Efficacy of Different Modalities and Frequencies of Physical Exercise on Glucose Control in People with Prediabetes (GLYCEX Randomised Trial)
Abstract
1. Introduction
2. Objectives
2.1. Main Aim
2.2. Secondary Aim
3. Experimental Design
3.1. Design
3.2. Participants
3.3. Sample Size and Randomization
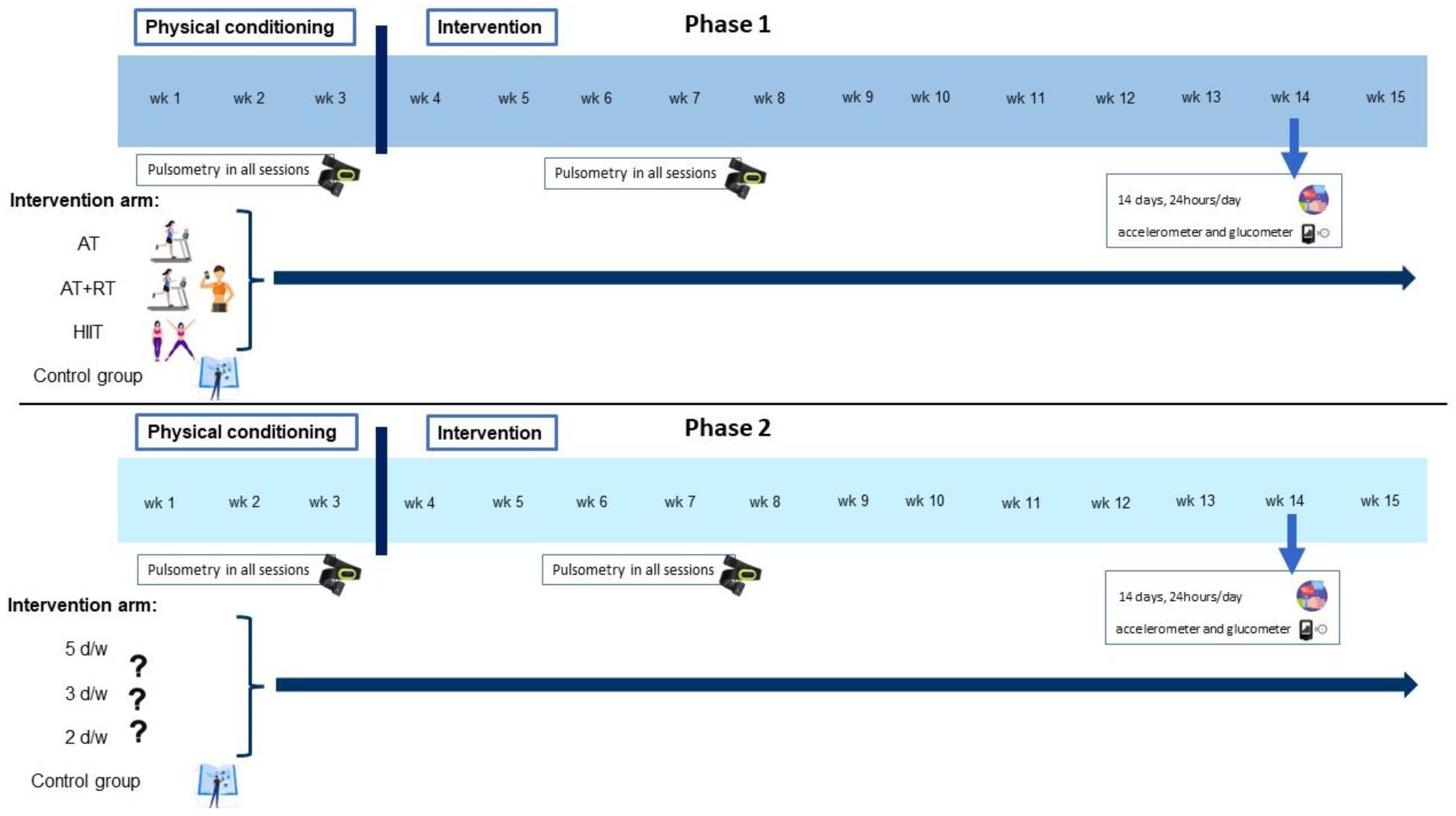
4. Procedure
4.1. Description of Interventions
4.1.1. Phase 1
- 1)
- Aerobic Training intervention (AT): Perform 50 min/day, 3 days/week, totalling 150 min/week [34,51] at moderate intensity, as recommended by WHO [26], in a range of 65–75% HRMax. Because any form of aerobic exercise involving large muscle groups and causing sustained increases in HR is likely to be beneficial [52], the type of aerobic exercise will be agreed on with each group of patients, varying from four to eight people. There will be a choice of exercises and participants will be able to choose a combination of up to two different exercises. The participants’ choice of the type of activity to be performed is expected to encourage greater adherence. The range of exercises will be brisk walking or running, swimming and/or aerobic dancing.
