An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glycomics Analysis
2.2. Proteomics Analysis
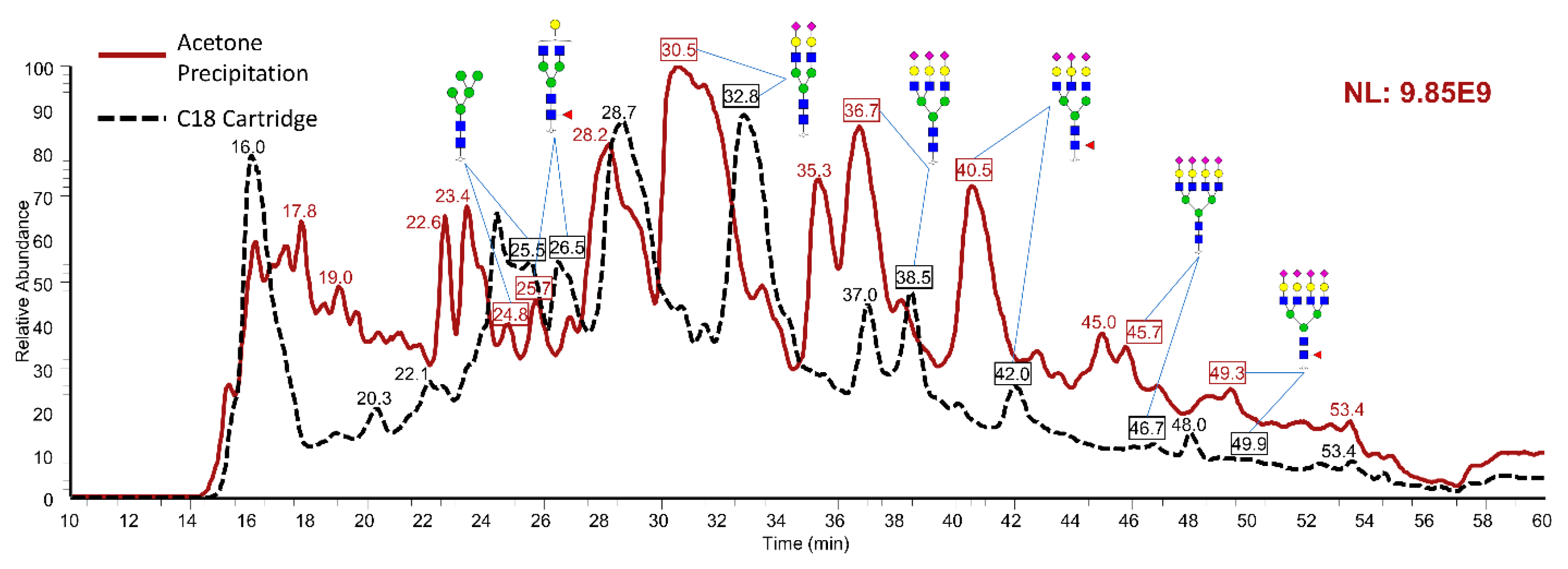
2.3. N-Glycomics of Egg Yolks
3. Materials and Methods
3.1. Materials and Reagents
3.2. Glycomics Sample Preparation
3.3. Proteomics Sample Preparation
3.4. N-Glycan Extractions from Egg Yolks
3.5. LC-MS/MS Conditions
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, C.H. Protein glycosylation: New challenges and opportunities. J. Org. Chem. 2005, 70, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids in human health and disease. Trends. Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veillon, L.; Huang, Y.; Peng, W.; Dong, X.; Cho, B.G.; Mechref, Y. Characterization of isomeric glycan structures by LC-MS/MS. Electrophoresis 2017, 38, 2100–2114. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhao, J.; Dong, X.; Banazadeh, A.; Huang, Y.; Hussien, A.; Mechref, Y. Clinical application of quantitative glycomics. Expert Rev. Proteom. 2018, 15, 1007–1031. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Cho, B.G.; Zhong, J.; Gautam, S.; Peng, W.; Williamson, S.D.; Banazadeh, A.; Torres-Ulloa, K.Y.; Mechref, Y. Advances in mass spectrometry-based glycomics. Electrophoresis 2018, 39, 3063–3081. [Google Scholar] [CrossRef] [PubMed]
- Donohoo, K.B.; Wang, J.; Goli, M.; Yu, A.; Peng, W.; Hakim, M.A.; Mechref, Y. Advances in mass spectrometry-based glycomics—An update covering the period 2017–2021. Electrophoresis 2022, 43, 119–142. [Google Scholar] [CrossRef]
- Dwek, R. Glycobiology:“towards understanding the function of sugars”. Biochem. Soc. Trans. 1995, 23, 1–25. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, X.; Veillon, L.; Huang, Y.; Mechref, Y. LC-MS/MS analysis of permethylated N-glycans facilitating isomeric characterization. Anal. Bioanal. Chem. 2017, 409, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Lowe, J.B.; Marth, J.D. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 2003, 72, 643–691. [Google Scholar] [CrossRef] [Green Version]
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Tsai, C.H.; Chao, T.K.; Chou, Y.C.; Yang, Y.C.; Tsai, M.H.; Cha, T.L.; Hsiao, P.W. O-glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 2018, 32, 6869–6882. [Google Scholar] [CrossRef] [PubMed]
- de Vreede, G.; Morrison, H.A.; Houser, A.M.; Boileau, R.M.; Andersen, D.; Colombani, J.; Bilder, D. A Drosophila tumor suppressor gene prevents tonic TNF signaling through receptor N-glycosylation. Dev. Cell 2018, 45, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, R.A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: The Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 1994, 4, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Huang, Y.; Dong, X.; Peng, W.; Veillon, L.; Kitagawa, D.A.S.; Aquino, A.J.A.; Mechref, Y. Isomeric Separation of Permethylated Glycans by Porous Graphitic Carbon (PGC)-LC-MS/MS at High Temperatures. Anal. Chem. 2017, 89, 6590–6597. [Google Scholar] [CrossRef]
