Abstract
In the face of climate change, progressive degradation of the environment, including agricultural land negatively affecting plant growth and development, endangers plant productivity. Seeking efficient and sustainable agricultural techniques to replace agricultural chemicals is one of the most important challenges nowadays. The use of plant growth-promoting microorganisms is among the most promising approaches; however, molecular mechanisms underneath plant–microbe interactions are still poorly understood. In this review, we summarized the knowledge on plant–microbe interactions, highlighting the role of microbial and plant proteins and metabolites in the formation of symbiotic relationships. This review covers rhizosphere and phyllosphere microbiomes, the role of root exudates in plant–microorganism interactions, the functioning of the plant’s immune system during the plant–microorganism interactions. We also emphasized the possible role of the stringent response and the evolutionarily conserved mechanism during the established interaction between plants and microorganisms. As a case study, we discussed fungi belonging to the genus Trichoderma. Our review aims to summarize the existing knowledge about plant–microorganism interactions and to highlight molecular pathways that need further investigation.
1. Introduction
Traditional agricultural techniques, such as chemical fertilizers, pesticides, fungicides, and herbicides, enable the protection of crop plants against pathogens and ensure better yield. Chemical compounds present in agricultural chemicals are harmful to the environment and cause soil, atmosphere, and water pollution [1]. These compounds are the reason for the extinction of fish [2], bees [3], and plants [4,5], and pose a threat to the biodiversity of soil bacterial [6,7,8] and fungal communities [9]. Chemical plant protection products negatively affect agricultural soils, i.e., they change soil physical properties, (e.g., texture, permeability, porosity), they disturb the cycle of the elements, such as phosphorus and nitrogen, and they decrease the complexity of soil microbiome [10]. In the face of a growing world population and increased demand for food both in terms of food quantity and food quality, the usage of bioinoculants, i.e., biofertilizers to increase the yield and biopesticides to protect plants, is the future of agriculture. Bioinoculants comprised of living or dormant microbes that are able to promote plant growth and development are called PGPM (plant growth-promoting microorganisms) and have great potential not only for enhancing plant yield but also for remediation of degraded soils [11,12,13]. Bioinoculants are cost-effective and environmental-friendly approaches in agriculture [14]. The first step in a bioinoculant formulation is the isolation and identification of a microbe. The further potential of a particular microorganism for plant growth promotion needs to be verified, and this ability should be confirmed in laboratory and field conditions. Moreover, potential risks to other organisms, such as animals and natural soil microbiomes, should be also determined [15]. The best-known examples of PGPM are mycorrhizal fungi and bacteria belonging to Rhizobium; however, plant growth-promoting microorganisms are found among varied taxa of bacteria, fungi, and algae [16]. In Table 1, several examples of PGPM are shown.

Table 1.
Examples of plant growth-promoting microorganisms. The effect of PGPM on plants and, if known, the mode of action of microorganisms is also included.
One of the major drawbacks of the application of bioinoculants is the fact that the number of bioinoculants tested in laboratory/greenhouse conditions fail in the field trials. This is mostly because microbes introduced to the environment have to compete for a niche with native microorganisms in order to attain sufficient abundance. The ability of introduced microbes to survive and thrive is variable and significantly depends on environmental conditions including temperature, rainfall, and soil type, as well as on interactions with the host plant and other organisms. A good example of this dependence is the field trial that showed that the promotion of plant growth by subtropical strains of Azotobacter chroococcum and Azospirillum brasilense was observed only when used in the same type of climate and not in the alpine region with a temperate climate [80]. Moreover, it was demonstrated that the high biodiversity of the native soil microbiome has a negative impact on the survivability of applied bioinoculants, i.e., there is a negative correlation between the diversity of the soil microbes and the survival rate of the introduced strain [81]. Therefore, when the native microbiome biodiversity is low, the chance for a new strain to thrive is higher which corresponds with better availability of nutrients and reduced competition among microorganisms for niche [82,83,84]. Moreover, several studies showed that PGPM that promotes the growth of a particular plant species might not be beneficial for other species of plants. For example, fungi belonging to Penicillium sp. and Trichoderma sp. showed diversified effectiveness in enhancing the growth of varied wheat (Triticum aestivum L.) cultivars [85]. Similarly, the effect of inoculation of winter and spring varieties of oilseed rape (Brassica napus L.) with different strains of PGPM on the germination rate and growth of seedlings depended on the plant variety [86]. On the other hand, different strains of the same microbial species might differ significantly in their ability to promote plant growth and development. For example, Znajewska et al. [59] showed that seven Trichoderma viride isolates had various effects on winter rapeseed germination and growth promotion depending on the used strain.
Microorganisms colonize not only roots but also other plant tissues and organs including stems, leaves, flowers, seeds, and fruits. The aerial part of plants colonized by microbes is called the phyllosphere, whereas the rhizosphere is the soil adjacent to the root [87]. In contrast to the rhizosphere, the above-ground parts of plants are scarce in water and nutrients. Only a small number of microorganisms that reach the surface of the plant will land on beneficial spots and will have conditions to survive [88]. As a consequence, the number of microorganisms living in the rhizosphere is much higher than in the phyllosphere. Microbes are present at every stage of plant development, from seed to fully developed plant producing a new generation of seeds [89]. Some microorganisms live on the surfaces of plant organs, i.e., epiphytes whereas others are able to colonize the internal tissues of plants, i.e., endophytes [90,91].
Plant growth-promoting microorganisms stimulate plant growth and development through various direct and indirect mechanisms (Figure 1, Table 1). Production of phytohormones [92,93], nitrogen assimilation [94], solubilization, and mineralization of macro- and micro-elements [95,96], and modulation of the endogenous level of ethylene in plants tissues [97] are examples of direct mechanisms. Examples of indirect mechanisms are inhibition of pathogens growth through antibiosis [98], secretion of lytic enzymes [99], and competition, e.g., via siderophores production [100], induction/inhibition of plant genes expression [101], induction of plant immune response [102], and manipulation of plant microbiome composition [103]. This work aims to summarize the current knowledge about the interactions of plants with beneficial microbes, and how those interactions affect the overall health of the plant. For further development of environmental-friendly methods of plant cultivation, is it crucial to deeply understand the molecular mechanisms underneath (i) the recruitment of useful microbes by plants, (ii) the interactions among microorganisms, and (iii) the plant-microorganism interplay. The interactions between plants and microorganisms can be divided into three types, i.e., interactions are either neutral, negative, or positive in their effects on the host plant. This review focuses exclusively on positive interactions and mechanisms underneath those interactions. In this work, we also discuss the role of stringent responses in interactions between plants and microorganisms.
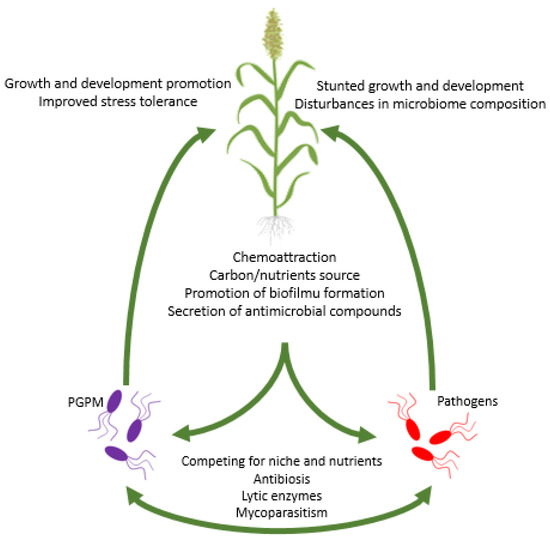
Figure 1.
Multidimensional network of interactions between plants and PGPM, between plants and pathogens, and between PGPM and pathogens.
2. Rhizosphere and Root Exudates
The rhizosphere is defined as “the field of action or influence of a root”, i.e., it is soil adjacent to the roots which are influenced by root exudates that are the mixture of several compounds produced and secreted by roots [104]. The main components of root exudates are water, enzymes, amino acids, nucleotides, vitamins, organic acids, fatty acids, sugars, phenolic compounds, anions, volatile organic compounds (VOCs), polysaccharides, and proteins [105,106,107,108,109,110,111,112]. The composition of exudates varies depending on the plant species, and even phenotype; it changes during plant development [107,113] and it is dependent on environmental conditions, such as temperature [114], light [115], the amount of nutrients [116,117,118,119], and stress factors [120,121,122]. Moreover, the type and composition of soil can also affect the composition of root exudates [123]. For instance, plants that grow in soil deprived of nitrogen probably do not secrete extra amino acids or proteins to the rhizosphere [119]. Root exudates are also an important source of elements present in the soil. It was estimated that about 10–44% of carbon compounds [124] and about 10–16% of nitrogen compounds [125] synthetized by the plant is secreted to the rhizosphere. In legumes, the rhizodeposition of nitrogen is estimated between 4% and 70%, depending on the plant species [126].
The rhizosphere is the hotspot of plant–microorganism interactions. Interactions between those organisms have a direct influence on the availability of soil nutrients for plants [95,127,128,129,130] and on plant tolerance toward biotic and abiotic stresses [131,132,133,134,135]. Exudates are a rich source of carbon and other nutrients and, therefore, the abundance of microorganisms in the rhizosphere can be up to a hundred times greater than in the bulk soil [136]. Moreover, root exudates allow plants to communicate with rhizosphere microorganisms and affect their behavior through the secretion of various signaling molecules [137,138,139,140]. For instance, barley (Hordeum vulgare L.) in response to infection by the fungus Pythium ultimum, secretes increased amounts of organic and phenolic acids, which activates the expression of the phlA gene in the endophytic bacterium Pseudomonas fluorescens CHA0. Hydroxymethylglutaryl-CoA synthase encoded by the phlA gene is involved in the synthesis of DAPG (1,4-diacetylphloroglucinol) that has antifungal activity [98]. Zhang et al. [141] demonstrated the role of organic acids in root exudates of cucumber (Cucumis sativus L.) and banana (Musa acuminata Colla). The citric acid present in cucumber root exudates attracted Bacillus amyloliquefaciens (isolated from cucumber rhizosphere) and B. subtilis (isolated from banana rhizosphere). Moreover, it induced the formation of the B. amyloliquefaciens biofilm. Fumaric acid present in banana root exudates promoted biofilm formation of both tested strains. Biofilm is a coherent multicellular structure embedded in a self-produced extracellular matrix that can be formed on the surface of plant organs. Members of biofilm are better protected from environmental factors; their nutritional status is enhanced and, therefore, the survival rate of members of biofilm is much higher than that of single bacteria cells, as reviewed in [142]. Another example of a molecule allowing plant–PGPM communication is polyamines present in root exudates, which inform rhizospheric microorganisms about the presence of a potential host plant [143]. For instance, putrescine and its precursor arginine attract Pseudomonas sp. and trigger a lifestyle change, promoting attachment to the root and formation of biofilm [144]. However, the best-known example is the mechanism of communication between Rhizobium and legumes. Flavonoids secreted by plants activate bacterial nod genes lead to secretion by bacteria of the nod factor. The nod factor promotes the formation of nitrogen-fixing nodules in roots [145,146].