- 2)
- Aerobic Training plus Resistance Training intervention (AT+RT): Perform 50 min/day, 3 days/week, starting with 50% of 1-repetition maximum (1-RM) and follow a progression of increasing loads up to 75% of 1-RM for optimal gains in strength and insulin action [51]. In each session, between five and ten exercises will be worked on, performing 10–15 repetitions, and progressing to 8–10 lifting as the weight increases, involving the major muscle groups from the core, lower body and upper body [53,54]. In all sessions there will be a 3 min warm-up at the beginning of the session and a 2 min cool-down at the end of the session [34].
- 3)
- High Intensity Interval Training intervention (HIIT): To be considered high intensity, the heart rate needs to be above ≥85% [19,34,55]. Perform 25 min/day, 3 days/week, totalling 75 min/week [34,51] at a vigorous intensity, as recommended by WHO [26]. Starting with four intervals lasting 1 min keeping in a range of 85–90% HRMax, separated by 1 min of low intensity activity (no static) (4 × 1 min intervals), a progression will be followed by increasing the number of circuits, up to ten (10 × 1 min intervals) [19,55,56]. In all sessions there will be a 3 min warm-up at the beginning of the session and a 2 min cool-down at the end of the session [19,34,55,56]. Although the target population is a sedentary population, we expect the HIIT approach, despite being high intensity, to be well received, due to its growing popularity, as demonstrated by a study in which 62% of inactive participants preferred HIIT to other types of exercise [57].
- 4)
- Participants in the control group will receive written standard PA recommendations in this phase.
4.1.2. Phase 2
4.2. Data Collection and Procedures
4.2.1. Visit −1 (V−1)
4.2.2. Visit 0 (V0):
4.2.3. Visit 1 (V1)
4.3. Data Collection
4.3.1. Biological Samples and Laboratory Procedures
4.3.2. Glycaemic Variability
4.3.3. Accelerometer
4.3.4. Diet
4.3.5. Physical Activity and Sedentary Behaviour
4.3.6. Anthropometric Measurements
4.3.7. Blood Pressure
4.3.8. Quality of Life and Sleep
5. Results
5.1. Main Dependent Variable
5.2. Main Independent Variable
5.3. Statistical Analysis
5.4. Ethical Considerations
5.5. Validity and Reliability
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF (International Diabetes Federation). IDF Diabetes Atlas Tenth Edition 2021. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 20 February 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiu, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castano, L.; Castell, C.; Catala, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B.; American Diabetes, A. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bennasar-Veny, M.; Fresneda, S.; Lopez-Gonzalez, A.; Busquets-Cortes, C.; Aguilo, A.; Yanez, A.M. Lifestyle and Progression to Type 2 Diabetes in a Cohort of Workers with Prediabetes. Nutrients 2020, 12, 1538. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Kivimaki, M.; Tabak, A.G. Does addressing prediabetes help to improve population health? Lancet Diabetes Endocrinol. 2018, 6, 354–356. [Google Scholar] [CrossRef]
- Andrews, R.C.; Cooper, A.R.; Montgomery, A.A.; Norcross, A.J.; Peters, T.J.; Sharp, D.J.; Jackson, N.; Fitzsimons, K.; Bright, J.; Coulman, K.; et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: The Early ACTID randomised controlled trial. Lancet 2011, 378, 129–139. [Google Scholar] [CrossRef]
