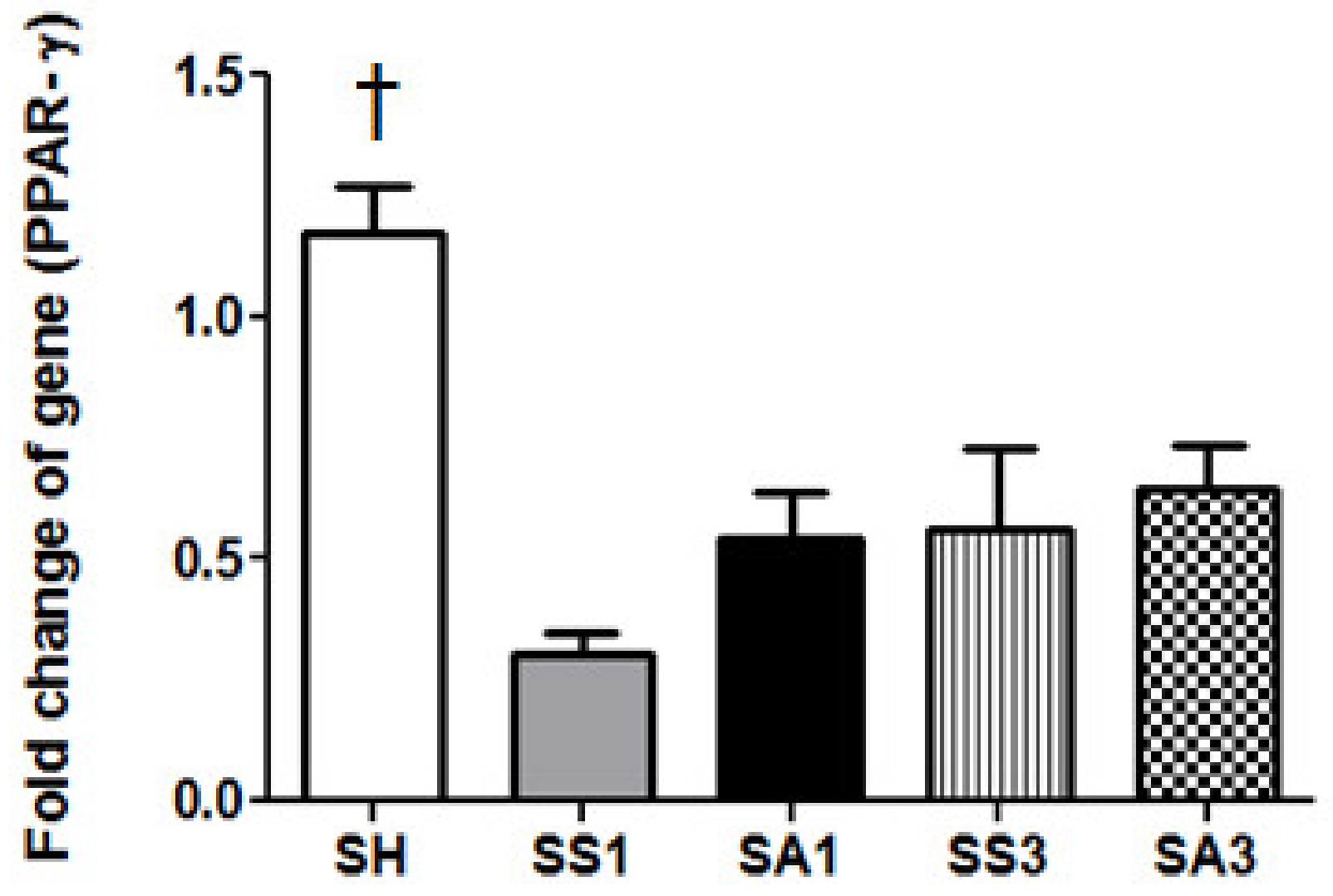
2.7. Messenger (m)RNA Expression of Peroxisome Proliferator-Activated Receptor (PPAR)-γ in Adipose Tissues
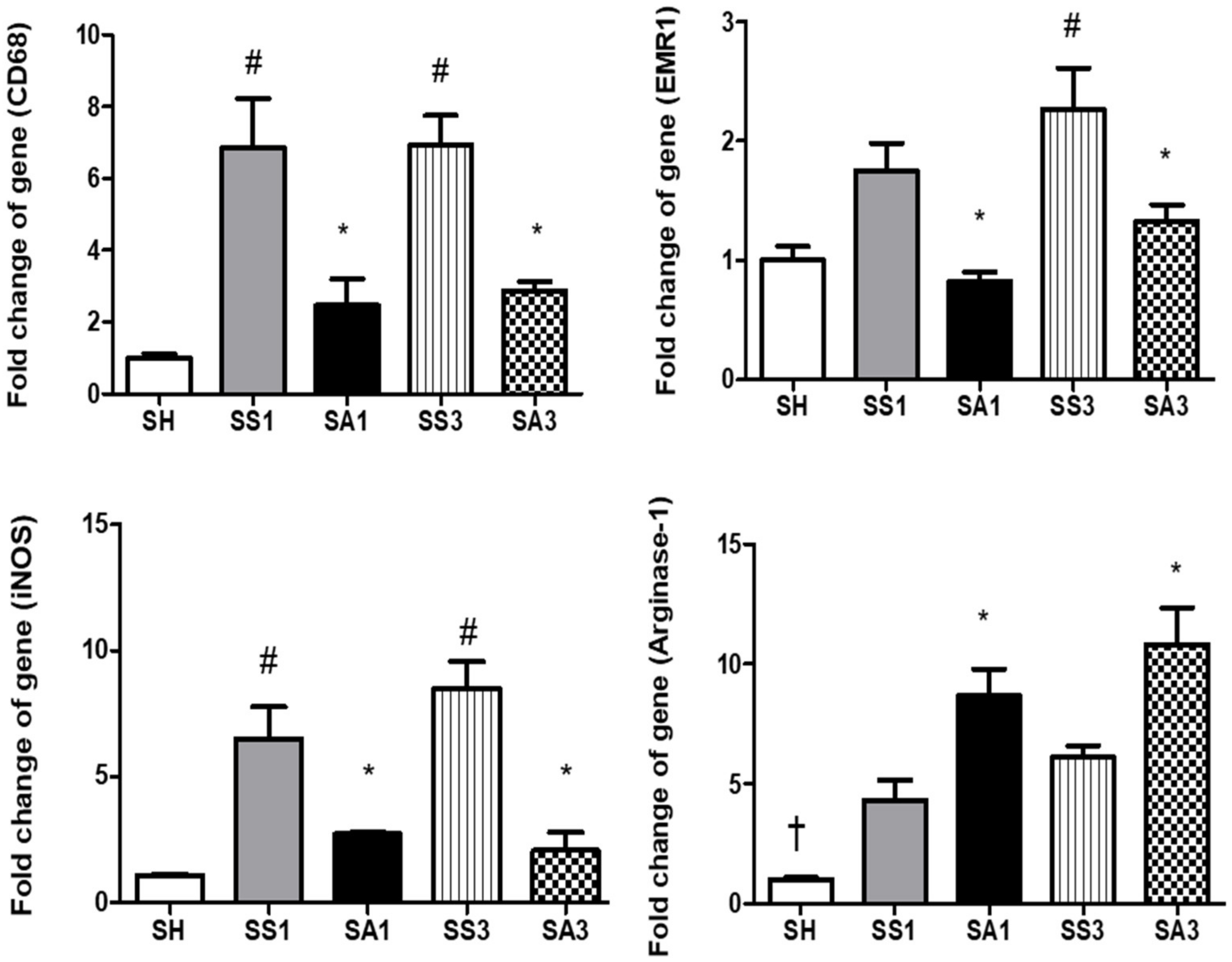
2.8. mRNA Expressions of Inflammatory Mediators and Macrophage Infiltration Markers in Adipose Tissues
2.9. Lipid Metabolism-Related Gene Expressions in the Liver
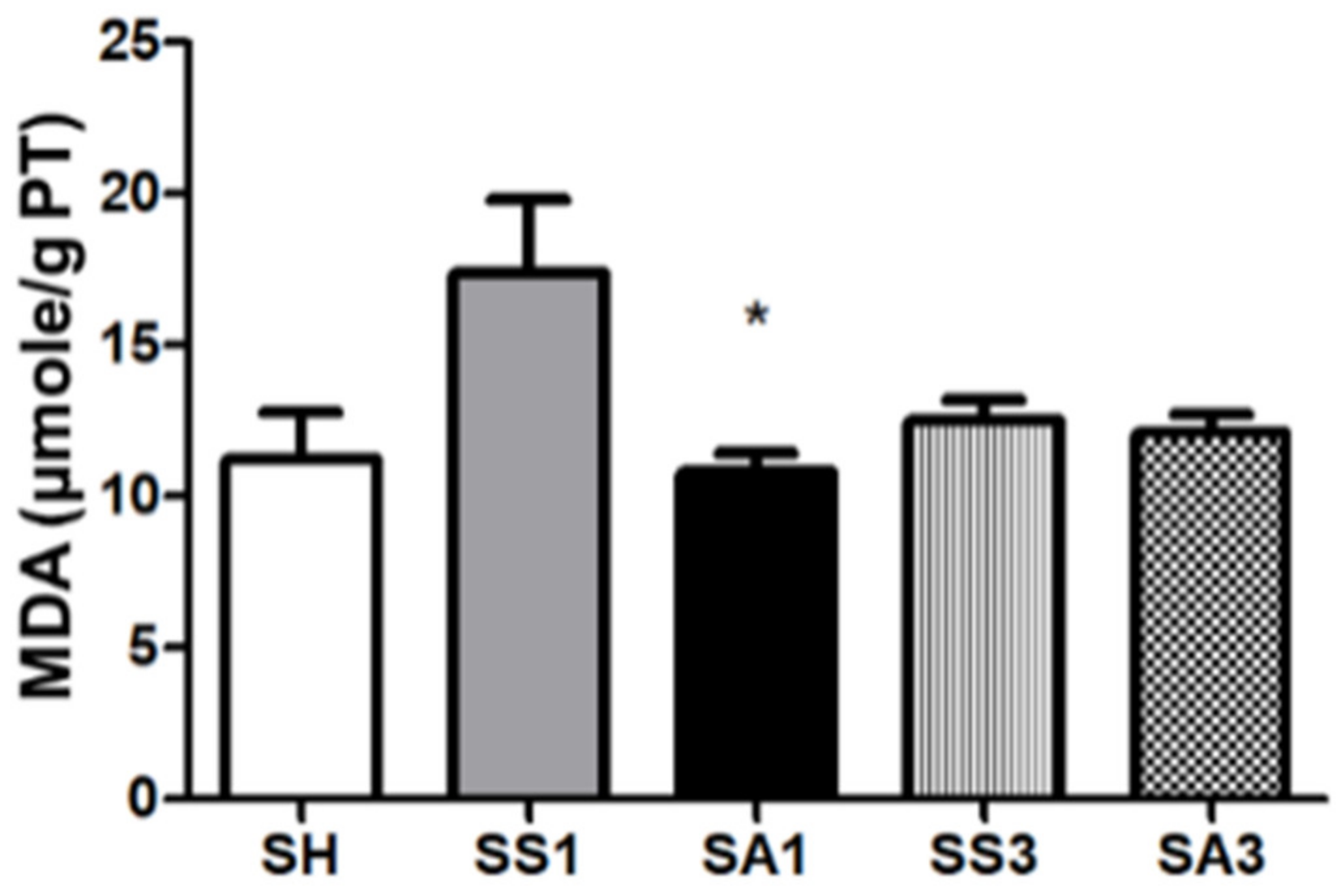
2.10. Lipid Peroxide Concentrations in Liver Tissues
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Grouping and Design
4.3. The IP Glucose Tolerance Test (IPGTT)
4.4. Measurements of Plasma Biochemical Markers and Adipokines
4.5. Plasma Concentrations of Amino Acids
4.6. Inflammatory Cytokine Levels in PLF
4.7. mRNA Extraction and a Real-Time Reverse-Transcription (RT) Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.8. Analysis of Thiobarbituric Acid-Reactive Substances (TBARS) in the Liver
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, H.C.; Yang, H.C.; Chang, H.Y.; Yeh, C.J.; Chen, H.H.; Huang, K.C.; Pan, W.H. Morbid obesity in taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS ONE 2017, 12, e0169577. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar]
- Tsai, A.G.; Wadden, T.A. The evolution of very-low-calorie diets: An update and meta-analysis. Obesity 2006, 14, 1283–1293. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hung, C.K.; Chang, Y.Y.; Tai, C.M.; Lin, J.T.; Wang, J.D. Health-related quality of life in adult patients with morbid obesity coming for bariatric surgery. Obes. Surg. 2010, 20, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, D.; Bellatorre, A.; Bellatorre, N. Sleeve gastrectomy as a stand-alone bariatric operation for severe, morbid, and super obesity. JSLS J. Soc. Laparoendosc. Surg. 2013, 17, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.J.; Kim, G.; Chew, C.A.Z.; San, M.T.; So, J.B.; Shabbir, A. Is laparoscopic sleeve gastrectomy for asian super obese a safe and effective procedure? Ann. Acad. Med. Singap. 2018, 47, 177–184. [Google Scholar] [PubMed]