Root exudates serve as a chemoattractant, by which plants “recruit” microorganisms (Figure 1). Several studies showed that the composition of root exudates has an enormous effect on the composition of plant microbiome [147,148,149,150,151,152]. The composition of root exudates is specific for each plant species, which enables plants to attract a particular set of microbes [153]. Moreover, plants can secrete substrates that are available only for selected microbial groups or compounds that are toxic for certain groups of microorganisms in order to inhibit their growth [154]. A prominent example is the amino acid canavanine, which is present in seeds and root exudates of legumes. Canavanine is toxic to a number of soil bacteria excluding rhizobia which possess the msiA gene encoding canavanine exporter that warrants canavanine resistance [155]. Mardani-Korrani et al. [156] demonstrated that canavanine is secreted by Vicia villosa Roth, significantly decreased the diversity and changed the composition of microbial communities in soil. The presence of canavanine caused an increase in the abundance of Firmicutes and Actinobacteria and decreased the number of Proteobacteria and Acidobacteria. Another example of the selective action of compounds present in root exudates is coumarin secreted by Arabidopsis thaliana (L.) Heynh. Coumarin selectively inhibits the proliferation of some pathogenic fungi, stimulates the growth of some Pseudomonas spp. and other microbes that belong to the PGPM group, and increases the bioavailability of iron by ferric ions reduction [157]. Coumarins are used by plants to increase the bioavailability of iron. Coumarins, such as scopoletin and esculetin, enable the mobilization of Fe from minerals in acidic and alkalic soil [158]. Moreover, the depletion of Fe in soils enhances the biosynthesis and secretion of coumarins in A. thaliana [157]. It is also worth noting that fungi also produce and secrete exudates that might affect other rhizosphere microorganisms. An interesting observation was made by Toljander et al. [159], who showed that arbuscular mycorrhizal fungi (AMF) affect the composition of bacterial community through mycelia exudates. Analysis of exudates produced by Glomus sp. showed that the main components are water, low molecular weight sugars, and organic acids. Moreover, it was shown that Glomus exudates inhibited the growth of several bacterial strains including opportunistic pathogens Flavobacterium spp. and increased the abundance of bacteria belonging to Gammaproteobacteria.
Root exudates are usually secreted without energy, mostly through diffusion, ionic channel, and vesicle transport. Among several transporters involved in the secretion of exudates by roots are ABC (ATP-binding cassette) transporters which transport lipids and flavonoids [160], and anion channels are involved in secreting carbohydrates [161]. Moreover, for the secretion of root exudates transmembrane proteins aquaporins (AQPs) that are related to the membrane reflection coefficient and root hydraulic conductivity seem to be of great importance, as reviewed in [162]. Aquaporins are present in endogenous and exogenous membranes of eukaryotes and prokaryotes and are responsible for symplastic transport of not only water but also low molecular weight compounds and non-charged molecules including urea, glycerol, hydrogen peroxide, ammonium ions, and some elements, e.g., silicon and boron, as reviewed in [163]. Interestingly, it was demonstrated that during ectomycorrhiza formation the expression of fungi Laccaria bicolor aquaporins significantly increased. Moreover, fungal aquaporins exhibit high permeability for NH3 and, therefore, it was suggested that they are involved in the transfer of this compound from fungal cytoplasm to plant [164]. Glycine max L. noduline 26 aquaglyceroporin (GmNod26) is located in the symbiosome membrane in N2-fixing nodules and is a transporter of ammonia. The C-terminal domain of GmNod26 interacts with the main ammonia assimilatory enzyme, i.e., glutamine synthetase, and this probably supports the effective assimilation of fixed nitrogen [165,166]. The potential of root exudates to determine the composition of the rhizosphere microbiome is also important for the biodegradation of various soil pollutants. Environmental pollution is a serious global challenge and needs urgent development of effective methods of remediation. An interesting observation was presented by Janczak et al. [167,168] who showed that the presence of plants had a positive effect on bacterial, (i.e., Arthrobacter sulfonivorans, and Serratia plymuthica), and fungal, (i.e., Clitocybe sp. and Laccaria laccata), ability to degrade polymers including polylactide (PLA) and poly(ethylene terephthalate) (PET). The effectiveness of biodegradation also significantly depended on the species, i.e., the presence of Salix viminalis L. (willow) enhanced the level of biodegradation more significantly that the presence of B. napus and Miscanthus x giganteus J.M.Greef, Deuter ex Hodk., Renvoize (giant miscanthus). It was suggested that root exudates probably support the growth of microorganisms and/or root exudates can activate microbial genes involved in the biodegradation of plastics, such as intra- and extra-cellular depolymerases. The breakdown of long polymers into oligo-, di-, and mono-mers enables uptake of those molecules by a bacterial cell which can be then utilized as a carbon and/or energy source [169]. Afzal et al. [170] reported that inoculation of Italian ryegrass (Lolium multiflorum Lam.) and birdsfoot trefoil (Lotus corniculatus L.) with alkane-degrading bacteria Pantoea ssp. and Pseudomonas sp. separately or in consortium resulted in higher biomass production by plants and bacterial consortium showed higher degradation ratio in comparison to single strain inoculants. The ability of these bacteria to degrade alkane was linked to the presence of genes encoding cytochrome p450 alkane hydroxylase (CYP153) and alkane monooxygenase (alkB). Enterobacter ludwigii possessing the CYP153 gene was able to degrade diesel fuel [171]. On the other hand, it was shown that the level of degradation of polycyclic aromatic hydrocarbon was reduced in the presence of ryegrass (Lolium perenne L.) root exudates [172] which suggest that plants might differ in their potential to enhance bioremediation potential of soil microorganisms. The PGPM are able to degrade various other compounds of different origins as a means for the promotion of plant growth and development. Allelochemical compounds secreted by plants can hinder the cultivation of other plant species in cropping systems. Trichoderma harzianum SQR-T037 was shown to degrade allelochemicals secreted by cucumber roots (4-hydroxybenzoic acid, vanillic acid, ferulic acid, benzoic acid, 3-phenylpropionic acid, and cinnamic acid). The use of strains able to biodegrade allelochemicals can ameliorate allelopathic stress in continuous cropping systems [173]. Among common soil contaminants, plant protection agents are of special interest since their persistence in the agricultural soil is high, and their concentration may increase with each application. Diuron, a phenylurea herbicide, has a mean half-life of 330 days. Inoculation of soil containing diuron with fungal endophyte Neurospora intermedia leads to degradation of 99% of diuron in the soil after 3 days. Moreover, the authors reported that this strain is able to degrade other phenylurea herbicides, e.g., fenuron, monuron, isoproturon, chlorbromuron, and chlortoluron [174]. Although organophosphorus pesticides are perceived as non-persistent, they are highly toxic for a wide variety of non-target organisms, including mammals and rhizospheric microbes. Tested strains of T. harzianum and Metarhizium anisopliae showed an ability to degrade a number of organophosphorus pesticides such as diazinon, profenofos, and malathion in a temperature range of 20–45 °C [175].
3. Microbiome and Holobiont
Plants provide a multitude of ecological niches for various organisms to thrive. All these organisms including bacteria, fungi, protists, and nematodes that live on the surface and inside tissues/organs of a certain plant form the plant microbiome [176]. Although each member of the microbiome might contain genes related to the promotion of plant growth and development, the expression of those genes is dependent on the composition of the whole microbiome, on the population dynamics of potential pathogens, and on environmental conditions [177]. For example, the consortium of six bacteria (Arthrobacter nitroguajacolicus, Bacillus cereus, Bacillus megaterium, Bacillus mojavensis, Pseudomonas azotoformans, and Pseudomonas frederiksbergensis) was much more effective in the protection of Nicotiana attenuata Torr. ex S.Watson (coyote tobacco) against fungal pathogens Fusarium sp. and Alternaria sp. than individual members of the consortium [178]. Analysis of P. fluorescens transcriptome in response to the presence of bacteria belonging to three different genera revealed significant differences in the transcription response of P. fluorescens to different competitors [179]. Moreover, another layer of microbiome complexity is added by the presence of microbial symbionts of the members of the plant microbiome. For example, plant-associated fungi are in symbiosis with bacteria, i.e., endofungal/endohyphal bacteria [180]. Interestingly, it was shown that endohyphal bacteria Luteibacter sp. significantly increases the production of indole-3-acetic acid (IAA) in fungi Pestalotiopsis sp. However, Luteibacter sp. is not able to synthesize IAA [181]. Viruses can also interact with the plant microbiome. Dichanthelium lanuginosum (Elliott) Gould (panic grass) grows in geothermal areas in consortium with its endophytic fungus Curvularia protuberata. When the fungus is infected with Curvularia thermal tolerance virus (CThTV), soil temperature tolerance of panic grass increases from 40 °C to 65 °C [182,183]. Those results clearly show that other microorganisms present in soil strongly affect the PGPM potential to promote plant growth and development.
Although plants recruit a number of diverse microorganisms, they show preferences toward specific bacteria species. Analysis of seeds of medicinal plant red sage (Salvia miltiorrhiza Bunge) collected at different locations revealed that there are no significant differences in the composition of the microbiome, whereas different plant species collected at the same location had significantly different microbiomes. This indicates that different species of plants exhibit a preference for specific groups of microorganisms [184]. Redford et al. [185] demonstrated the structure of microbial communities present on Pinus ponderosa Dougl. Ex C. Lawson needles are very similar regardless of geographic location. However, there are also examples of substantial differences in the microbiome of different cultivars of one plant species described in the literature. For example, Germida et al. [186] showed that root microbiomes of modern and older wheat cultivars were significantly different. Moreover, some microorganisms interact only with single plant species. For instance, a number of ectomycorrhizal fungi form mycorrhiza only with one species of a tree, e.g., Suillus grevillei and larch (Larix sp.) (as reviewed in [187]). Species of Pinaceae are the only hosts for the fungal genus Rhizopogon [188]. The most known example is the highly specialized relationship between legumes and rhizobia [189]. Wicaksono et al. [190], by studying bog ecosystems, found that microbiomes of vascular plants are less diversified than those of non-vascular plants including bryophytes. Specificity between host and microbes seems to be a plastic trait modulated by the environment [191]. The mechanisms underneath the preferences of the host plant toward a specific set of microorganisms are not well understood, but recently Salas-González et al. [192] showed that mechanisms involved in the maintenance of plant mineral nutrient homeostasis also contribute to microbiome assembly.
