Visualization of Glutamate Decarboxylase Activity in Barley Seeds under Salinity Stress Using Mass Microscope
Abstract
1. Introduction
2. Experimental Design
2.1. Chemicals and Reagents
2.2. Plant Growth Conditions
2.3. Measurement of GAD Activity Using LC/MS
2.4. Creating Sections of Barley Seeds
2.5. On-Tissue Enzyme Reaction
2.6. MALDI-MSI Analysis
2.7. Statistical Analysis
3. Results and Discussion
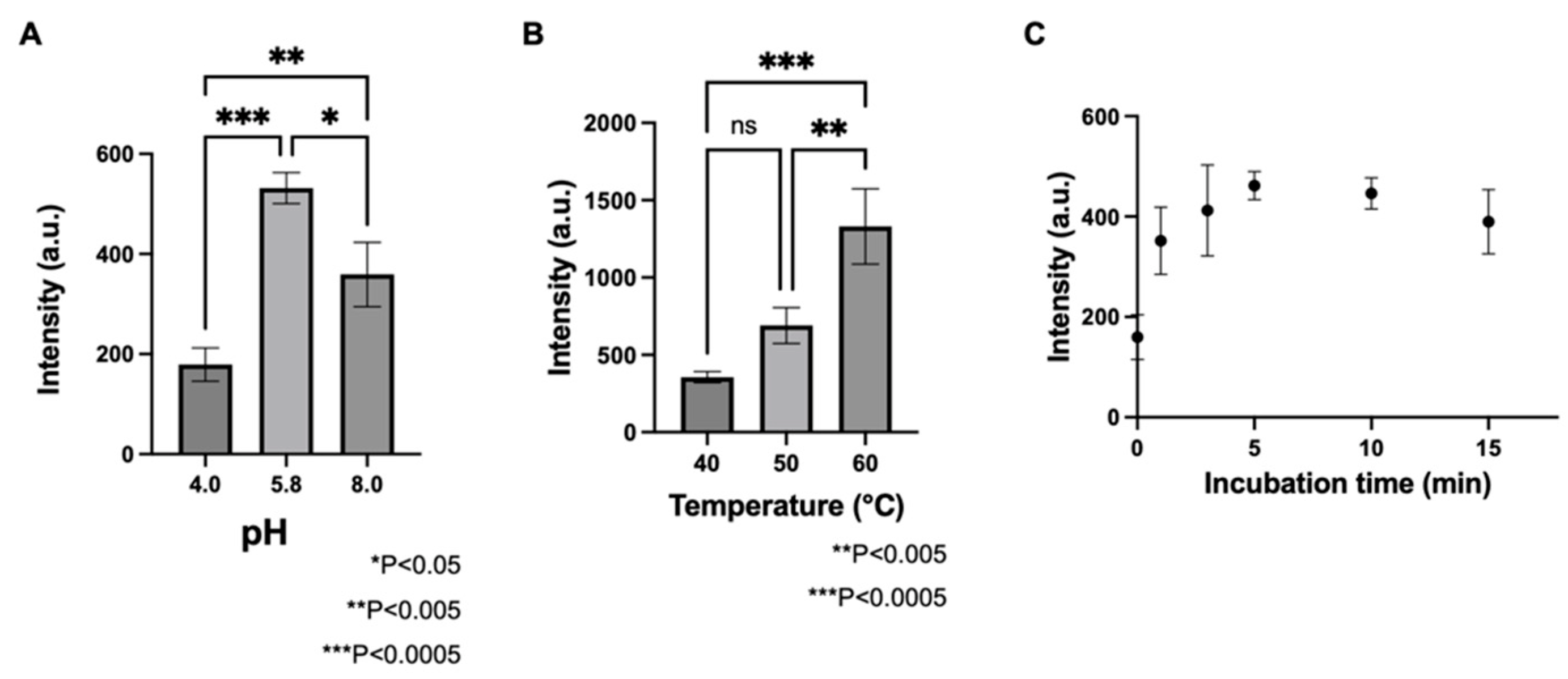
3.1. Determination of GAD Activity Using LC/MS
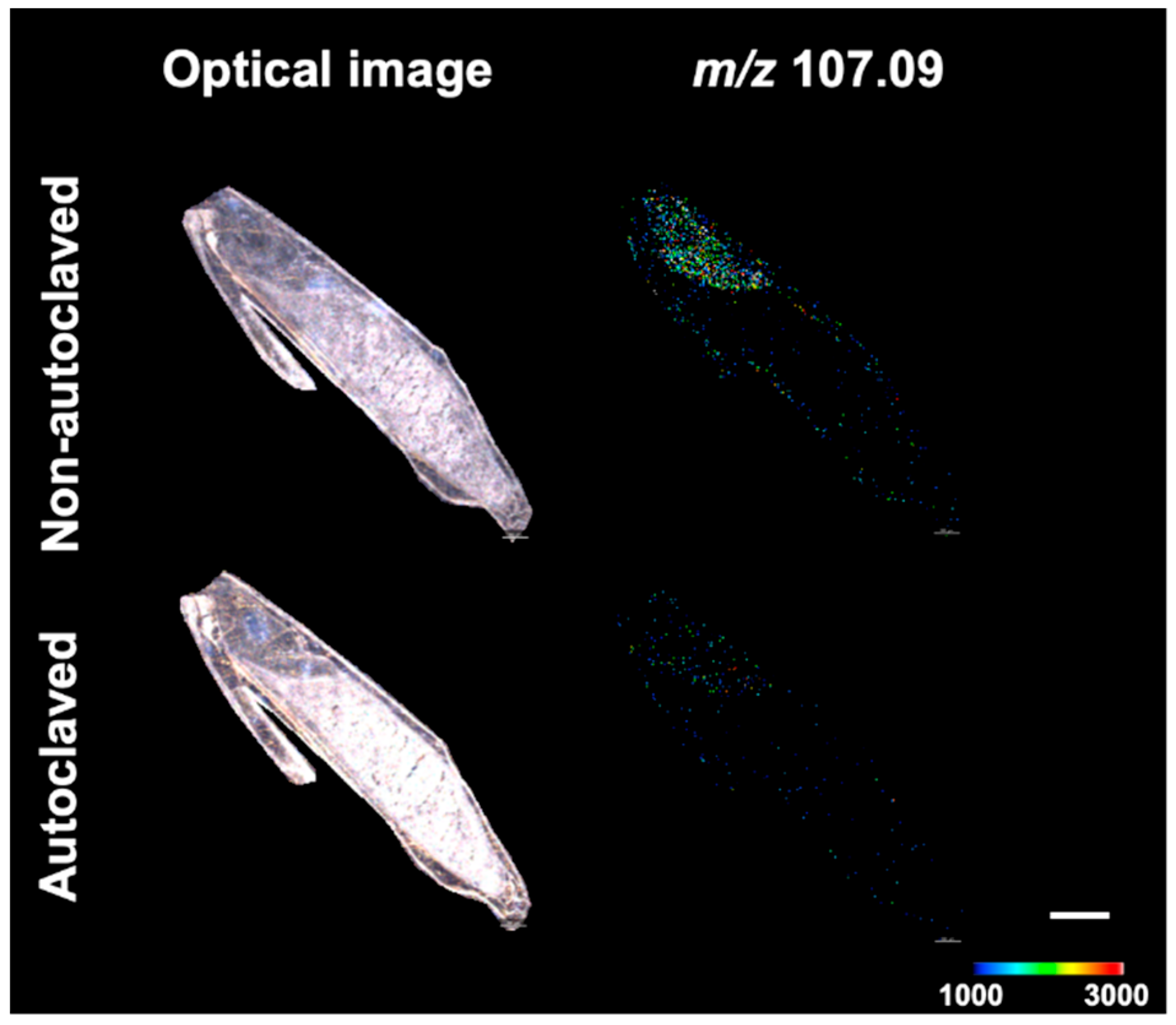
3.2. Construction of a Method for the Visualization of GAD Activity in Barley Seeds
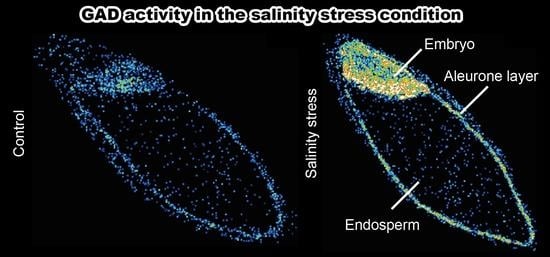
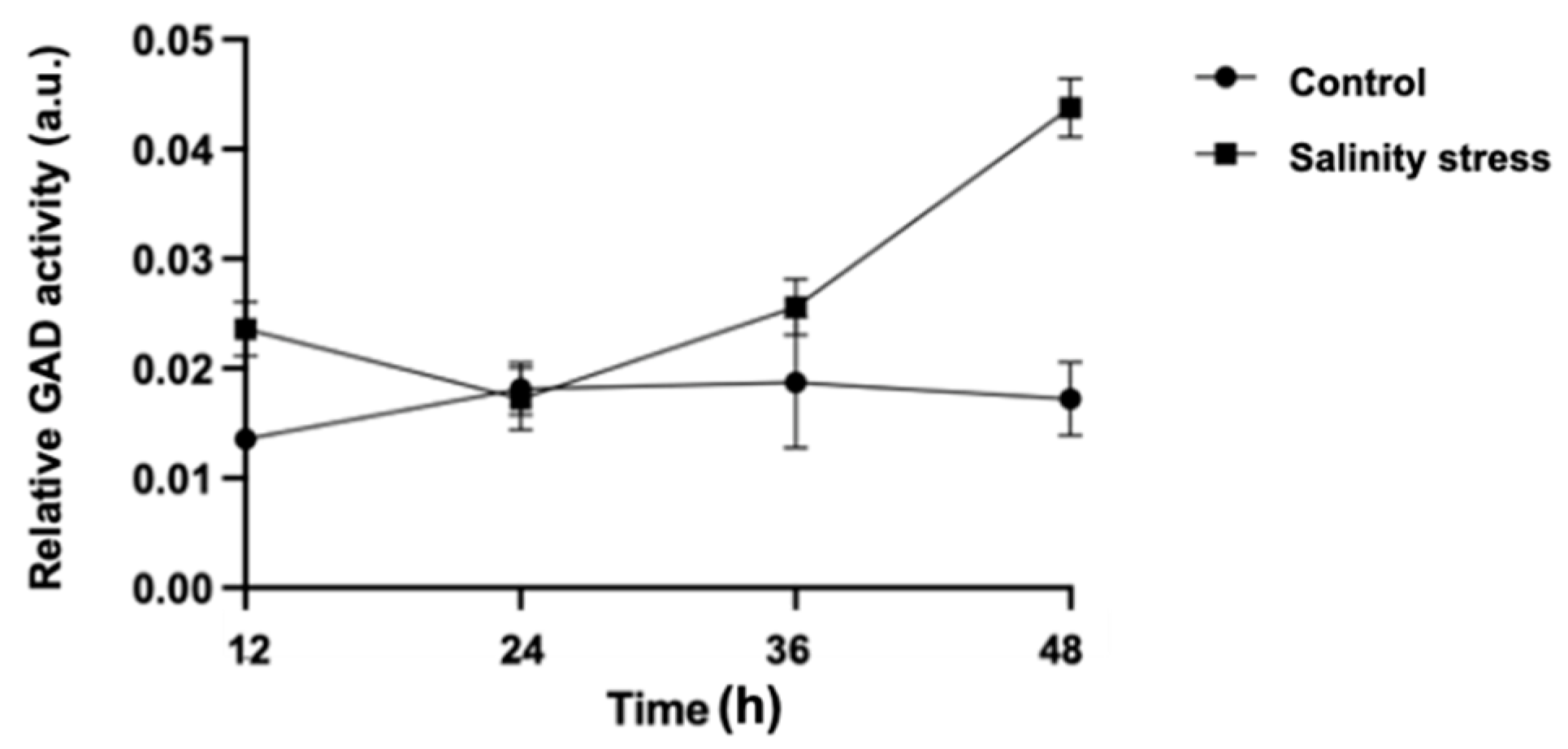
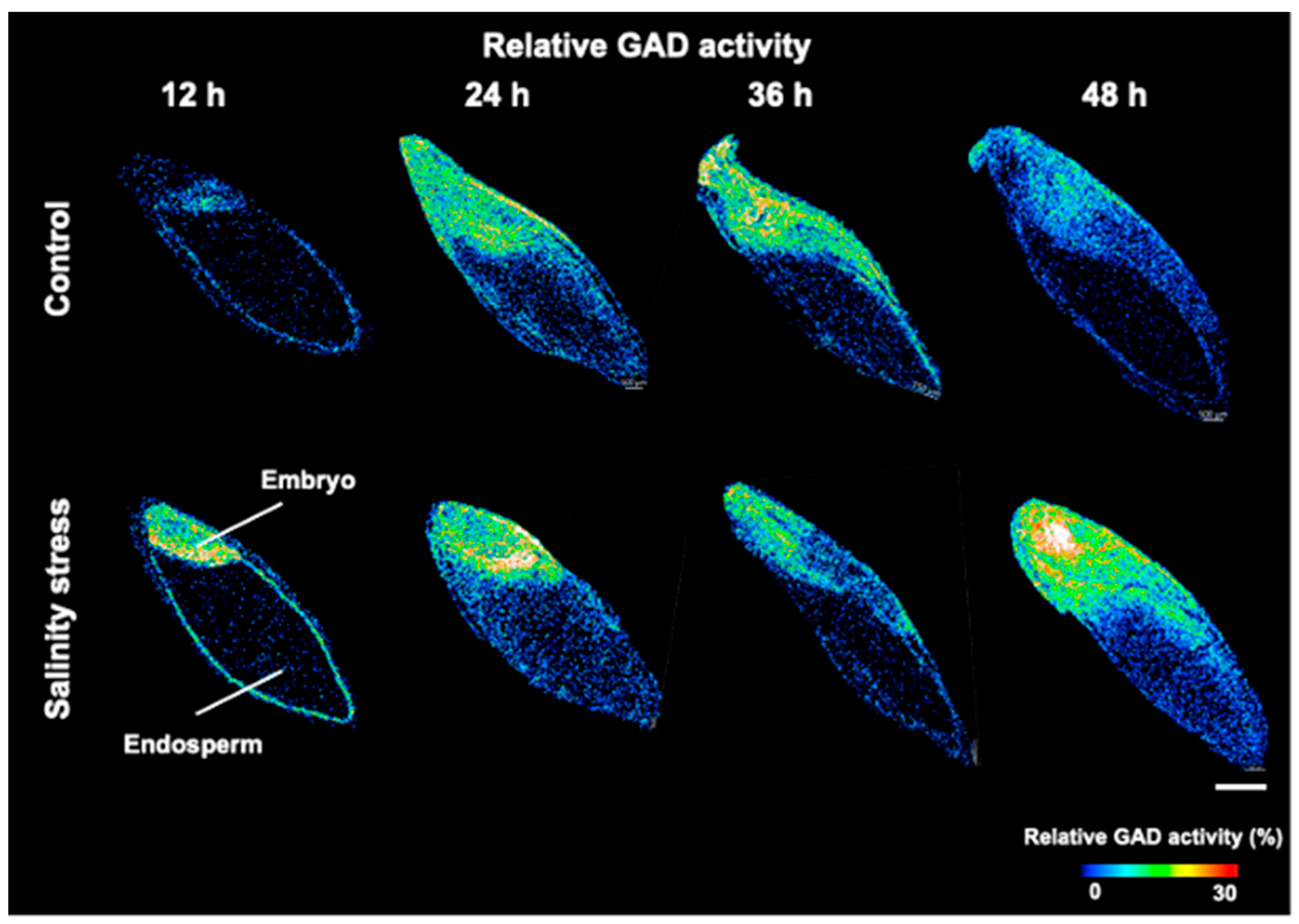
3.3. Visualization of GAD Activity during Germination under Salinity Stress
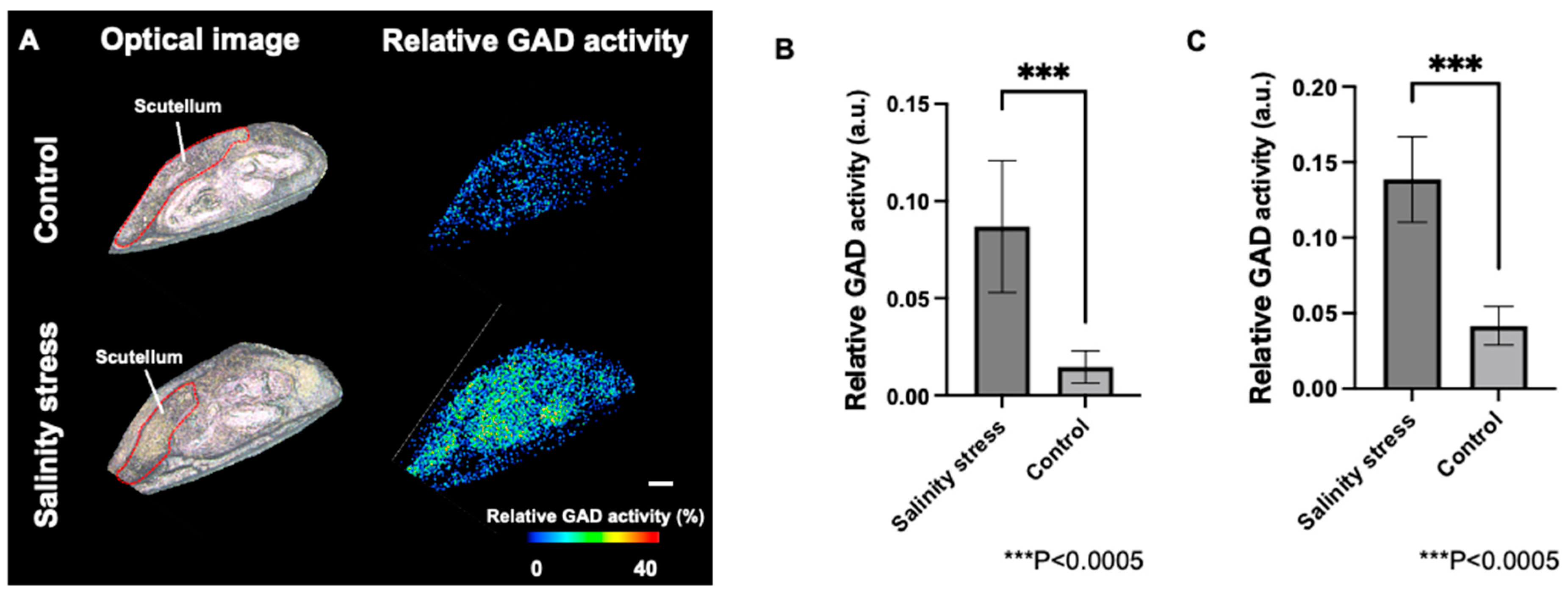
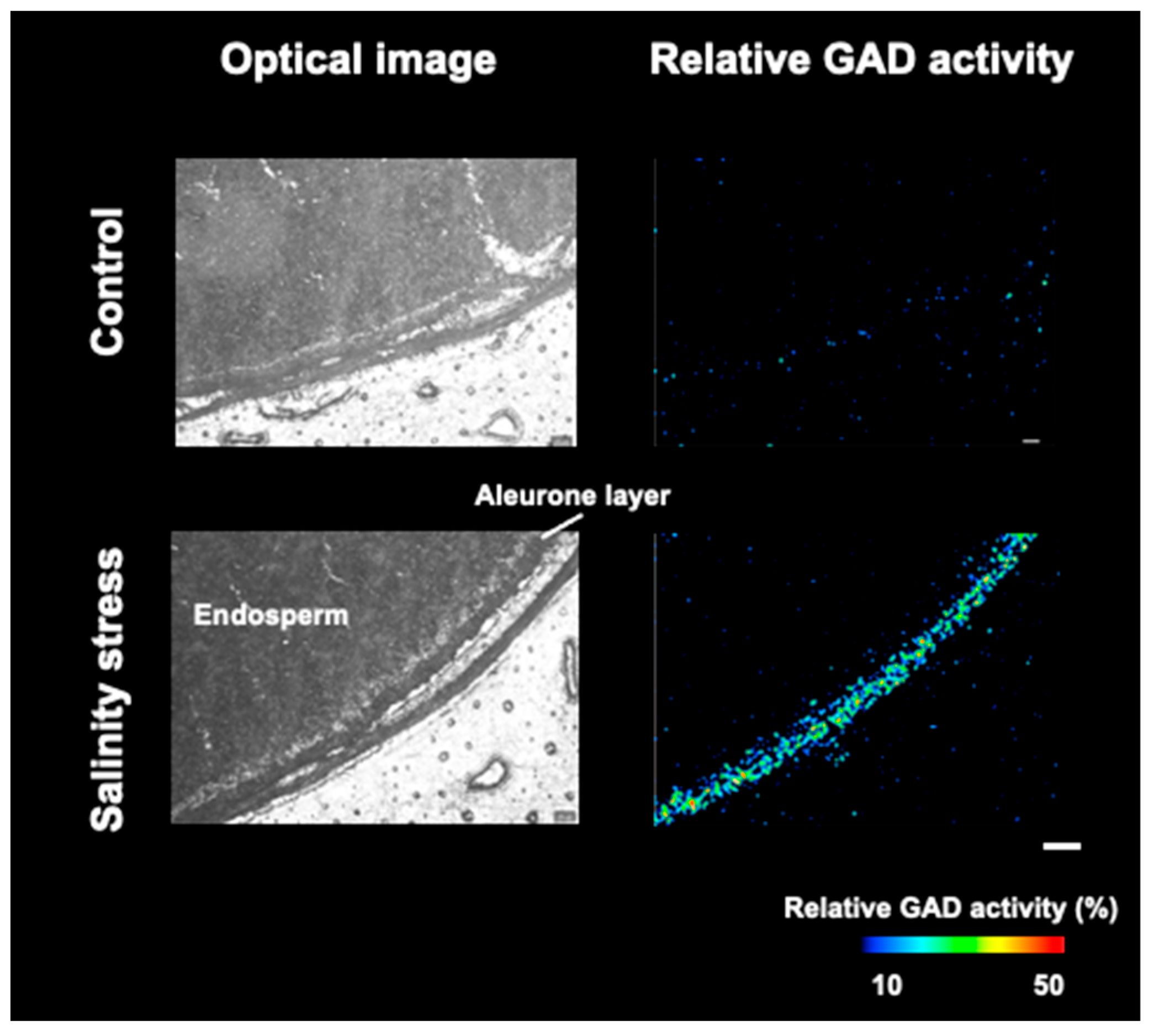