- Gautam, S.; Banazadeh, A.; Cho, B.G.; Goli, M.; Zhong, J.; Mechref, Y. Mesoporous Graphitized Carbon Column for Efficient Isomeric Separation of Permethylated Glycans. Anal. Chem. 2021, 93, 5061–5070. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Yu, A.; Huang, Y.; Peng, W.; Mechref, Y. Isomeric separation of permethylated glycans by extra-long reversed-phase liquid chromatography (RPLC)-MS/MS. Analyst 2022, 147, 2048–2059. [Google Scholar] [CrossRef]
- Kirmiz, C.; Li, B.; An, H.J.; Clowers, B.H.; Chew, H.K.; Lam, K.S.; Ferrige, A.; Alecio, R.; Borowsky, A.D.; Sulaimon, S. A Serum Glycomics Approach to Breast Cancer Biomarkers* S. Mol. Cell. Proteom. 2007, 6, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Vercoutter-Edouart, A.S.; Slomianny, M.C.; Dekeyzer-Beseme, O.; Haeuw, J.F.; Michalski, J.C. Glycoproteomics and glycomics investigation of membrane N-glycosylproteins from human colon carcinoma cells. Proteomics 2008, 8, 3236–3256. [Google Scholar] [CrossRef]
- Isailovic, D.; Kurulugama, R.; Plasencia, M.; Stokes, S.; Kyselova, Z.; Goldman, R.; Mechref, Y.; Novotny, M.; Clemmer, D. Profiling of human serum glycans associated with liver cancer and cirrhosis by IMS− MS. J. Proteome Res. 2008, 7, 1109–1117. [Google Scholar] [CrossRef]
- Xia, B.; Royall, J.A.; Damera, G.; Sachdev, G.P.; Cummings, R.D. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology 2005, 15, 747–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertozzi, C.R.; Freeze, H.H.; Varki, A.; Esko, J.D. Glycans in Biotechnology and the Pharmaceutical Industry. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2009. [Google Scholar]
- Yu, A.; Zhao, J.; Zhong, J.; Wang, J.; Yadav, S.P.S.; Molitoris, B.A.; Wagner, M.C.; Mechref, Y. Altered O-glycomes of Renal Brush-Border Membrane in Model Rats with Chronic Kidney Diseases. Biomolecules 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, W.; Yu, A.; Fokar, M.; Mechref, Y. Glycome Profiling of Cancer Cell Lines Cultivated in Physiological and Commercial Media. Biomolecules 2022, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.; Wu, Y.; Sha, Q.; Liu, B.F.; Lin, Y.; Liu, X. Microwave irradiation-assisted high-efficiency N-glycan release using oriented immobilization of PNGase F on magnetic particles. J. Chromatogr. A 2020, 1619, 460934. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ju, H.; Lasanajak, Y.; Kudelka, M.R.; Smith, D.F.; Cummings, R.D. Oxidative release of natural glycans for functional glycomics. Nat. Methods 2016, 13, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Pereira, W.; Hoyano, Y.; Summons, R.; Bacon, V.; Duffield, A. Chlorination studies II. The reaction of aqueous hypochlorous acid with α-amino acids and dipeptides. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1973, 313, 170–180. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Hypochlorite-induced damage to proteins: Formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem. J. 1998, 332, 617–625. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Hypochlorite-induced oxidation of proteins in plasma: Formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochem. J. 1999, 340, 539–548. [Google Scholar] [CrossRef]