The composition of the plant microbiome is largely dependent on the phase of plant growth and development. The mature seed is colonized by microorganisms that were associated with the mother plant, (i.e., microbes were transferred vertically), and those microorganisms are the first members of the microbiome of a newly emerging plant [193]. During germination, microbes transferred vertically have an advantage in plant colonization over the microorganisms present in the soil. During plant development, new symbiotic microorganisms transferred horizontally, (i.e., from the soil), appear [136,194]. Interestingly, it was demonstrated that microbes transferred vertically usually inhabit the phyllosphere, while the rhizosphere and root are colonized by the microorganisms from the soil. A study on oak (Quercus robur L.) microbial inheritance showed that microbial composition of the phyllosphere was very similar to the composition of the embryo [195]. Sánchez-López et al. [196] showed that seed endophyte Methylobacterium sp. Cp3 was transferred via seeds across three generations of the plant Crotalaria pumila Ortega. Moreover, when Methylobacterium sp. Cp3 was inoculated to the soil at the time of C. pumila, flowering migration of bacteria from soil to seeds was observed. Moreover, the microbiome strongly differs among plant organs and tissues, i.e., the surface of the leaf is colonized by different microorganisms in comparison to the rhizosphere microbes [177]. A study on native and cultivated Agave species showed differences in bacterial taxa colonizing the rhizosphere, the phyllosphere, and the leaf and root endosphere. Interestingly, a composition of the fungal microbiome was affected mainly by the host plant biogeography [197]. Zarraonaindia et al. [198] demonstrated that below- and above-ground microbial communities of grapevine (Vitis vinifera L.) were significantly different. Moreover, the composition of microbiomes of leaves, grapes, and flowers was more similar to the composition of soil microbiomes than to each other. Similarly, in sugarcane (Saccharum sp.), clear differences in microbial taxa between organ types were observed. Interestingly, the microbiome composition of the young shoots formed from the underground ratoon was very similar to the microbiome of roots [199]. The phyllosphere is a hostile and dynamic environment. Microbes present on plant above-ground surfaces are subjected to irregular nutrient availability and changeable environmental conditions. The phyllosphere microbiome seems to be even more dependent on environmental conditions than the root microbiome [88,185]. It should be pointed out that the data regarding phyllosphere microbiomes other than leaf microbiomes is still rather limited. A study on the microbiome of apple (Malus domestica Borkh.) flowers showed that the most popular are bacteria belonging to the extremophilic phylum Deinococcus-Thermus. Moreover, the composition of the flower microbiome was dependent on the phase of apple flower development [200].
The composition of the plant microbiome is also strongly influenced by various environmental factors, such as climate, soil properties [201,202,203], water [204], and nutrient availability [205,206]. The adaptation of plant metabolism to environmental change is strongly supported by rapidly changing microbial communities [204]. Analysis of the lettuce (Lactuca sativa L.) microbiome showed that the composition of the phyllosphere microbiome is strongly dependent on the time of the year [207]. Drought is one of the most important stress factors significantly affecting not only plant growth and the yield of crops [208], but also the plant microbiome. Drought affects the microbiome directly because a low level of soil moisture inhibits the growth of several microorganisms. In drought conditions, plants recruit stress microbiomes, i.e., the most beneficial group of microbes allowing the plant to adapt to a particular set of environmental conditions. For instance, the abundance of Actinobacteria increased in drought-treated roots and the rhizosphere of 18 species belonging to Poaceae. The results suggest that although the microbiome is species-specific, drought caused a relatively conserved response in different hosts [209]. In drought stress conditions, an increase in the abundance of the rhizospheric drought stress-resistant bacteria in Oryza sativa L. was observed, mainly members of Actinobacteria and Chloroflexi, whereas the abundance of Acidobacteria and Deltaproteobacteria decreased [210]. Nelson et al. [211] reported enormous changes in microbial composition in forest soils after wildfires which occur more often due to climate change. As expected, an overall decrease in the abundance and biodiversity of bacterial and fungal communities was observed one year after the fire. Both the carbon and nitrogen cycle were found to be impaired not only by the loss of microbial taxa involved in geochemical cycles, (e.g., no expression of nifA genes in tested soils was detected), but also by the activity of viruses. The presence of plant pathogens has also a substantial effect on the microbiome (Figure 1). In Gossypium hirsutum L. (cotton) infected with the pathogenic fungus Verticillium, the abundance of arbuscular mycorrhiza fungi and plant growth-promoting bacteria (PGPB) was lowered [212]. A study on strawberries (Fragaria x ananassa Duchesne) infected with Verticillium dahliae and Macrophomina phaseolina showed that plants without symptoms of infection had a higher abundance of PGPB than plants with visible symptoms of infection. The microbiome of healthy plants includes more bacteria antagonistic or competitive towards pathogens [213]. Moreover, herbivores can shape the plant microbiome. Kong et al. [214] showed that whitefly infestation of Capsicum annuum L. (pepper) changed the overall microbiome composition. Pseudomonas spp. that are recruited by the plant to the rhizosphere microbiome increased the mortality of whitefly.
A particular part of the microbiome is the core microbiome which consists of those species of microbes that regularly and ubiquitously appear in the microbiomes of particular plant species. The concept of the core microbiome was first coined by a scientist involved in the Human Microbiome Project, with the goal to identify microbial taxa and/or genes that are shared by all or most humans [215]. Microbial taxa that are commonly found in a number of environments or host types can be assigned to a core microbiome. The most common approach to verifying whether a species belong to the core microbiome is to determine microbial groups that are shared among two or more microbiomes of a particular host in various environments [216]. For example, analysis of the B. napus rhizosphere microbiome grown in different conditions, i.e., the level of fertilization and the level of plant density, revealed that the core root microbiome of this plant is composed of microbes belonging to genera Streptomyces, Cryocola, Arthrobacter, Flavobacterium, Janthinobacterium, Serratia, Kaistobacter, Pseudomonas, Pedobacter, Agrobacterium, Burkholderia, Acidovorax, Erwinia, and Stentrophomonas [217]. Analysis of the composition of the microbiome of various plant species including A. thaliana, rice, sugarcane, grapevine, barley, and soybean has revealed that the core microbiome included microbes belonging to Pseudomonas, Agrobacterium, Methylobacterium, Sphingomonas, Erwinia, Cladosporium, Conithyrium, Resinicium, and Fusarium [177]. A special part of the core microbiome is a group of microorganisms called “hub microorganisms”. Those microorganisms strongly shape the composition of the microbiome through biotic interactions with host plants and other microbes. Microorganisms belonging to the hub species are called keystone species since they serve as mediators between the plant and members of the plant microbiome. Through the hub species, the host plant can selectively affect the composition of the associated microbiome. Removal of keystone species can result in the loss of interaction and a disturbance in the whole microbiome [218]. The analysis of the phyllosphere microbiome of A. thaliana showed that plant-parasitic oomycetes Albugo laibachii is a hub species that strongly affects the whole microbial community. As a consequence of infection with A. laibachii, high divergence between the composition of the microbiome of control and of infected plants was observed. Moreover, less variability among microbiomes of infected plants was shown [219]. In a study on corn (Zea mays L.) hub species, the elimination of Enterobacter cloaceae from inoculum containing seven microbes resulted in a loss of a few other microbes from the microbiome. Removal of other bacteria from this system did not significantly change the microbial community which suggests that E. cloaceae functions as a keystone species [220].
A far wider concept than microbiome is the concept of holobiont which was introduced in 1991 [221]. Currently, a holobiont is defined as an organism composed of the plant host and of all the microorganisms that are associated with that particular plant. Natural selection between the plant and microbes supports the system and its stability throughout the evolution of a holobiont. In a holobiont, intricate networks of interactions between microorganisms and plant host are observed (as reviewed in [177,218,222,223]). All the genes present in the holobiont, i.e., plant genes and genes in the microbiome, constitute the hologenome [224,225]. The concept of a hologenome suggests that plants’ adaptability to the environment is determined not only by plant genes but also by genes of microorganisms. Hologenomes are responsible for shaping the phenotype of holobionts in response to a particular set of environmental conditions [226].
4. Plant Immune System in Plant–PGPM Interactions
The plant immune system plays a key role in plant–microorganism interactions. It is crucial not only for controlling pathogenic microorganisms but also for balancing the homeostasis of the microbiome and for overseeing commensal microbes [227]. The prominent role in plant–PGPM interactions play patterns recognizing receptors (PRRs), which recognize conserved microorganisms-specific molecules referred to as pathogen-/microbe-associated molecular patterns (P/MAMPs), such as flagellin, lipopolysaccharides, antibiotics, and VOCs [228]. PRRs are transmembrane multimeric protein complexes located at the plasma membranes present in all plant organs and tissues. Plant PRRs are either surface-localized receptor kinases that contain ligand-binding ectodomain and intracellular kinase domain or receptor-like proteins that do not have any intracellular signaling domain. PRRs contain various ligand-binding ectodomains that allow for the recognition of a wide range of P/MAMPs [228] and activate pattern-triggered immunity (PTI) [229]. Activation of PTI via MAMPs inhibits intensive proliferation of most microorganisms via synthesis and secretion of low-molecular-weight compounds, e.g., phytoanticipins and proteins, e.g., defensins. Moreover, plants synthesize cuticles which lead to the thickening of the cell wall [227]. Some pathogens secrete effector molecules, which disturb PTI functioning and thus allow for the infection of the plant [222]. Effector-triggered immunity (ETI) is activated by recognition of pathogen effector proteins via intracellular receptors R proteins encoded by resistance genes (R genes) [230,231] and via nucleotide-binding leucine-rich repeat receptors (NLR) located in the cytoplasm [229]. ETI leads to the overproduction of reactive oxygen species (ROS) and ion fluxes. As a consequence, hypersensitive response (HR) is activated which leads to apoptosis of infected plant cells that restrict the spread of infection [232].