3.4. Visualization of Relative GAD Activity in the Embryo and Periphery of Seed Coat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuan, P.A.; Sun, M.; Nguyen, T.-N.; Park, S.; Ayele, B.T. Molecular Mechanisms of Seed Germination. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 1–24. [Google Scholar]
- Tester, M.; Davenport, R. Na+ Tolerance and Na+ Transport in Higher Plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yang, R.; Guo, Q.; Gu, Z. NaCl stress and supplemental CaCl2 regulating GABA metabolism pathways in germinating soybean. Eur. Food Res. Technol. 2014, 238, 781–788. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Chen, H.; Song, Y.; Gu, Z. Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 234, 679–687. [Google Scholar] [CrossRef]
- Yin, Y.; Cheng, C.; Fang, W. Effects of the inhibitor of glutamate decarboxylase on the development and GABA accumulation in germinating fava beans under hypoxia-NaCl stress. RSC Adv. 2018, 8, 20456–20461. [Google Scholar] [CrossRef]
- Yang, R.; Wang, S.; Yin, Y.; Gu, Z. Hypoxia treatment on germinating faba bean (Vicia faba L.) seeds enhances GABA-related protection against salt stress. Chil. J. Agric. Res. 2015, 75, 184–191. [Google Scholar] [CrossRef][Green Version]
- Dash, M.; Panda, S.K. Salt Stress Induced Changes in Growth and Enzyme Activities in Germinating Phaseolus Mungo Seeds. Biol. Plant. 2001, 44, 587–589. [Google Scholar] [CrossRef]
- Caprioli, R.M. Imaging Mass Spectrometry: A Perspective. J. Biomol. Tech. 2019, 30, 7–11. [Google Scholar] [CrossRef]
- Takeo, E.; Fukusaki, E.; Shimma, S. Mass Spectrometric Enzyme Histochemistry Method Developed for Visualizing In Situ Cholinesterase Activity in Mus musculus and Drosophila melanogaster. Anal. Chem. 2020, 92, 12379–12386. [Google Scholar] [CrossRef]
- Takeo, E.; Sugiura, Y.; Ohnishi, Y.; Kishima, H.; Fukusaki, E.; Shimma, S. Mass Spectrometric Enzyme Histochemistry for Choline Acetyltransferase Reveals De Novo Acetylcholine Synthesis in Rodent Brain and Spinal Cord. ACS Chem. Neurosci. 