- Tretter, V.; Altmann, F.; März, L. Peptide-N4-(N-acetyl-β-glucosaminyl) asparagine amidase F cannot release glycans with fucose attached α1→ 3 to the asparagine-linked N-acetylglucosamine residue. Eur. J. Biochem. 1991, 199, 647–652. [Google Scholar] [CrossRef]
- Dell, A.; Morris, H.R. Glycoprotein structure determination by mass spectrometry. Science 2001, 291, 2351–2356. [Google Scholar] [CrossRef]
- Banazadeh, A.; Peng, W.; Veillon, L.; Mechref, Y. Carbon nanoparticles and graphene nanosheets as MALDI matrices in glycomics: A new approach to improve glycan profiling in biological samples. J. Am. Soc. Mass Spectrom. 2018, 29, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Wu, Y.; Wang, C.; Sun, B.; Zhang, Z.; Zhang, L.; Lin, Y.; Liu, X. Cellulose microspheres-filled pipet tips for purification and enrichment of glycans and glycopeptides. J. Chromatogr. A 2018, 1569, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, K.H.; İzzet, A.; Bekir, S. A New titania glyco-purification tip for the fast enrichment and efficient analysis of glycopeptides and glycans by MALDI-TOF-MS. J. Pharm. Biomed. Anal. 2019, 174, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kayili, H.M.; Ertürk, A.S.; Elmacı, G.; Salih, B. Poly (amidoamine) dendrimer-coated magnetic nanoparticles for the fast purification and selective enrichment of glycopeptides and glycans. J. Sep. Sci. 2019, 42, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Benktander, J.D.; Gizaw, S.T.; Gaunitz, S.; Novotny, M.V. Comprehensive analytical approach toward glycomic characterization and profiling in urinary exosomes. Anal. Chem. 2017, 89, 5364–5372. [Google Scholar] [CrossRef]
- Tao, S.; Huang, Y.; Boyes, B.E.; Orlando, R. Liquid chromatography-selected reaction monitoring (LC-SRM) approach for the separation and quantitation of sialylated N-glycans linkage isomers. Anal. Chem. 2014, 86, 10584–10590. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Zhang, M.; Wang, J.; Schlüter, H. Comparative Analysis of Different N-glycan Preparation Approaches and Development of Optimized Solid-Phase Permethylation Using Mass Spectrometry. J. Proteome Res. 2021, 20, 2914–2922. [Google Scholar] [CrossRef]
- Valk-Weeber, R.L.; Dijkhuizen, L.; van Leeuwen, S.S. Large-scale quantitative isolation of pure protein N-linked glycans. Carbohydr. Res. 2019, 479, 13–22. [Google Scholar] [CrossRef]
- Takakura, D.; Harazono, A.; Hashii, N.; Kawasaki, N. Selective glycopeptide profiling by acetone enrichment and LC/MS. J. Proteom. 2014, 101, 17–30. [Google Scholar] [CrossRef]
- Green, E.D.; Adelt, G.; Baenziger, J.U.; Wilson, S.; Van Halbeek, H. The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J. Biol. Chem. 1988, 263, 18253–18268. [Google Scholar] [CrossRef]