- Thomas, D.E.; Elliott, E.J.; Naughton, G.A. Exercise for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Gilbertson, N.M.; Mandelson, J.A.; Hilovsky, K.; Akers, J.D.; Hargens, T.A.; Wenos, D.L.; Edwards, E.S. Combining supervised run interval training or moderate-intensity continuous training with the diabetes prevention program on clinical outcomes. Eur. J. Appl. Physiol. 2019, 119, 1503–1512. [Google Scholar] [CrossRef]
- Gidlund, E.K.; von Walden, F.; Venojarvi, M.; Riserus, U.; Heinonen, O.J.; Norrbom, J.; Sundberg, C.J. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol. Rep. 2016, 4, e13063. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Zhong, S.G.; Li, P.; Chen, W.B.; Cheng, C.; Wang, Y.G.; Wu, P.S.; Xiao, C. Effects and mechanism of moderate aerobic exercise on impaired fasting glucose improvement. Lipids Health Dis. 2015, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Pitocco, D.; Ghirlanda, G. Glycemic risk factors of diabetic vascular complications: The role of glycemic variability. Diabetes Metab. Res. Rev. 2009, 25, 199–207. [Google Scholar] [CrossRef]
- Faerch, K.; Vaag, A.; Holst, J.J.; Hansen, T.; Jorgensen, T.; Borch-Johnsen, K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: The Inter99 study. Diabetes Care 2009, 32, 439–444. [Google Scholar] [CrossRef]
- Faerch, K.; Vaag, A.; Witte, D.R.; Jorgensen, T.; Pedersen, O.; Borch-Johnsen, K. Predictors of future fasting and 2-h post-OGTT plasma glucose levels in middle-aged men and women-the Inter99 study. Diabet. Med. 2009, 26, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, J.W.; Manders, R.J.; Canfora, E.E.; Mechelen, W.V.; Hartgens, F.; Stehouwer, C.D.; Van Loon, L.J. Exercise and 24-h glycemic control: Equal effects for all type 2 diabetes patients? Med. Sci. Sports Exerc. 2013, 45, 628–635. [Google Scholar] [CrossRef]
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R.J. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014, 39, 835–841. [Google Scholar] [CrossRef]
- O’Hagan, C.; De Vito, G.; Boreham, C.A. Exercise prescription in the treatment of type 2 diabetes mellitus: Current practices, existing guidelines and future directions. Sports Med. 2013, 43, 39–49. [Google Scholar] [CrossRef]
- Tonyan, Z.N.; Nasykhova, Y.A.; Danilova, M.M.; Barbitoff, Y.A.; Changalidi, A.I.; Mikhailova, A.A.; Glotov, A.S. Overview of Transcriptomic Research on Type 2 Diabetes: Challenges and Perspectives. Genes 2022, 13, 1176. [Google Scholar] [CrossRef]
- Liew, C.C.; Ma, J.; Tang, H.C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Reynes, B.; Diaz-Rua, R.; Cifre, M.; Oliver, P.; Palou, A. Peripheral blood mononuclear cells as a potential source of biomarkers to test the efficacy of weight-loss strategies. Obesity 2015, 23, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Gjevestad, G.O.; Hamarsland, H.; Raastad, T.; Ottestad, I.; Christensen, J.J.; Eckardt, K.; Drevon, C.A.; Biong, A.S.; Ulven, S.M.; Holven, K.B. Gene expression is differentially regulated in skeletal muscle and circulating immune cells in response to an acute bout of high-load strength exercise. Genes Nutr. 2017, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- WHO (World Health Organization). Guidelines on Physical Activity and Sedentary Behaviour. 2020. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 20 February 2022).