2021, 12, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Wisman, A.P.; Minami, M.; Tamada, Y.; Hirohata, S.; Gomi, K.; Fukusaki, E.; Shimma, S. Visualization of dipeptidyl peptidase B enzymatic reaction in rice koji using mass spectrometry imaging. J. Biosci. Bioeng. 2022, 134, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, S.; Shinohara, N.; Fukusaki, E.; Shimma, S. Mass spectrometry imaging enables visualization of the localization of glutamate decarboxylase activity in germinating legume seeds. J. Biosci. Bioeng. 2022, 134, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Dermendjiev, G.; Schnurer, M.; Weiszmann, J.; Wilfinger, S.; Ott, E.; Gebert, C.; Weckwerth, W.; Ibl, V. Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley. Int. J. Mol. Sci. 2021, 22, 9642. [Google Scholar] [CrossRef]
- Kihara, M.; Okada, Y.; Iimure, T.; Ito, K. Accumulation and degradation of two functional constituents, GABA and beta-glucan, and their varietal differences in germinated barley Hordeum vulgare grains. Breed Sci. 2007, 57, 85–89. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Al-Ajlouni, Z.I.; Obedat, D.I. The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci. Res. 2019, 29, 250–260. [Google Scholar] [CrossRef]
- Krasensky-Wrzaczek, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef]
- Lamkin, W.M.; Nelson, S.W.; Miller, B.S.; Pomeranz, Y. Glutamic Acid Decarboxylase Activity as a Measure of Percent Germination for Barley. Cereal Chem. 1983, 60, 166–171. [Google Scholar]
- Pramai, P.; Thanasukarn, P.; Thongsook, T.; Jannoey, P.; Chen, F.; Jiamyangyuen, S. Glutamate Decarboxylase (GAD) Extracted from Germinated Rice: Enzymatic Properties and Its Application in Soymilk. J. Nutr. Sci. Vitaminol. 2019, 65, S166–S170. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, R.; Han, Y.; Gu, Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous calcium on the germinating soybean response to salt stress. J. Proteom. 