- Palmisano, G.; Larsen, M.R.; Packer, N.H.; Thaysen-Andersen, M. Structural analysis of glycoprotein sialylation–part II: LC-MS based detection. RSC Adv. 2013, 3, 22706–22726. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.Y.; Mayampurath, A.; Hu, Y.; Zhou, S.; Mechref, Y.; Tang, H. Automated annotation and quantification of glycans using liquid chromatography–mass spectrometry. Bioinformatics 2013, 29, 1706–1707. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chmil, C.; Pierce, F.; Ganapathy, K.; Gump, B.B.; MacKenzie, J.A.; Mechref, Y.; Bendinskas, K. Immobilized metal affinity chromatography and human serum proteomics. J. Chromatogr. B 2013, 934, 26–33. [Google Scholar] [CrossRef]
- Aguilar, M.-I. Reversed-phase high-performance liquid chromatography. In HPLC of Peptides and Proteins; Springer: Berlin/Heidelberg, Germany, 2004; pp. 9–22. [Google Scholar]
- Kita, Y.; Miura, Y.; Furukawa, J.I.; Nakano, M.; Shinohara, Y.; Ohno, M.; Takimoto, A.; Nishimura, S.-I. Quantitative glycomics of human whole serum glycoproteins based on the standardized protocol for liberating N-glycans. Mol. Cell. Proteom. 2007, 6, 1437–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, J.I.; Tsuda, M.; Okada, K.; Kimura, T.; Piao, J.; Tanaka, S.; Shinohara, Y. Comprehensive glycomics of a multistep human brain tumor model reveals specific glycosylation patterns related to malignancy. PloS ONE 2015, 10, e0128300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gizaw, S.T.; Ohashi, T.; Tanaka, M.; Hinou, H.; Nishimura, S.-I. Glycoblotting method allows for rapid and efficient glycome profiling of human Alzheimer’s disease brain, serum and cerebrospinal fluid towards potential biomarker discovery. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1716–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Zhou, S.; Mechref, Y. LC-MS/MS analysis of permethylated free oligosaccharides and N-glycans derived from human, bovine, and goat milk samples. Electrophoresis 2016, 37, 1532–1548. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Mechref, Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis 2012, 33, 1768–1777. [Google Scholar] [CrossRef] [Green Version]
- Mechref, Y.; Kang, P.; Novotny, M.V. Solid-phase permethylation for glycomic analysis. Methods Mol. Biol. 2009, 534, 53–64. [Google Scholar]
- Dong, X.; Mondello, S.; Kobeissy, F.; Talih, F.; Ferri, R.; Mechref, Y. LC-MS/MS glycomics of idiopathic rapid eye movement sleep behavior disorder. Electrophoresis 2018, 39, 3096–3103. [Google Scholar] [CrossRef]
- Peng, W.; Mirzaei, P.; Zhu, R.; Zhou, S.; Mechref, Y. Comparative Membrane N-Glycomics of Different Breast Cancer Cell Lines to Understand Breast Cancer Brain Metastasis. J. Proteome Res. 2020, 19, 854–863. [Google Scholar] [CrossRef] [PubMed]
 , N-acetylglucosamine
(GlcNAc);
, N-acetylglucosamine
(GlcNAc);  , Galactose (Gal);
, Galactose (Gal);  , Fucose (Fuc);
, Fucose (Fuc);  , Mannose (Man);
, Mannose (Man);  , N-acetylneuraminic
acid (NeuAc/Sialic Acid).
, N-acetylneuraminic
acid (NeuAc/Sialic Acid).
 , N-acetylglucosamine
(GlcNAc);
, N-acetylglucosamine
(GlcNAc);  , Galactose (Gal);
, Galactose (Gal);  , Fucose (Fuc);
, Fucose (Fuc);  , Mannose (Man);
, Mannose (Man);  , N-acetylneuraminic
acid (NeuAc/Sialic Acid).