- Nascimento, A.P.; Costa, A.M. Overweight induced by high-fat diet delays rat cutaneous wound healing. Br. J. Nutr. 2006, 96, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Böger, R.H. The pharmacodynamics of l-arginine. Altern. Ther. Health Med. 2014, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Evoy, D.; Lieberman, M.D.; Fahey, T.J., 3rd; Daly, J.M. Immunonutrition: The role of arginine. Nutrition 1998, 14, 611–617. [Google Scholar] [CrossRef]
- Yeh, C.L.; Pai, M.H.; Shih, Y.M.; Shih, J.M.; Yeh, S.L. Intravenous arginine administration promotes proangiogenic cells mobilization and attenuates lung injury in mice with polymicrobial sepsis. Nutrients 2017, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.F.; Yeh, C.L.; Shih, J.M.; Shih, Y.M.; Yeh, S.L. Arginine pretreatment enhances circulating endothelial progenitor cell population and attenuates inflammatory response in high-fat diet-induced obese mice with limb ischemia. Nutr. Res. 2018, 53, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Breuillard, C.; Belabed, L.; Bonhomme, S.; Blanc-Quintin, M.C.; Neveux, N.; Couderc, R.; De Bandt, J.P.; Cynober, L.; Darquy, S. Arginine availability modulates arginine metabolism and tnfα production in peritoneal macrophages from zucker diabetic fatty rats. Clin. Nutr. 2012, 31, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.C.; Feng, R.N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Hurt, R.T.; Ebbert, J.O.; Schroeder, D.R.; Croghan, I.T.; Bauer, B.A.; McClave, S.A.; Miles, J.M.; McClain, C.J. L-arginine for the treatment of centrally obese subjects: A pilot study. J. Diet. Suppl. 