Much less is known about the action of the plant immune system in the context of commensal microorganisms; however, there is some evidence that the plant immune system is crucial for microbiome assembly. Some strains of bacteria are able to modulate plant receptors, transcription factors, and molecules involved in the functioning of an immune system which allows for the colonization of plant tissues by other symbiotic microbes [233]. Moreover, some mechanisms used by the members of the microbiome to evade or suppress the plant immune system were also described. For example, in some symbiotic microorganisms, MAMP variants that do not activate plant immune response via PRRs have evolved. In addition, some commensal fungi are able to convert chitin into chitosan via deacetylation which induces a weaker immune response. MAMPs could be also degraded or sequestered by microbial proteases and other enzymes in order to evade recognition by PRR, as reviewed in [222]. It was also suggested that plants are able to actively ignore the presence of microbial commensals [234]. In A. thaliana, outer layers of roots low expression of PRRs and a lack of immune response in presence of pathogen- and commensal-derived MAMPs were reported. Neighboring cells harbor a high number of PRRs and show a rapid MAMP-triggered response [235].
5. Mechanisms Underneath PGPM–Plant Interactions
Plant growth-promoting microorganisms affect various aspects of plant growth and development. PGPM enhances the germination ratio [18,23,236], increases the elongation growth of the shoot and root [46,48,237], increases the biomass production [20,26,37], accelerates flowering [56,73], and increases the photosynthesis rate [27,42]. The examples of the mechanisms of action of plant growth-promoting fungi (PGPF) and bacteria (PGPB) are presented in Table 2 and Table 3, respectively.

Table 2.
Examples of mechanisms of plant growth promotion by PGPF.

Table 3.
Examples of mechanisms of plant growth promotion by PGPB.
Several mechanisms underneath the promotion of plant growth and development by microorganisms are employed by both bacteria and fungi for example degradation of ethylene via ACC deaminase, production of phytohormones, and solubilization of various soil compounds to increase the bioavailability of nutrients (Table 2 and Table 3). For sure the mechanism of greatest importance is the fixation of atmospheric nitrogen via nitrogenase, a mechanism that is specific to some specialized groups of prokaryotes (as reviewed in [271,272]). On the other hand, the fungi-specific mechanisms that allow for the promotion of plant growth and development includes the production of hydrophobins, swollenins, and peptaibols (please see the section Trichoderma-plant interaction—a case study, for details).
5.1. Plant Antioxidant Defence System
One of the best-known mechanisms to improve plant growth and development by PGPM is the modification of the level of antioxidants including antioxidative enzymes, e.g., superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), catalase (CAT), glutathione reductase (GR), and non-enzymatic antioxidants, e.g., proline, glutathione (GSH), ascorbic acid, carotenoids, and phenolics [25,273,274,275,276,277]. Islam et al. [278] demonstrated that inoculation of Vigna radiata (L.) R. Wilczek with Bacillus cereus Pb25 increased dry biomass and yield in salt stress conditions. Salt-induced oxidative damage was reduced by enhancing the activity of plant POD, SOD, and CAT and by increasing proline content in plants. Inoculation of O. sativa with Bacillus pumilus increased the activity of rice catalase and superoxide dismutase in salt stress conditions. Moreover, inoculation of rice with B. pumilus promoted the synthesis of photosynthetic pigments and proline [275]. Accumulation of phenols and proline was observed in cucumber inoculated with arbuscular mycorrhizal fungi in salt conditions [279]. A potato co-inoculated with T. viride and plant pathogen Alternaria solani showed improved redox homeostasis via increased activity of CAT and SOD, and enhanced concentration of free phenolics. Moreover, co-inoculation with T. viride and A. solani resulted in increased H2O2 production which induced the expression of plant defense genes [274]. Chen et al. [25] reported increased salt stress tolerance in maize after inoculation with B. amyloliquefaciens SQR9. Inoculated plants showed a reduced level of Na+, a higher glutathione content, a higher concentration of soluble sugars, and enhanced activities of peroxidase and catalase. B. amyloliquefaciens enhanced chlorophyll content and promoted the overall growth of inoculated plants in comparison to control plants. In addition to the well-known elements of the antioxidant system, there are also other proteins exhibiting antioxidant activity, including small cysteine-rich proteins metallothioneins (MTs). MTs act as direct antioxidants since the reduced thiol groups (-SH) can be oxidized by ROS. MTs also serve as a donor of zinc and copper to other antioxidative enzymes [280,281]. Inoculation of rapeseed with fungal strains isolated from forest soil showed varied expressions of B. napus metallothioneins (BnMT1-BnMT3). L. laccata inoculated plants showed significant upregulation of BnMT2 expression with a decrease in BnMT3 transcripts [43].
5.2. Phytohormones
Some bacteria are able to synthesize and secrete phytohormones and thus regulate plant growth and development. For example, B. subtills synthesizes auxins and gibberellins [282], and A. brasilense [283,284], Peanibacillus polymyxa [285], and P. fluorescens [286] produce cytokinins. Inoculation of S. tuberosum damaged by insect attack (beetle Leptinotarsa decemlineata) with B. subtilis 26D led to increased concentration of zeatin-riboside but not of abscisic acid (ABA) and indole acetic acid (IAA). The inoculation of potatoes with B. subtilis increased the mass of roots [287]. In the culture of Bacillus aryabhattai abscisic acid, indole acetic acid, cytokinins, and gibberellic acids were detected. Soybean inoculated with these bacteria produced more IAA, jasmonic acid, and some gibberellic acids. Moreover, inoculated plants displayed increased tolerance to heat stress possibly due to the ABA-induced closure of stomata [288].
Although different stressors affect plant organisms in various ways, most of them lead to increased ethylene production in plants. Weak stress factors can cause small overproduction of ethylene which leads to the activation of plant stress-related genes. Long periods of stress and severe stressors cause a high level of ethylene production, which might lead to senescence, chlorosis, and organ abscission [136]. Some PGPM are able to lower the level of ethylene through secretion of ACC deaminase, i.e., the enzyme that breaks down ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC). Inoculation of plants with microbes producing ACC deaminase effectively increased the plant resistance to stress caused by fungal pathogens [289], nematodes [290], and several abiotic stresses such as flooding [291], drought [135], salination [133], heavy metals [292], and toxic contaminates [134]. For example, inoculation of pearl millet seed with ACC deaminase-producing B. amyloliquefaciens increased plant growth in drought stress via increasing the level of enzymatic and non-enzymatic antioxidants [272]. Isolated from Brassica rapa L. rhizosphere bacteria belonging to Pseudomonas sp. improved biomass and yield of B. rapa. The positive effect of analyzed strains on B. rapa is possibly due to the production of IAA, ACC deaminase, and siderophores [95].
5.3. Availability of Micro- and Macronutrients
In most terrestrial ecosystems, nitrogen is the major nutrient limiting plant growth. PGPMs might increase the pool of nitrogen available for plants thorough various mechanisms. It is estimated that in nature more than 60% of fixed nitrogen is a result of biological nitrogen fixation [272]. A well-known example is the Rhizobia present in root and stem nodules of legumes that is able to reduce N2 to ammonia. There are also other symbiotic, plant-endophytic, and free-living bacteria able to fix molecular nitrogen, including bacteria belonging to Frankia, Cyanobacteria, Azotobacter, Bacillus, and Azospirillum, as reviewed in [293]. The Co-inoculation of beans with Rhizobium phaseoli and bacteria belonging to Bacillus and Pseudomonas improved plant growth by enhancing the total content of nitrogen in plant tissues more efficiently than inoculation with R. phaseoli. Only [127]. Hungria et al. [130] reported that the co-inoculation of soybean seeds with Azospirillum and Bradyrhizobium significantly enhances the yield without any input of nitrogen fertilizers. Several lines of evidence showed that rhizobia are susceptible to drought-stress and the efficiency of N2-fixation dramatically declines in low-water conditions [294]. For example, co-inoculation of a common bean with Rhizobium tropici and two strains of P. polymyxa more effectively increased nitrogen content and promoted plant growth than inoculation with Rhizobium only especially in drought conditions [295].
Phosphorus is also considered as element limiting plant growth in most ecosystems. Although phosphorus is an abundant element in ecosystems, most of it is not bioavailable [296]. There is a group of microbes, phosphate solubilizing microorganisms (PSM), that are able to increase the available fraction of phosphorus for plants via solubilization and mineralization mediated by secretion of organic acids, phosphatases, protons, and exopolysaccharides [297]. Red clover (Trifolium pratense L.) inoculated with phosphate solubilizing fungi Penicillium albidum showed a significant increase in root biomass. In soil inoculated with fungi, the phosphatase activity was 1.5-fold higher than in the non-inoculated soil [129]. In soil inoculated with Burkholderia sp., Gluconobacter sp., and Pseudomonas striata, higher activity of dehydrogenase and phosphatase and a higher level of available P were detected. Vigna unguiculata (L.) Walp. grown in inoculated soil had noticeably higher biomass and yield than plants grown in non-inoculated soil. Moreover, tested microorganisms enhance uptake not only of phosphorus but also of nitrogen [298]. Inoculation of T. aestivum with Serratia marcescens enhanced plant growth and nutrient uptake (P, N, and K) in low temperatures. The ability of tested bacteria to solubilize P decreased at low temperatures [299].
Soil minerals, such as feldspars and micas, are the most common form of potassium in soils. Up to 90–98% of soil potassium is present in a form unavailable for plants [300]. By secretion of organic acids and capsular polysaccharides, some PGPM are able to solubilize potassium rocks, e.g., bacteria belonging to Acidothiobacillus, Bacillus, Pseudomonas, Burkholderia, and Peanibacillus [301]. Ali et al. [302] reported that inoculation of potatoes with K-solubilizing B. cereus resulted in significantly improved plant growth and yield. Moreover, the content of K in potato tubers and the content of N, P, and K in leaves was higher in comparison to control plants. Inoculation of ryegrass with Mesorhizobium sp., Peanibacillus sp., and Arthrobacter sp. isolated from canola rhizosphere improved biomass and yield. The content of available K in soil was much higher and resulted in increased K content in plants [303]. Basak and Biswas [304] demonstrated that treatment of waste mica with Bacillus mucilaginosus led to the transformation of K forms into water-soluble forms. This had a positive effect on K uptake and biomass of sudan grass (Sorghum vulgare Pers.). Similar effects were observed by Raji and Thangavelu [305], who analyzed the effect of inoculation of tomato (Lycopersicon esculentum L.) grown in Alfisol and Vertisol soils with B. subtilis, B. cereus, Bacillus licheniformis, and Burkholderia cenocepacia. Inoculated plants showed higher K content in tissues and improved growth.