, N-acetylneuraminic
acid (NeuAc/Sialic Acid).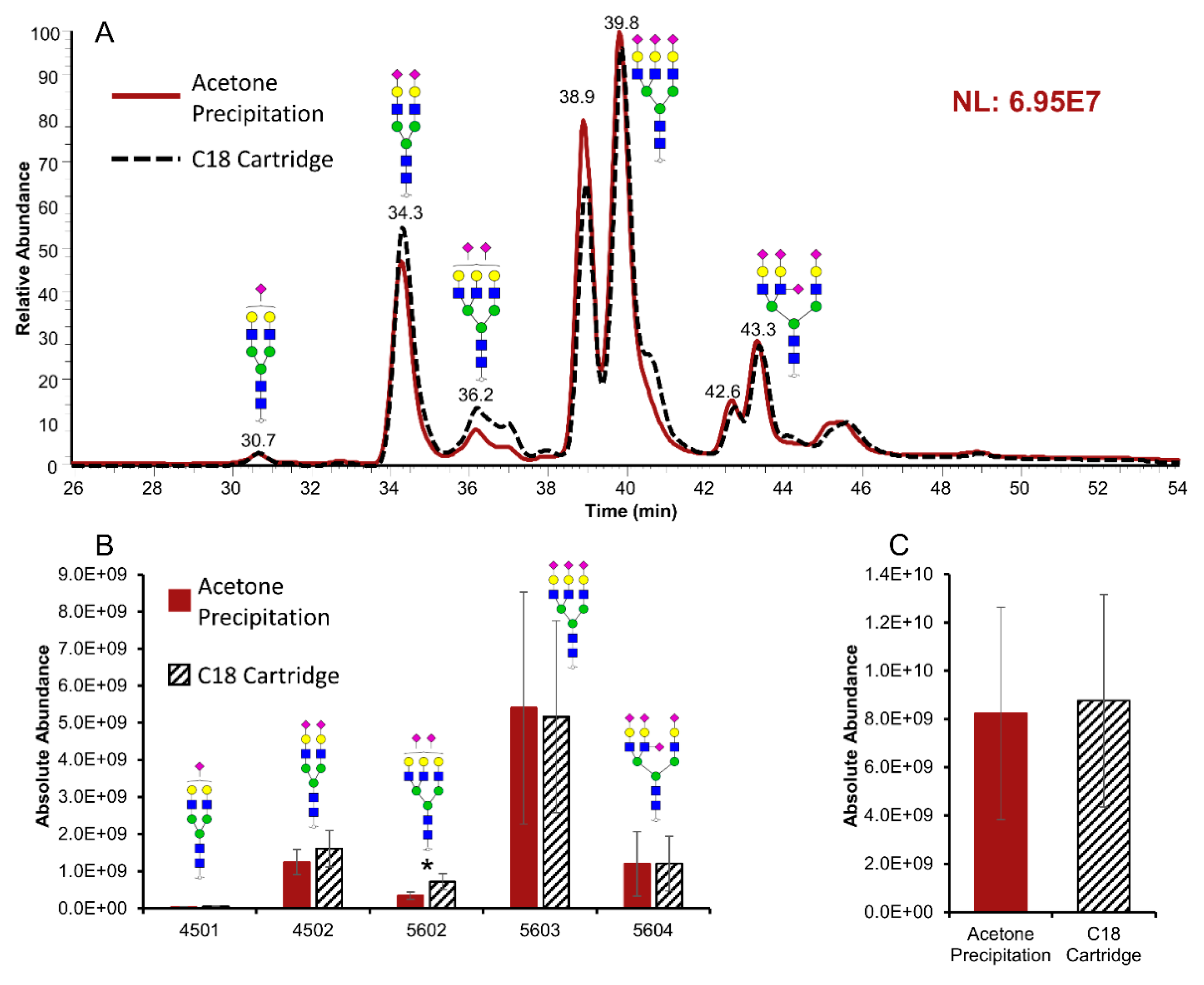


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Peng, W.; Fowowe, M.; Daramola, O.; Mechref, Y. An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation. Metabolites 2022, 12, 1285. https://doi.org/10.3390/metabo12121285
Wang J, Peng W, Fowowe M, Daramola O, Mechref Y. An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation. Metabolites. 2022; 12(12):1285. https://doi.org/10.3390/metabo12121285
Chicago/Turabian StyleWang, Junyao, Wenjing Peng, Mojibola Fowowe, Oluwatosin Daramola, and Yehia Mechref. 2022. "An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation" Metabolites 12, no. 12: 1285. https://doi.org/10.3390/metabo12121285
APA StyleWang, J., Peng, W., Fowowe, M., Daramola, O., & Mechref, Y. (2022). An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation. Metabolites, 12(12), 1285. https://doi.org/10.3390/metabo12121285