2014, 11, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary l-arginine supplementation reduces fat mass in zucker diabetic fatty rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary l-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 2009, 139, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, J.M.; Gauthier, M.S. Regulation of fat metabolism in the liver: Link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell. Mol. Life Sci. 2006, 63, 1393–1409. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Braley, S.; Fleming, N.W.; Lambourne, L.; Rivers, R.; Wolfe, B.M. Comparison of postoperative hepatic function after laparoscopic versus open gastric bypass. Am. J. Surg. 2003, 186, 40–44. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, c57bl/6, balb/c and icr. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. Cd68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of ppar isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, M.; Audano, M.; Sahebkar, A.; Sirtori, C.R.; Mitro, N.; Ruscica, M. Ppar agonists and metabolic syndrome: An established role? Int. J. Mol. Sci. 2018, 19, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025s–2031s. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D. M1 and m2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.J.; Kim, M.; Park, H.S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.S.; Oh, G.T.; et al. Ampk activation increases fatty acid oxidation in skeletal muscle by activating pparalpha and pgc-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Durandt, C.; Kallmeyer, K.; Ambele, M.A.; Pepper, M.S. The role of pref-1 during adipogenic differentiation: An overview of suggested mechanisms. Int. J. Mol. Sci. 2020, 21, 4104. [Google Scholar] [CrossRef]