The deficiency of microelements, including copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn), is also a prominent factor that negatively affects plant health. In calcareous soils (widespread in arid and semiarid regions of the world), the high content of calcium carbonate which acts as a buffer and maintains a pH above 7.5, is correlated with decreased bioavailability of Fe, Zn, and Mn [306]. Plants possess mechanisms allowing them to increase amounts of bioavailable microelements in soils. For example, to facilitate Fe acquisition, plants secrete siderophores, organic acids, and flavonoids [307], whereas the amount of bioavailable Zn is increased by the secretion of organic acids, such as propionic acid, formic acid, lactic acid, citric acid, succinic acid, malic acid, oxalic acid, and gluconic acid as well as by secretion of siderophores, as reviewed in [308]. A tomato grown in hydroponic culture with the addition of soil minerals in Cu-deficient conditions showed increased biomass and Cu uptake after inoculation with Trichoderma harzianum SQR-T037 in comparison to non-inoculated plants. Interestingly, inoculation of a tomato with T. harzianum SQR-T037 grown in an Fe-deficient hydroponic culture with the addition of solid mineral increased the Fe content in plant tissue, but the biomass of seedling was unaffected. In addition, inoculation of tomatoes grown in Zn-deficient hydroponic conditions did not increase the biomass and the concentration of Zn in plant tissues was decreased. Those observations suggest that in element-deficient conditions fungi compete with plants for nutrients [309]. Rana et al. [128] showed enhanced yield and increased concentrations of Fe, Zn, Cu, and Mn by 13–16% in rice grains after inoculation with Brevundimonas diminuta, Ochrobactrum anthropic, and Providencia sp. Wheat inoculation with Providencia sp. significantly increased yield and the content of Fe and Cu in grains was 45% higher than in control plants. Singh et al. [310] reported that endophytic bacteria isolated from wheat, such as B. subtilis, Arthrobacter sp., A. sulfonivorans, and Enterobacter hirae exhibiting plant growth-promoting traits, enhanced Fe and Zn fortification as well as yield and dry/fresh weight in pot experiment and field conditions. Moreover, a decrease in phytic acid was observed in grains of wheat plants inoculated with endophytes. A field study on common bean and wheat fortification showed that inoculation with A. brasilense and T. harzianum significantly enhanced micronutrients, i.e., Fe, Mn, and Zn content in both tested plants [311]. Enhanced content of selenium was observed in lettuce inoculated with bacteria Bacillus sp., Klebsiella sp., Acinetobacter sp., and with fungus Rhizophagus intraradices in drought stress conditions. Results showed that plants inoculated with bacteria showed higher biomass production in comparison to plants inoculated with fungus, and Klebsiella sp. was the most effective in the induction of Se accumulation in lettuce. Moreover, tested microbes increased drought stress resistance, the chlorophyll and carotenoid content, and enhanced the level of antioxidant enzymes [312]. The ability of some PGPM to increase the content of macro- and micro-elements in different parts of plants is also important for human nutrition. Microelements deficiency (especially zinc and iron) is widespread all over the world. Biofortification, i.e., approaches to enhance the nutritional value of crops, with microelements in edible parts of plants, is the most promising approach to fight against microelements deficiency, as reviewed in [313,314,315]. Therefore, PGPM seems to be of crucial importance for both food quantity, i.e., enhanced plant yield and food quality, i.e., edible parts of plants with high content of minerals.
5.4. Direct Interactions of PGPM with Plant Pathogens
PGPM might directly interact with various plant pathogens. Through various mechanisms, including the production and secretion of antimicrobial metabolites, antagonisms, mycoparasitisms, and competing for niche PGPM can enhance plant biotic stress resistance. Cabrefiga et al. [316] reported an antagonistic interaction between P. fluorescens EPS62e and Erwinia amylovora, bacteria causing fire blight disease on pear trees (Pyrus sp.). P. fluorescens EPS62e did not produce antibiotics and required cell-to-cell contact with the pathogen in order to inhibit their growth on pear flowers and fruits. Interestingly, antagonistic activity was not shown when bacteria were grown in an Fe-rich medium, which suggested that the production of siderophores is responsible for the P. fluorescens EPS62e ability to inhibit the growth of E. amylovora. Inoculation of plants with antibiotic-producing microorganisms might lead to the suppression of various plant diseases. From the roots and rhizosphere soil of cucumber grown in soil inoculated with biocontrol agent B. subtilis, antibiotics surfactin and iturin A were extracted [317]. Bais et al. [318] showed that the biocontrol ability of B. subtilis against Pseudomonas syringae is tightly linked with the production of surfactin involved in biofilm formation on A. thaliana roots. B. subtilis mutant with a deletion in the surfactin synthase gene was unable to form biofilm and was ineffective in protection against P. syringae attack. Rhizospheric S. plymuthica HRO-C48 isolated from the rhizosphere of rapeseed produces antibiotic pyrrolnitrin and can protect plants against Verticillium wilt [319]. Kurze et al. [320] demonstrated that S. plymuthica HRO-C48 protects strawberries against fungal pathogens V. dahliae and Phytophtora cactorum. Moreover, the inoculation of strawberries with S. plymuthica promoted plant growth and improved yield. Inoculation of wheat seedlings with Trichoderma sp. resulted in an increase in plant resistance markers when plants were infected with Fusarium spp. Moreover, a decrease in IAA was observed. Biocontrol activity of Trichoderma was connected with the secretion of lytic enzymes and fungal elicitors as well as mycoparasitism [49]. An interesting observation was made by Chen et al. [321] who showed that Pseudomonas piscium isolated from wheat is able to alter histone acetylation in pathogenic Fusarium gramineareum and, therefore, reduce the fungus level of virulence and mycotoxin production by the fungus. An identified compound secreted by P. piscium, i.e., phenazine-1-carboxamide, disturbs the activity of fungal histone acetyltransferase FgGcn5, responsible for the regulation of gene expression involved not only in virulence and the growth of the mycelium, but also in asexual and sexual reproduction and stress response.
5.5. Induction of the Plant Resistance by Microbial Elicitors
Although the use of living microorganisms is a potent tool in the development of sustainable agriculture, it has also numerous constraints due to legal regulations [322]. As an alternative to the living microbes and cell wall polymers (CWP) of bacteria, fungi can be employed as elicitors [323,324,325]. Elicitors trigger the immune response of the plant via numerous mechanisms including accumulation of lignin, antimicrobial enzymes, e.g., chitinases, glucanases, phytoalexins, and proteins related to the response to the presence of pathogens, guaiacol, and ribonuclease [326]. One of the efficient elicitors is chitin and its deacetylated derivative chitosan (N-acetylglucosamine subunits are linked by (1 → 4) -β bonds). For example, chitosan led to an increase in the systemic resistance in tomatoes and an increase in the plant resistance towards Alternaria solani and Xanthomonas vesicatoria [327]. Treatment of Psammosilene tunicoides with chitosan led to an increased expression of genes encoding antioxidant enzymes and transcription factors controlling stress-response genes. Moreover, the content of secondary metabolites terpenoid saponins increased [328]. The cost-effective and efficient source of elicitors are fungi belonging both to Ascomycota and Basidiomycota. Research by Nowak et al. [326] showed that (1 → 3) -α-D-glucooligosaccharides (GOS) obtained by hydrolysis of (1→3)- α-D-glucan from Laetiporus sulphureus induced the growth of wheat seedlings. Moreover, GOS caused the increase in the activity of CAT and APX, in the activity of chitinase, and higher activity of enzymes activating phenylpropanoid-producing pathways. Laminarin, a polysaccharide, consists of β-(1-3)-glucan with β-(1-6)-linkages of 20–25 units isolated from brown algae, which is an example of elicitor belonging to β-glucans. Treatment of grapevine-cultured cells with laminarin led to calcium influx, an oxidative burst, and the induction of pathogen-related genes. Laminarin by the induction of plant resistance indirectly contributes to the reduction of the growth of B. cinerea and Plasmopara viticola on grapevine plants [329].
6. The Stringent Response in Plant–Microorganism Interactions
Among several plant mechanisms regulating growth, development, and the response to environmental factors, the stringent response is of particular interest. The stringent response was first discovered in Escherichia coli in response to amino acid starvation [330]. The hallmark of the stringent response is the accumulation of atypical regulatory nucleotides guanosine tetra- (ppGpp) and pentaphosphates (pppGpp) called alarmones that are responsible for pleiotropic adaptation to nutrient deficiency and stress factors [331,332]. Moreover, the bacterial stringent response through regulation of quorum sensing indirectly affects the formation of microcolonies and the development and functioning of biofilm [333]. Alarmones are synthesized from ATP/GTP or GDP by enzymes possessing active synthetase domain (SYNTH) and are hydrolyzed to GTP/GDP and pyrophosphate by enzymes containing active hydrolytic domain (HD). Gram-negative bacteria usually possess two separate enzymes, i.e., alarmones synthetase RelA and alarmones hydrolase SpoT. Gram-positive bacteria usually possess one bifunctional Rel protein [334,335]. RelA and SpoT belong to the long RSH (RelA/SpoT homologue), i.e., enzymes possessing HD and SYNTH domains. In bacteria, there are also short RSH, i.e., enzymes containing either SYNTH domain, small alarmone synthases (SAS) or HD domain, or small alarmone hydrolases (SAH), whereas in animals, to date only small alarmone hydrolases were identified (Mesh1—metazoan SpoT homologue) [336]. Alarmones regulate transcription, translation, and DNA replication, and trigger metabolical and physiological changes in response to unfavorable environmental conditions. Upon accumulation of alarmones, bacteria change their lifestyle from growth and proliferation to survival mode [335]. Sanchez-Vazquez et al. [337] demonstrated that in E. coli, the elevated (p)ppGpp level affected the expression of 757 genes five minutes after the induction of the stringent response and after another five minutes the expression of 1 224 genes was affected. Activation of the stringent response can be triggered by a deficiency of amino acids [330], fatty acids [338,339], iron [340], carbon [341], nitrogen [342], phosphorus [343], and by other types of stress, e.g., increased temperature [344,345], cell wall antibiotics, ethanol and acid treatments, superoxide stress [346], and alkaline shock [347].