- Hansen, E.; Landstad, B.J.; Gundersen, K.T.; Torjesen, P.A.; Svebak, S. Insulin sensitivity after maximal and endurance resistance training. J. Strength Cond. Res. 2012, 26, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Yang, H.Y.; Shun, S.C. Effect of exercise intervention dosage on reducing visceral adipose tissue: A systematic review and network meta-analysis of randomized controlled trials. Int. J. Obes. 2021, 45, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M.; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health and Cardiovascular, Exercise, Cardiac Rehabilitation; et al. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart Association. Circulation 2013, 128, 2259–2279. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar]
- Korhonen, M.; Halmesmaki, K.; Lepantalo, M.; Venermo, M. Predictors of failure of endovascular revascularization for critical limb ischemia. Scand. J. Surg. 2012, 101, 170–176. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S.; American College of Sports, M. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Davies, D.S.C.; Atherton, F.; McBride, M.; Calderwood, C. UK Chief Medical Officers’ Physical Activity Guidelines. UK Chief Medical Officers. 2009. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed on 10 January 2022).
- Jung, M.E.; Bourne, J.E.; Beauchamp, M.R.; Robinson, E.; Little, J.P. High-intensity interval training as an efficacious alternative to moderate-intensity continuous training for adults with prediabetes. J. Diabetes Res. 2015, 2015, 191595. [Google Scholar] [CrossRef]
- Pan, B.; Ge, L.; Xun, Y.Q.; Chen, Y.J.; Gao, C.Y.; Han, X.; Zuo, L.Q.; Shan, H.Q.; Yang, K.H.; Ding, G.W.; et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 72. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports, M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- RezkAllah, S.S.; Takla, M.K. Effects of Different Dosages of Interval Training on Glycemic Control in People With Prediabetes: A Randomized Controlled Trial. Diabetes Spectr. 2019, 32, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhai, L.; Chen, Q.; Miller, J.D.; Lu, L.; Hsue, C.; Liu, L.; Yuan, X.; Wei, W.; Ma, X.; et al. Two-year-supervised resistance training prevented diabetes incidence in people with prediabetes: A randomised control trial. Diabetes Metab. Res. Rev. 2019, 35, e3143. [Google Scholar] [CrossRef] [PubMed]
- Viskochil, R.; Malin, S.K.; Blankenship, J.M.; Braun, B. Exercise training and metformin, but not exercise training alone, decreases insulin production and increases insulin clearance in adults with prediabetes. J. Appl. Physiol. 2017, 123, 243–248. [Google Scholar] [CrossRef]
- Faerch, K.; Vistisen, D.; Johansen, N.B.; Jorgensen, M.E. Cardiovascular risk stratification and management in pre-diabetes. Curr. Diab. Rep. 2014, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, C.; Fiorini, L.; Rosato, R.; Audenino, M.; Valpreda, M.; Vineis, P. Randomized controlled trial: Effect of nutritional counselling in general practice. Int. J. Epidemiol. 2006, 35, 409–415. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Gotzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hrobjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Rowan, C.P.; Riddell, M.C.; Jamnik, V.K. The Prediabetes Detection and Physical Activity Intervention Delivery (PRE-PAID) program. Can. J. Diabetes 2013, 37, 415–419. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.A. OxMaR: Open source free software for online minimization and randomization for clinical trials. PLoS ONE 2014, 9, e110761. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, I.T.; Hanoun, S.; Nahavandi, D.; Nahavandi, S. Validation of Polar OH1 optical heart rate sensor for moderate and high intensity physical activities. PLoS ONE 2019, 14, e0217288. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Landwehr, R. The surprising history of the “HRmax = 220-age” equation. J. Exerc. Physiol. 2002, 5, 1–10. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Colberg, S.R.; Albright, A.L.; Blissmer, B.J.; Braun, B.; Chasan-Taber, L.; Fernhall, B.; Regensteiner, J.G.; Rubin, R.R.; Sigal, R.J.; American College of Sports, M.; et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2010, 42, 2282–2303. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Solomon, C.; Liu, S.; Colditz, G.A.; Speizer, F.E.; Willett, W.C.; Manson, J.E. Physical activity and risk for cardiovascular events in diabetic women. Ann. Intern. Med. 2001, 134, 96–105. [Google Scholar] [CrossRef]
- Gordon, B.A.; Benson, A.C.; Bird, S.R.; Fraser, S.F. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2009, 83, 157–175. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Little, J.P.; Punthakee, Z.; Tarnopolsky, M.A.; Riddell, M.C.; Gibala, M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Bourne, J.E.; Little, J.P. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS ONE 2014, 9, e114541. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendia, L.E.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Ramos-Zavala, M.G.; Hernandez-Gonzalez, S.O.; Jacques-Camarena, O.; Rodriguez-Moran, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Sanchez, J.; Priego, T.; Pico, C.; Ahrens, W.; De Henauw, S.; Fraterman, A.; Marild, S.; Molnar, D.; Moreno, L.A.; Peplies, J.; et al. Blood cells as a source of transcriptional biomarkers of childhood obesity and its related metabolic alterations: Results of the IDEFICS study. J. Clin. Endocrinol. Metab. 2012, 97, E648–E652. [Google Scholar] [CrossRef]
- Szostaczuk, N.; van Schothorst, E.M.; Sanchez, J.; Priego, T.; Palou, M.; Bekkenkamp-Grovenstein, M.; Faustmann, G.; Obermayer-Pietsch, B.; Tiran, B.; Roob, J.M.; et al. Identification of blood cell transcriptome-based biomarkers in adulthood predictive of increased risk to develop metabolic disorders using early life intervention rat models. FASEB J. 2020, 34, 9003–9017. [Google Scholar] [CrossRef]
- Wadwa, R.P.; Laffel, L.M.; Shah, V.N.; Garg, S.K. Accuracy of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System During 10 Days of Use in Youth and Adults with Diabetes. Diabetes Technol. 2018, 20, 395–402. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2022. [CrossRef]
- Faerch, K.; Amadid, H.; Nielsen, L.B.; Ried-Larsen, M.; Karstoft, K.; Persson, F.; Jorgensen, M.E. Protocol for a randomised controlled trial of the effect of dapagliflozin, metformin and exercise on glycaemic variability, body composition and cardiovascular risk in prediabetes (the PRE-D Trial). BMJ Open 2017, 7, e013802. [Google Scholar] [CrossRef]
- Galmes-Panades, A.M.; Varela-Mato, V.; Konieczna, J.; Warnberg, J.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Corella, D.; Schroder, H.; Vioque, J.; Alonso-Gomez, A.M.; et al. Isotemporal substitution of inactive time with physical activity and time in bed: Cross-sectional associations with cardiometabolic health in the PREDIMED-Plus study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V.; Mirkes, E.M.; Yates, T.; Clemes, S.; Davies, M.; Khunti, K.; Edwardson, C.L. Accelerometer-assessed Physical Activity in Epidemiology: Are Monitors Equivalent? Med. Sci. Sports Exerc. 2018, 50, 257–265. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; da Silva, I.C.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Zomeno, M.D.