- Yin, D.P.; Boyd, K.L.; Williams, P.E.; Abumrad, N.N.; Wasserman, D.H. Mouse models of bariatric surgery. Curr. Protoc. Mouse Biol. 2012, 2, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, K.F.; Shih, J.M.; Shih, Y.M.; Pai, M.H.; Yeh, S.L. Arginine administration increases circulating endothelial progenitor cells and attenuates tissue injury in a mouse model of hind limb ischemia/reperfusion. Nutrition 2018, 55–56, 29–35. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wu, J.M.; Su, L.H.; Yang, P.J.; Lee, P.C.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Intravenous calcitriol administration regulates the renin-angiotensin system and attenuates acute lung injury in obese mice complicated with polymicrobial sepsis. Biomed. Pharmacother. 2021, 141, 111856. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Weights | SS1 | SA1 | SS3 | SA3 |
|---|---|---|---|---|
| BW (g) | ||||
| Before SG | 41.7 ± 2.6 | 40.2 ± 2.3 | 39.5 ± 0.6 | 39.1 ± 0.9 |
| After SG | 39.1 ± 2.4 | 37.4 ± 1.9 | 34.9 ± 1.3 | 34.8 ± 1.5 |
| BW change (g) | −2.65 ± 0.48 | −2.82 ± 0.39 | −4.78 ± 1.07 | −4.17 ± 0.75 |
| Epididymal fat (g) | 2.54 ± 0.56 | 2.65 ± 0.51 | 2.04 ± 0.35 | 1.84 ± 0.25 |
| Parameters | NC | SH | SS1 | SA1 | SS3 | SA3 |
|---|---|---|---|---|---|---|
| AST (UL) | 54.5 ± 7.8 | 100.2 ± 23.6 | 224.6 ± 85.3 # | 171.4 ± 68.8 | 237.8 ± 55.2 # | 135.4 ± 37.7 |
| ALT (U/L) | 12.1 ± 1.0 | 31.5 ± 14.1 | 389.7 ± 122.6 # | 188.6 ± 48 #,* | 171.8 ± 62.5 # | 27.1 ± 8.7 * |
| TGs (mg/dL) | 65.4 ± 18.1 | 60.3 ± 14.6 | 45.6 ± 12.8 | 37.4 ± 15.1 # | 89.4 ± 9.2 # | 60.5 ± 5.1 * |
| TC (mg/dL) | 103.5 ± 8.8 | 125.3 ± 13.2 † | 107.2 ± 18.3 | 78.4 ± 3.4 #,* | 142.1 ± 33.8 | 101.9 ± 25.2 |
| LDL-C (mg/dL) | 14.5 ± 2.1 | 24.0 ± 6.6 † | 20.5 ± 4.1 | 21.7 ± 2.9 | 35.8 ± 9.1 # | 35.1 ± 7.4 # |
| HDL-C (mg/dL) | 84.3 ± 8.6 | 95.3 ± 5.4 | 71.8 ± 10.8 # | 76.1 ± 9.7 # | 70.7 ± 11.1 # | 83.8 ± 7.3 * |
| Glucose (mg/dL) | 110.2 ± 6.1 | 204.4 ± 11.4 † | 103.7 ± 9.1 # | 103.8 ± 9.7 # | 73.4 ± 14.6 # | 87.37 ± 24.9 # |