2015, 113, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Che-Othman, M.H.; Millar, A.H.; Taylor, N.L. Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C 3 plants. Plant Cell Environ. 2017, 40, 2875–2905. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Gupta, S.; Rupasinghe, T.; Callahan, D.; Natera, S.H.A.; Smith, P.; Hill, C.; Roessner, U.; Boughton, B.A. Spatio-Temporal Metabolite and Elemental Profiling of Salt Stressed Barley Seeds During Initial Stages of Germination by MALDI-MSI and µ-XRF Spectrometry. Front. Plant Sci. 2019, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B. Molecular and Cellular Biology Associated with Endosperm Mobilization in Germinating Cereal Grains. Annu. Rev. Plant Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Asakura, T.; Hirose, S.; Asatsuma, S.; Nanjo, Y.; Nakaizumi, T.; Itoh, K.; Hori, H.; Komatsu, S.; Mitsui, T. Proteomic Characterization of Tissue Expansion of Rice Scutellum Stimulated by Abscisic Acid. Biosci. Biotechnol. Biochem. 2007, 71, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, S.; Fukusaki, E.; Shimma, S. Visualization of Glutamate Decarboxylase Activity in Barley Seeds under Salinity Stress Using Mass Microscope. Metabolites 2022, 12, 1262. https://doi.org/10.3390/metabo12121262
Ikuta S, Fukusaki E, Shimma S. Visualization of Glutamate Decarboxylase Activity in Barley Seeds under Salinity Stress Using Mass Microscope. Metabolites. 2022; 12(12):1262. https://doi.org/10.3390/metabo12121262
Chicago/Turabian StyleIkuta, Soichiro, Eiichiro Fukusaki, and Shuichi Shimma. 2022. "Visualization of Glutamate Decarboxylase Activity in Barley Seeds under Salinity Stress Using Mass Microscope" Metabolites 12, no. 12: 1262. https://doi.org/10.3390/metabo12121262
APA StyleIkuta, S., Fukusaki, E., & Shimma, S. (2022). Visualization of Glutamate Decarboxylase Activity in Barley Seeds under Salinity Stress Using Mass Microscope. Metabolites, 12(12), 1262. https://doi.org/10.3390/metabo12121262