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Corella, D.; Vioque, J.; Romaguera, D.; Martinez, J.A.; Tinahones, F.J.; Miranda, J.L.; et al. Validity of the energy-restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Penafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schroder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Lopez-Fontana, C.; Varo, J.J.; Sanchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Kelly, J.; Metcalfe, J. Validity and Reliability of Body Composition Analysis Using the Tanita BC 418-MA. J. Exerc. Physiol. Online 2012, 15, 74–83. [Google Scholar]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, J. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry (ISAK): Wellington, New Zeland, 2011. [Google Scholar]
- Devlin, N.; Parkin, D.; Janssen, B. Methods for Analysing and Reporting EQ-5D Data; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hays, R.D.; Martin, S.A.; Sesti, A.M.; Spritzer, K.L. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005, 6, 41–44. [Google Scholar] [CrossRef]
- Kim, S.S.; Won, J.C.; Kwon, H.S.; Kim, C.H.; Lee, J.H.; Park, T.S.; Ko, K.S.; Cha, B.Y. Validity of the medical outcomes study sleep scale in patients with painful diabetic peripheral neuropathy in Korea. J. Diabetes Investig. 2013, 4, 405–409. [Google Scholar] [CrossRef]
- Royston, P.; White, I.R. Multiple Imputation by Chained Equations (MICE): Implementation in Stata. J. Stat. Softw. 2011, 45, 1–20. [Google Scholar] [CrossRef]
| Initial Evaluation | Start | ||
|---|---|---|---|
| Visit number | −1 | 0 | 1 |
| Time | −Day 7 | Day 0 | Week 15 |
| Informed consent | X | ||
| Inclusion/exclusion criteria | X | ||
| Randomization | X | ||
| Blood sample | X | X | |
| Glucose monitoring | X | X | |
| Baseline data | X | ||
| Cites management | X | ||
| Adverse events | X | ||
| Anthropometric measurements | X | X | |
| Blood pressure | X | X | |
| Physical activity questionnaire | X | X | |
| Sedentary behaviour questionnaire | X | X | |
| Diet, alcohol and smoking questionnaire | X | X | |
| Quality of life questionnaire | X | X | |
| Accelerometer | X | X | |
| Pulsometer | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmes-Panades, A.M.; Bennasar-Veny, M.; Oliver, P.; Garcia-Coll, N.; Chaplin, A.; Fresneda, S.; Gallardo-Alfaro, L.; García-Ruano, C.; Konieczna, J.; Leiva, A.; et al. Efficacy of Different Modalities and Frequencies of Physical Exercise on Glucose Control in People with Prediabetes (GLYCEX Randomised Trial). Metabolites 2022, 12, 1286. https://doi.org/10.3390/metabo12121286
Galmes-Panades AM, Bennasar-Veny M, Oliver P, Garcia-Coll N, Chaplin A, Fresneda S, Gallardo-Alfaro L, García-Ruano C, Konieczna J, Leiva A, et al. Efficacy of Different Modalities and Frequencies of Physical Exercise on Glucose Control in People with Prediabetes (GLYCEX Randomised Trial). Metabolites. 2022; 12(12):1286. https://doi.org/10.3390/metabo12121286
Chicago/Turabian StyleGalmes-Panades, Aina M, Miquel Bennasar-Veny, Paula Oliver, Natalia Garcia-Coll, Alice Chaplin, Sergio Fresneda, Laura Gallardo-Alfaro, Carmen García-Ruano, Jadwiga Konieczna, Alfonso Leiva, and et al. 2022. "Efficacy of Different Modalities and Frequencies of Physical Exercise on Glucose Control in People with Prediabetes (GLYCEX Randomised Trial)" Metabolites 12, no. 12: 1286. https://doi.org/10.3390/metabo12121286
APA StyleGalmes-Panades, A. M., Bennasar-Veny, M., Oliver, P., Garcia-Coll, N., Chaplin, A., Fresneda, S., Gallardo-Alfaro, L., García-Ruano, C., Konieczna, J., Leiva, A., Masmiquel, L., Pico, C., Ricci-Cabello, I., Romaguera, D., Rivera, R., Sanchis, P., Vidal-Conti, J., & Yañez, A. M. (2022). Efficacy of Different Modalities and Frequencies of Physical Exercise on Glucose Control in People with Prediabetes (GLYCEX Randomised Trial). Metabolites, 12(12), 1286. https://doi.org/10.3390/metabo12121286