| Insulin (µIU/dL) | 208.3 ± 63.3 | 202.7 ± 89.2 | 537.9 ± 93.5 # | 592.6 ± 98.1 # | 218.9 ± 35.9 | 207.6 ± 49.6 |
| Parameters | NC | SH | SS1 | SA1 | SS3 | SA3 |
|---|---|---|---|---|---|---|
| NO (µmol/dL) | 42.2 ± 7.2 | 34.5 ± 8.1 | 45.7 ± 12.3 | 48.1 ± 10.5 | 22.3 ± 4.9 | 40.3 ± 2.7 * |
| Leptin (ng/dL) | 11.1 ± 5.7 | 34.6 ± 18.4 | 406.2 ± 101.2 # | 302.7 ± 71.8 #,* | 31.2 ± 15.1 | 32.6 ± 12.7 |
| Adiponectin (µg/dL) | 11.2 ± 0.6 | 9.2 ± 1.4 † | 3.9 ± 0.5 # | 3.7 ± 0.3 # | 4.3 ± 1.3 # | 6.1 ± 0.9 #,* |
| Ingredient | 60% High-Fat Diet | Normal Diet |
|---|---|---|
| Casein, lactic (g) | 258.45 | 189.56 |
| Cysteine, L (g) | 3.88 | 2.84 |
| Corn starch (g) | - | 521.30 |
| Maltodextrin (g) | 161.53 | 142.17 |
| Sucrose (g) | 94.08 | 3.79 |
| Cellulose (g) | 64.61 | 47.39 |
| Lard (g) | 316.60 | 18.96 |
| Soybean oil (g) | 32.31 | 23.70 |
| Mineral mix 1 (g) | 64.61 | 47.39 |
| Choline bitartrate (g) | 2.58 | 1.90 |
| Vitamin mix 2 (g) | 1.29 | 0.95 |
| Dye (g) | 0.06 | 0.06 |
| Total (g) | 1000 | 1000 |
| Protein/Fat/Carbohydrate (%) | 20/60/20 | 20/10/70 |
| Energy density (kcal/g) | 5.21 | 3.82 |
| Gene Name | Accession No. | 5′→3′ Primer Sequence |
|---|---|---|
| ACC-1 | XM_036156218.1 | F: ATGGGCGGAATGGTCTCTTTC R: TGGGGACCTTGTCTTCATCAT |
| ACOX-1 | NM_001377522.1 | F: GGATGGTAGTCCGGAGAACA R: AGTCTGGATCGTTCAGAATCAAG |
| Adiponectin | NM_009605.5 | F: ATCTGGAGGTGGGAGACCAA R: GGGCTATGGGTAGTTGCAGT |
| Arginase-1 | AH011507.2 | F: AGCAGAAGGCTTTGTCAGCA R: ACCCAAAGTGGCACAACTCA |
| CD68 | NM_001291058.1 | F: TGTTCAGCTCCAAGCCCAAA R: ACTCGGGCTCTGATGTAGGT |
| CPT-1 | NM_013495.2 | F: GAGCCAGACCTTGAAGTAACG R: GAGACAGACACCATCCAACAC |
| EMR1 | U66889.1 | F: ACCTTGTGGTCCTAACTCAGTC R: ACAAAGCCTGGTTGACAGGTA |
| FAS | NM_007988.3 | F: GGAGGTGGTGATAGCCGGTAT R: TGGGTAATCCATAGAGCCCAG |
| IL-1β | NM_008361.4 | F: TGCCACCTTTTGACAGTGATG R: ATGTGCTGCTGCGAGATTTG |