The presence of (p)ppGpp in photosynthetic Eucaryota was confirmed in the algae Chlamydomonas reinharditi [348]. In higher plants, RSH genes were identified for the first time in A. thaliana [349] and subsequently in other plant species, e.g., Nicotiana tabacum L. [350], rice [351], Ipomoea nil L. Roth [352], Sueda japonica Forssk. ex J.F. Gmel. [353], pepper [354], and non-vascular plants, e.g., Physcomitrella patens (Hedw.) Bruch and Schimp [355]. Plant RSH proteins are divided into three groups, i.e., RSH1, RSH2/3, and CRSH. All identified plant RSH proteins belong to the long RSH, i.e., possess both HD and SYNTH domains. In model plants, the A. thaliana SYNTH domain of RSH1 (AtRSH1) is Inactive due to the substitution of conserved glycine residue required for its activity. AtCRSH does not possess a functional hydrolase domain and in AtRSH2/3, both SYNTH and HD domains are active [356]. In addition to HD and SYNTH domains, RSH possess chloroplast transit peptide [357], TGS (RSH1, RSH2/3), and ACT domains (RSH1) which were proposed to act as regulatory- or ligand-binding domains [358]. CRSH are the only proteins involved in alarmones metabolism that possess two EF hand motifs and are activated by Ca2+ ions [359]. The domain structure of RSH proteins is highly conserved across plant species. It is now widely accepted that in plants, the place of alarmones action are chloroplasts [360,361,362,363]. Alarmones act as regulators of plant development and growth, i.e., (p)ppGpp coordinate micro- and macro-elements redistribution during senescent [364]. They modulate the level of phytohormones [365], lipids [366,367], and secondary metabolites [368] in chloroplasts. Moreover, alarmones promote the replication of plastidial DNA [364]. Plant RSH genes are differently expressed in presence of biotic and abiotic stress factors, which suggest that alarmones contribute to plant response in a number of stress factors, i.e., oxidative stress [369], nitrogen starvation [369,370], wounding, salination, drought, UV radiation, heat shock, heavy metals, and abrupt change from light to darkness [371]. Masuda et al. [372] showed that RSH probably play a role in plant reproduction, as the AtCRSH knockdown mutant produced smaller siliques and a lower number of seeds. An interesting observation was made by Ono et al. [373], who demonstrated an increased (p)ppGpp concentration in chloroplast upon a light-to-dark transition. The alarmones accumulation was due to higher activity of CRSH caused by elevated levels of Ca2+ in chloroplasts. As a consequence, the expression of plastidial genes is adapted to darkness. Interestingly, it was demonstrated that the accumulation of alarmones in plants leads to a decreased expression of genes involved in the defense system, which can lead to higher susceptibility to infections [365].
Plants are probably able to manipulate the level of alarmones synthesis in members of their own microbiome but also in pathogenic bacteria. Through modulation of (p)ppGpp production in bacteria, plants may be able to decrease virulence and inhibit the growth of pathogens. Nowicki et al. [374] demonstrated the activation of stringent response in E. coli cells by plant secondary metabolites isothiocyanates (ITC). ITC-induced stringent response in E. coli led to growth inhibition, disturbed transcription, and DNA replication. The induction of the E. coli stringent response may be the result of a direct interaction of ITC with cellular proteins. That idea is plausible since sulforaphane (one of the ITCs) inhibits the growth of numerous bacteria and recently potential target proteins of ITC were identified [375]. Mwita et al. [376] showed that the expression of SasA (short alarmone synthase) of PGPB Bacillus atrophaeus UCMB-5137 is considerably upregulated by maize root exudates. Further implications of observation on these bacteria metabolisms need, however, further evaluation. On the other hand, inoculation of plants with PGPB can affect plant RSH gene expression; however, the data are scarce. Dąbrowska et al. [377] demonstrated that B. napus inoculated with S. plymuthica and Serratia liquefaciens exhibited elevated mRNA levels of BnRSH1 in cotyledons and roots, whereas inoculation with Massilia timonae increased BnRSH1 expression only in roots. Moreover, S. plymuthica seemed to affect also the expression of BnRSH2 and BnRSH3 in cotyledons and roots. The relative transcript level of BnCRSH was elevated in cotyledons in presence of S. plymuthica and S. liquefaciens. Inoculation of canola grown in salt-stress conditions with endophytic Pseudomonas stutzeri ISE12 significantly increased mRNA levels of BnRSH1 and BnRSH3 in roots in comparison to non-inoculated plants grown in salt stress [34]. Moreover, Givens et al. [350] observed a 10-fold increase in RSH2 protein level in N. tabacum after infecting the plant with the bacterial pathogen Erwinia carotovora.
Microbes might directly and/or indirectly activate the stringent response in other microorganisms. A study on the effect of the pathogenic fungus Rhizoctonia solani on the rhizosphere microbiome of sugar beet (Beta vulgaris L.) showed that in several rhizobacteria, the expression of genes involved in (p)ppGpp metabolism is upregulated. It is not clear whether the activation of the stringent response in bacteria present in the rhizosphere is triggered directly by oxalic and phenylacetic acids secreted by R. solani or indirectly by signaling molecules in root exudates [378]. Interestingly, the relA and relA/spoT mutants of Pseudomonas sp. DF41 and Pseudomonas chlororaphis PA23, showed increased antifungal activity against Sclerotinia sclerotiorum. All mutants produced an increased level of antifungal antibiotic pyrrolnitrin, lipase, and protease in comparison to wild-type bacteria. The lack of (p)ppGpp led also to reduced transcription of rpoS [379,380]. Selin et al. [381] showed that expression of rsmZ, rsmE, and rsmA, i.e., elements involved in the regulation of several processes such as virulence, motility, and biocontrol abilities, was regulated via the stringent response in P. fluorescens, a PGPB inhibiting the growth of a number of pathogenic fungi including S. sclerotiorum. Takeuchi et al. [382] reported that a mutant of P. fluorescens CHA0 lacking the ability to synthesize alarmones, produced significantly fewer antibiotics and had lower biocontrol activity against P. ultimum. Moreover, the ability of the tested mutant to colonize cucumber roots, both in the presence and absence of P. ultimum, was reduced possibly due to impaired motility. The stringent response was found to strongly influence the production of antibiotics also in Streptomyces which are common members of the plant microbiome. Ochi [383] reported that rel mutant of Streptomyces antibioticus showed induction of phenoxazinone synthase, an enzyme involved in the production of actinomycin. Interestingly, this mutation did not affect the activity of another enzyme participating in the biosynthesis of actinomycin, i.e., kynurenine formamidase. Moreover, RSH genes regulated the morphological and physiological differentiation of Streptomyces clavuligerus; the lack of (p)ppGpp affected spore formation [384].
Recent studies showed that the stringent response may play a crucial role in the interaction between legumes and rhizobia. At the beginning of the interaction, legumes produce antimicrobial compounds that lead to nutritional, osmotic, and oxidative stress in rhizobia [385,386,387]. Based on the literature, it might be hypothesized that in order to survive these unfavorable conditions, rhizobia activates the stringent response. Soybean inoculated with the rsh knockout Bradyrhizobium diazoefficiens formed smaller nodules than those present in plants inoculated with wild-type B. diazoefficiens. Moreover, the biomass of plants inoculated with mutant bacteria was smaller in comparison to wild-type bacteria, but still higher than the biomass of non-inoculated plants. Those results suggest that the lack of alarmone signaling altered nodulation and, as a consequence, decreased N2 fixation. Interestingly, in plants co-inoculated with wild-type bacteria and the rsh mutant in equal proportion, only 26% of nodules were infected by mutant bacteria. This observation strongly suggests that the stringent response is crucial to win the competition for a niche with other rhizobia [388]. In the early phase of nodulation, establishing a symbiotic relationship between rhizobia and legumes requires plant-bacteria signaling that allows the recognition of bacteria by the host. It was shown that relA mutant of Sinorhizobium meliloti failed to form nodules on M. sativa due to disturbed stringent response, but remarkably the nodulation of Medicago truncatula Gaertn. was successful. The N2 fixation capacity of mutant bacteria was reduced in comparison to the wild type. It is not clear why there is a difference in nodulation between those two Medicago species; however, it might be hypothesized that different plants influenced bacterial stringent response at different stages of invasion [389]. Another study also found that S. meliloti mutant unable to synthesize (p)ppGpp did not establish a symbiotic relationship with M. sativa. The relA mutant of S. meliloti produced more succinoglycan, an exopolysaccharide needed for colonization of the host, than the wild-type bacteria [390]. The inactivation of relA in Rhizobium etli caused nodules on P. vulgaris form, but the level of nitrogen fixation was significantly reduced. Mutants showed significantly lower expression of raiI and cinI genes which encode regulators of quorum sensing in rhizobia [391]. Calderón-Flores et al. [392] reported that in P. vulgaris inoculated with R. etli, the rsh mutant nodulation and nitrogen fixation were disturbed. Qiu et al. [393] demonstrated that the addition of water-soluble humic materials into inoculum containing Sinorhizobium fredii significantly downregulated RSH expression, increased survivability of bacteria in soil, and promoted rhizoplane colonization of Glycine max (L.) Merr.
The stringent response is also a significant mechanism contributing to the virulence of various plant pathogens. A study on E. amylovora showed that cells of relA and relA/spoT mutants were significantly longer both in nutrient-rich and nutrient-limited conditions in comparison to the wild type. Moreover, it was demonstrated that the proliferation rate of relA/spoT mutant in pear fruits was 1000 times slower than the proliferation rate of the wild type. In minimal medium double, the relA/spoT mutant was unable to grow. Small-sized cells are more resistant to stress, and bigger relA/spoT knockouts are unable to survive on plant surfaces. It seems that (p)ppGpp are important regulators of cell growth in E. amylovora during plant infection [394]. A similar observation was made for P. syringae, one of the most common plant pathogens. P. syringae single (relA) and double (relA/spoT) knockout mutants grown on nutrient-rich medium were slightly bigger in comparison to the wild-type bacteria. An in vivo study demonstrated that relA/spoT mutants, even though they had bigger cells, were unable to survive on the surface of a tomato leaf. These findings suggest that the stringent response is a crucial element of plant surface colonization [395,396]. A great induction of relA and spoT genes in gram-negative bacterium Pectobacterium atrosepticum, able to degrade plant cell walls, was observed when bacteria were grown in high-density culture in carbon-deficient media [397,398]. Zhang et al. [399] reported that the Xanthomonas citri double knockout spoT/relA mutants showed a significant decrease in pathogenicity and inhibited growth in planta. Interestingly, the deletion of only the main alarmones synthase in the X. citri relA did not affect the virulence of the bacterium.
Several lines of evidence showed that the stringent response plays a crucial role in the adaptation of bacterial growth and metabolism to nutrient-limited conditions. The mentioned studies above show the importance of the stringent response for the establishment of plant–PGPM interactions and proper functioning of the plant microbiome. We hypothesize that the stringent response probably allows members of the microbiome to survive in unfavorable conditions during the first stages of interaction establishment. The data about the possible role of alarmones in an intricate network of interactions between plants and microorganisms, among members of microbiomes, and between PGPM and plant pathogens are rather limited. The possible crucial role of the plant and the microbial stringent response in the functioning of microbiomes is an exciting but rather overlooked research area that needs further experiments.