| IL-6 | NM_031168.2 | F: TCCTACCCCAACTTCCAATGCTC R: TTGGATGGTCTTGGTCCTTAGCC |
| IL-10 | M37897.1 | F: AGGCGCTGTCATCGATTTCT R: ATGGCCTTGTAGACACCTTGG |
| iNOS | U58677.1 | F: ACTAGGGCACCTCCATCACT R: TAATGGGGAGCGCAAAGTCT |
| Leptin | NM_008493.3 | F: TCTGAAAGATCCCACGTGCC R: AAGGCTCAGGACATTCCAGC |
| PPAR-α | XM_030248421.2 | F: AGAGCCCCATCTGTCCTCTC R: ACTGGTAGTCTGCAAAACCAAA |
| PPAR-γ | XM_006505743.4 | F: ATTGAGTGCCGAGTCTGTGG R: ACCTGATGGCATTGTGAGACA |
| TNF-α | NM_013693.3 | F: ATGGCCTCCCTCTCATCAGT R: TTTGCTACGACGTGGGCTAC |
| GAPDH | BC023196.2 | F: GAAGGTCGGTGTGAACGGAT R: AATCTCCACTTTGCCACTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Lin, M.-T.; Wang, W.-H.; Yeh, S.-L.; Yeh, C.-L. Intravenous Arginine Administration Attenuates the Inflammatory Response and Improves Metabolic Profiles in Diet-Induced Obese Mice after Sleeve Gastrectomy. Metabolites 2022, 12, 153. https://doi.org/10.3390/metabo12020153
Chen Y-L, Lin M-T, Wang W-H, Yeh S-L, Yeh C-L. Intravenous Arginine Administration Attenuates the Inflammatory Response and Improves Metabolic Profiles in Diet-Induced Obese Mice after Sleeve Gastrectomy. Metabolites. 2022; 12(2):153. https://doi.org/10.3390/metabo12020153
Chicago/Turabian StyleChen, Ya-Ling, Ming-Tsan Lin, Wan-Hsuan Wang, Sung-Ling Yeh, and Chiu-Li Yeh. 2022. "Intravenous Arginine Administration Attenuates the Inflammatory Response and Improves Metabolic Profiles in Diet-Induced Obese Mice after Sleeve Gastrectomy" Metabolites 12, no. 2: 153. https://doi.org/10.3390/metabo12020153
APA StyleChen, Y.-L., Lin, M.-T., Wang, W.-H., Yeh, S.-L., & Yeh, C.-L. (2022). Intravenous Arginine Administration Attenuates the Inflammatory Response and Improves Metabolic Profiles in Diet-Induced Obese Mice after Sleeve Gastrectomy. Metabolites, 12(2), 153. https://doi.org/10.3390/metabo12020153