7. Trichoderma–Plant Interaction—A Case Study
Among several other microbes belonging to the PGPM group, fungi from the genus Trichoderma are of great interest. Adaptability to unfavorable environmental conditions and the ability to utilize many different substrates as nutrients determine the ubiquitous occurrence of fungi belonging to Trichoderma in soils. It is estimated that one gram of soil contains 10–103 CFU (colony-forming unit) of fungi belonging to Trichoderma [400,401]. It was estimated that there are around 438 species in the genus Trichoderma, grouped into 10 phylogenetic lineages: Brevicompactum, Deliquescens, Harzianum, Hypocreanum, Longibrachiatum, Polysporum, Psychrophilum, Semiorbis, Stromaticum, and Viride [402].
Through interaction with plant fungi belonging to Trichoderma gains, a convenient niche for growth and development since root exudates are a rich source of carbon and other nutrients. The presence of fungi belonging to Trichoderma enhances growth and increases yield [403,404], improves uptake of nutrients by plants [405,406], and leads to a higher vigor and germination ratio of seeds [407,408]. In addition, fungi belonging to Trichoderma increase the level of photosynthesis [409], the level of amino acid synthesis [410], the level of transpiration [411], and the water content in tissues in drought conditions [412]. Fungi from the genus Trichoderma can colonize the roots of mono- [56,57,413] and di-cots [58,131,132] and when plants are grown in acidic soils [414], alkaline soils [415,416], and soils contaminated with heavy metals [417,418]. Trichoderma are potential symbionts of non-mycorrhizal plants belonging to Brassicaceae [59], Chenopodiaceae [302], Caryophyllaceae [419], Polygonaceae [420], and others. Recently, marine isolates of Trichoderma have been identified [421,422,423,424,425], which have the potential to serve as plant growth-promoting fungi for plants grown in saline soils [426]. Several mechanisms have been shown to contribute to the promotion of plant growth and development by fungi belonging to Trichoderma. Colonization of plants by Trichoderma changes host proteome [413] and secretome [427], affecting the level of synthesis of phytohormones [428] in soluble sugars [409] and phenolic compounds [173,429]. The inoculation of A. thaliana seedlings with Trichoderma virens and Trichoderma atroviride increased biomass production and promoted lateral root growth. Mutations in plant genes involved in auxin transport and signaling, i.e., AUX1, BIG, EIR1, and AXR1 caused reduced stimulation of root growth and development by tested Trichoderma isolates [92]. Fungi belonging to Trichoderma compete with pathogens for ecological niches and nutrients, which efficiently limits the growth of pathogens. Secreting antibiotics, siderophores, a range of volatile and non-volatile metabolites (n-alkanes, cyclohexane, cyclopentane, esters, alcohols, sulfur-containing compounds, pyrane, and benzene derivatives), and through mycoparasitism fungi belonging to Trichoderma, protect plants against various pathogens, such as R. solani [430,431,432], Rhizopus oryzae [433], Fusarium spp. [434,435], Alternaria alternate [436], S. sclerotiorum [432,437], Botrytis cinerea [438,439], Pythium spp. [433], and Ustilago maydis [440]. Several secondary metabolites produced by fungi belonging to Trichoderma peptaibols seem to be of great importance for Trichoderma biocontrol activity. Peptaibols are amphipathic polypeptides composed of 5–10 amino acids with molecular masses between 500 and 2200 Da. These non-ribosomally synthesized polypeptides contain not only typical amino acids but also non-proteinogenic amino acids and α-aminoisobutyric acid. Peptaibols are synthesized not only by fungi belonging to Trichoderma but also by other soil-born fungi as well as by plant-pathogen fungi [441,442]. Several lines of evidence have confirmed that peptaibols exhibit antibacterial and antifungal properties. Trichoderma pseudokoningii produces trichokonin VI that induces apoptotic cell death in F. oxysporum [443]. Trichokonins A produced by Trichoderma longibrachiatum damages the cell membrane of Gram-negative pathogenic bacteria Xanthomonas oryzae pv. Oryzae, leading to a significant reduction of the pathogenicity of these bacteria [444]. The same inhibitory effect was observed for several other peptaibols produced by various Trichoderma species against a range of plant pathogens, e.g., B. cinerea [445], Septoria tritici [446], A. solani, and R. solani [447]. Moreover, it was also demonstrated that peptaibols can act against viruses. Luo et al. [448] showed that trichokonins isolated from T. pseudokoningii induces resistance of tobacco against the tobacco mosaic virus probably via induction of reactive oxygen species and phenolic compound production. Peptaibols isolated from T. virens might act as elicitors and induce a defense response in cucumber against pathogenic bacteria Pseudomonas syringae pv. lachrymans via up-regulation of hydroxyperoxide lyase, phenylalanine ammonia lyase, and peroxidase gene expression. In addition, T. virens mutant tex1 lacking one of the non-ribosomal peptide synthetases was less effective in the inhibition of P. syringae pv. lachrymans growth [247]. It should be pointed out that high concentrations of peptaibols might have a negative impact on the growth of plants, as shown for peptaibols produces by Trichoderma reesei and their negative effect on A. thaliana. However, peptaibols at lower concentrations are still sufficient to inhibit the growth of plant pathogens with no adverse effect on plant growth [449].
Moreover, some strains of Trichoderma can induce ISR (induced systemic resistance) and/or SAR (systemic acquired resistance) in the host plant through the secretion of fungal elicitors. Shoresh et al. [450] demonstrated that the treatment of cucumber with T. asperellum T203 activated ISR via the JA/ethylene signaling pathway. Inoculated plants were more resistant to P. syringae than non-inoculated control plants. Another study showed that soil inoculation with T. harzianum enhanced tomato defense against root-knot nematode (Meloidogyne incognita) by SAR activation and increased ethylene synthesis [132]. Inoculation of canola with T. harzianum TH12 triggered SAR and ISR defense mechanisms and decreased the severity of disease symptoms caused by S. sclerotiorum [132]. Changes in host cells caused by inoculation with Trichoderma are also visible in tissues distant from the penetration site. Yedidia et al. [451] demonstrated that the cell walls of cucumber root epidermis and cortical cells after T. harzianum inoculation were strengthened also beyond the penetration site. The activity of plant peroxidases and chitinases was upregulated by the presence of a fungus both in roots and leaves. These findings are in agreement with the fact that microbial invasion of host plant cells induces systemic resistance mechanisms. Huang et al. [452] reported that inoculation of cucumber with T. harzianum SQR-T37 significantly promoted growth as well as suppressed the damping off disease caused by R. solani. It was shown that the main mechanism of biocontrol was mycoparasitism. Between the pathogen and T. harzianum SQR-T37, a direct interaction was observed. The hyphae of T. harzianum were densely coiled, and hooks and appressorium-like bodies were formed. As a consequence, the cell walls of R. solani were broken and leakage of cytoplasm was noted.
Fungi belonging to Trichoderma are able to colonize plants due to the mechanisms allowing the recognition and the adhesion to the surface of the root (Figure 2). Moreover, these fungi are able to penetrate the root tissues and suppress the plant’s immune system in order to avoid strong responses from the host, as reviewed in [453,454]. The plant’s immune system recognizes Trichoderma MAMPs, including swolenin [250], alamethicin [455], xylanase [456], cellulases [457], and polygalacturonase [458]. Plant responses to MAMPs are quick and transitional in the early stages, they involve ion level fluctuation, overproduction of ROS, nitric oxide, and ethylene [459]. Later stages involve the production of callus wall and antifungal compounds and, as a consequence, further penetration of the plant by hyphae is stopped [429,460]. It was demonstrated that salicylic acid has a particular role in callus synthesis during the colonization of plants by fungi. In a A. thaliana sid2 (salicylic acid induction-deficient2) mutant with a disturbed SA signalization, T. harzianum penetrated root vascular tissues whereas in wild-type plants, penetration of plants by fungi were restricted to outer root layers [460]. Inoculation of Z. mays with T. virens caused the reduction of plant secretome by 36%, which deprives plants of essential signaling molecules and proteins crucial for proper plant growth and development. At the same time, a fungus secretes similar compounds which do not activate the plant’s immune system [427]. The ability to colonize plant tissues by Trichoderma is tightly linked with the fungal capacity to tolerate secreted plants’ antimicrobial compounds [459]. An ABC transporter system is crucial for the resistance of Trichoderma to antifungal compounds secreted by plant pathogens. Deletion in the Taabc2 gene in T. atrioviride caused the loss of the ability of the fungus to protect the tomato against P. ultimum and R. solani attacks [461]. Another mechanism exploited by fungi belonging to Trichoderma is directly decreasing the synthesis of the antifungal compound. For example, Masunaka et al. [462] reported that inoculation of Lotus japonicus L. with Trichoderma koningi down-regulated the production of isoflavonoid vesitol, i.e., the main phytoalexin produced by lotus species. Fungi belonging to Trichoderma were found proficient in the degradation of allelochemicals secreted by plants which exhibit fungitoxicity to a number of fungi [173].
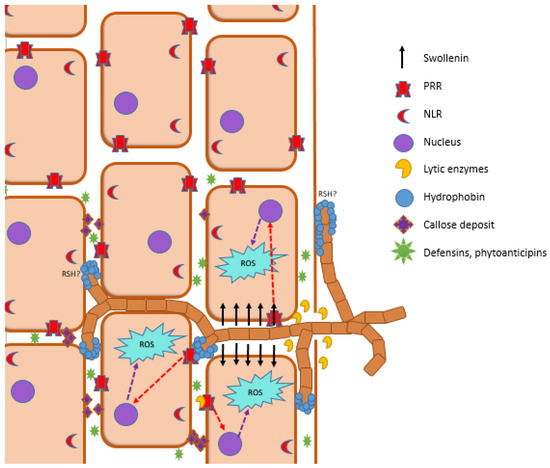
Figure 2.
Colonization of root by a fungus belonging to Trichoderma. Adhesion and protection of hyphae are mediated by the layer of hydrophobins, whereas lytic enzymes enable penetration of the epidermis. Swollenins facilitate penetration of apoplast through an expansion-like effect on plant cell walls. Recognition of Trichoderma-derived MAMP molecules (swollenins, hydrophobins, cellulolytic enzymes, and chitin) triggers plant responses to infection, i.e., synthesis of antimicrobial compounds (defensins and phytoanticipins), synthesis of the callose wall in order to physically inhibit further penetration, and overproduction of ROS and possibly also alarmones. See text for more details.
Adhesion of fungi belonging to Trichoderma to the surface of plants is mediated by hydrophobins, i.e., small, cysteine-rich hydrophobic proteins (Figure 2). Hydrophobins are synthesized by filamentous fungi. The amino acid sequence of hydrophobins is highly evolutionarily conserved. Hydrophobins are classified into two classes based on the arrangement of cysteine residues, differences in solubility, and physical properties. Hydrophobins form amphipathic monolayers at hydrophobic-hydrophilic interfaces. Those proteins are involved in the formation of aerial hyphae, fruiting bodies, and spores as reviewed in [463,464]. It was shown that class I hydrophobins produced by T. asperellum enabled adhesion to cucumber roots. The authors hypothesized that hydrophobins protect hyphae against antimicrobial compounds were secreted by the host during colonization [244]. Inoculation of tomato and cucumber with a T. harzianum mutant with a deletion in the gene encoding hydrophobin showed that the mutant fungi were able to colonize the roots of both plants; however, the lateral roots were significantly shorter than those present in plants inoculated with the wild-type fungus [245]. Further penetration of roots by hyphae is possible due to fungal proteolytic enzymes, e.g., aspartyl protease (PapA) [465], and cellulolytic enzymes, e.g., endopolygalacturonase (ThPG1) [458], and arabinofuranosidases (Abf1, Abf2) [465] that allow for degradation of the plant cell wall. Another important element allowing Trichoderma for tissue penetration are swolenins (Figure 2), i.e., proteins possessing a cellulose-binding domain (CBD), similar to plant proteins—expansins. Swollenins disrupt the structure of the cellulose which results in changes to the plant cell wall architecture and the expansion of intercellular space [466]. Swollenins facilitate penetration of apoplast by the hyphae and give fungi an advantage during the competition for the niche with other microbes. Brotman et al. [250] reported that T. asperellum overexpressing swollenin showed a significantly improved ability to colonize cucumber roots, whereas swolenin knockout mutants showed a reduced ability to colonize roots. Moreover, it was found that the CBD domain acts as the MAMP, and can induce plant defense against B. cinerae and P. syringae. Similarly, T. atroviride overexpressing the swolenin-coding gene Taswo1 improved the colonization rate and enhanced the growth of tomatoes and peppers. Moreover, the induction of the plant immune system was stronger by mutants overexpressing swollenins than by knockouts and wild-type fungi [467].
Sucrose plays important role in plant colonization by Trichoderma. As demonstrated by Macías-Rodríguez et al. [468], the concentration of carbohydrates, mainly arabinose, xylose, myo-inositol, fructose, and glucose in root exudates of L. esculentum is higher before colonization by T. atrioviride. Colonization of the root by T. atrioviride changed the exudation pattern and sucrose became a major component of exudates. Root-derived sucrose specifically enables better growth of fungi belonging to Trichoderma, since AMF fungi prefer glucose and fructose as a source of carbon. Analysis of the proteome of maize inoculated with T. harzianum T22 showed that 40 proteins involved in carbohydrate/starch metabolism were upregulated and 13 proteins were downregulated, which suggests that T. harzianum is able to modulate carbohydrate metabolism in colonized roots [413]. T. virens was shown to produce invertase that hydrolyses plant-derived sucrose. The sucrolytic activity of fungal cells is crucial for root colonization but also to increase the photosynthetic rate in maize leaves [409].
Author Contributions
Conceptualization, G.B.D.; investigation, M.A. and A.M.-A.; data curation, M.A.; writing—original draft preparation, M.A. and G.B.D.; writing—review and editing, G.B.D. and A.M.-A.; supervision, G.B.D. and A.M.-A.; funding acquisition G.B.D. and A.M.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Ani, L.K.T. Trichoderma: Beneficial role in sustainable agriculture by plant disease management. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; Volume 5, pp. 105–126. [Google Scholar]
- Weltje, L.; Simpson, P.; Gross, M.; Crane, M.; Wheeler, J.R. Comparative acute and chronic sensitivity of fish and amphibians: A critical review of data. Environ. Toxicol. Chem. 2013, 32, 984–994. [Google Scholar] [CrossRef]
- Mukherjee, R.K.; Kumar, V.; Roy, K. Chemometric modeling of plant protection products (PPPs) for the prediction of acute contact toxicity against honey bees (A. mellifera): A 2D-QSAR approach. J. Hazard. Mater. 2022, 423, 127230. [Google Scholar] [CrossRef]
- Bruni, I.; Gentili, R.; de Mattia, F.; Cortis, P.; Rossi, G.; Labra, M. A multi-level analysis to evaluate the extinction risk of and conservation strategy for the aquatic fern Marsilea quadrifolia L. in Europe. Aquat. Bot. 2013, 111, 35–42. [Google Scholar] [CrossRef]
- Matarczyk, J.A.; Willis, A.J.; Vranjic, J.A.; Ash, J.E. Herbicides, weeds and endangered species: Management of bitou bush (Chrysanthemoides monilifera ssp. rotundata) with glyphosate and impacts on the endangered shrub. Pimelea spicata. Biol. Conserv. 2002, 108, 133–141. [Google Scholar]
- Bikrol, A.; Saxena, N.; Singh, K. Response of Glycine max in relation to nitrogen fixation as influenced by fungicide seed treatment. Int. J. Biochem. Biotechnol. 2020, 9, 1–5. [Google Scholar]
- Santos, M.S.; Rodrigues, T.F.; Ferreira, E.; Megias, M.; Nogueira, M.A.; Hungria, M. Method for recovering and counting viable cells from maize seeds inoculated with Azospirillum brasilense. J. Pure Appl. Microbiol. 2020, 14, 195–204. [Google Scholar] [CrossRef]
- Zilli, J.É.; Ribeiro, K.G.; Campo, R.J.; Hungria, M. Influence of fungicide seed treatment on soybean nodulation and grain yield. Rev. Bras. Cienc. Solo 2009, 33, 917–923. [Google Scholar] [CrossRef]
- Streletskii, R.; Astaykina, A.; Krasnov, G.; Gorbatov, V. Changes in bacterial and fungal community of soil under treatment of pesticides. Agronomy 2022, 12, 124. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for improving formulations of bioinoculants. 3 Biotech. 2020, 10, 199. [Google Scholar] [CrossRef]
- Romano, I.; Ventorino, V.; Pepe, O. Effectiveness of plant beneficial microbes: Overview of the methodological approaches for the assessment of root colonization and persistence. Front. Plant Sci. 2020, 11, 6. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants-natural biological resources for sustainable plant production. Microorganisms 2022, 10, 51. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Maymon, M.; Pule-Meulenberg, F.; Hirsch, A.M. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can. J. Microbiol. 2019, 65, 91–104. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.F.; Bonilla, R.; Dussán, J. Effect of inoculation and co-inoculation of Acinetobacter sp. RG30 and Pseudomonas putida GN04 on growth, fitness, and copper accumulation of maize (Zea mays). Water Air Soil Pollut. 2014, 225, 2232. [Google Scholar] [CrossRef]
- Patel, P.; Gajjar, H.; Joshi, B.; Krishnamurthy, R.; Amaresan, N. Inoculation of salt-tolerant Acinetobacter sp. (RSC9) improves the sugarcane (Saccharum sp. hybrids) growth under salinity stress condition. Sugar Tech 2022, 24, 494–501. [Google Scholar] [CrossRef]
- Chihaoui, S.A.; Trabelsi, D.; Jdey, A.; Mhadhbi, H.; Mhamdi, R. Inoculation of Phaseolus vulgaris with the nodule-endophyte Agrobacterium sp. 10C2 affects richness and structure of rhizosphere bacterial communities and enhances nodulation and growth. Arch. Microbiol. 2015, 197, 805–813. [Google Scholar] [CrossRef]
- Sharma, M.; Mishra, V.; Rau, N.; Sharma, R.S. Increased iron-stress resilience of maize through inoculation of siderophore-producing Arthrobacter globiformis from mine. J. Basic Microbiol. 2016, 56, 719–735. [Google Scholar] [CrossRef]
- Fan, P.; Chen, D.; He, Y.; Zhou, Q.; Tian, Y.; Gao, L. Alleviating salt stress in tomato seedlings using Arthrobacter and Bacillus megaterium isolated from the rhizosphere of wild plants grown on saline–alkaline lands. Int. J. Phytoremediation 2016, 18, 1113–1121. [Google Scholar] [CrossRef]
- Parmasi, Z.; Tahmasebi, Z.; Zare, M.J.; Nourollahi, K.; Kanouni, H. Biocontrol of Ascochyta blight by Azospirillum sp. depending on the degree of resistance of chickpea genotypes. J. Phytopathol. 2019, 167, 601–607. [Google Scholar] [CrossRef]
- Mazhar, R.; Ilyas, N.; Saeed, M.; Bibi, F.; Batool, N. Biocontrol and salinity tolerance potential of Azospirillum lipoferum and its inoculation effect in wheat crop. Int. J. Agric. Biol. 2015, 18, 494–500. [Google Scholar] [CrossRef]
- Shirinbayan, S.; Khosravi, H.; Malakouti, M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019, 133, 138–145. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Safdar, M.E.; Chaudhry, A.U.; Malik, K.A. Rhizobial inoculation improves seedling emergence, nutrient uptake and growth of cotton. Aust. J. Exp. Agric. 2004, 44, 617–622. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Bernabeu, P.R.; Pistorio, M.; Torres-Tejerizo, G.; Estrada-De los Santos, P.; Galar, M.L.; Boiardi, J.L.; Luna, M.F. Colonization and plant growth-promotion of tomato by Burkholderia tropica. Sci. Hortic. 2015, 191, 113–120. [Google Scholar] [CrossRef]
- Tsuda, K.; Kosaka, Y.; Tsuge, S.; Kub, Y.; Horin, O. Evaluation of the endophyte Enterobacter cloacae SM10 isolated from spinach roots for biological control against Fusarium wilt of spinach. J. Gen. Plant Pathol. 2001, 67, 78–84. [Google Scholar] [CrossRef]
- Ngom, M.; Gray, K.; Diagne, N.; Oshone, R.; Fardoux, J.; Gherbi, H.; Hocher, V.; Svistoonoff, S.; Laplaze, L.; Tisa, L.S.; et al. Symbiotic performance of diverse Frankia strains on salt-stressed Casuarina glauca and Casuarina equisetifolia plants. Front. Plant Sci. 2016, 7, 1331. [Google Scholar] [CrossRef]
- Grossi, C.E.M.; Fantino, E.; Serral, F.; Zawoznik, M.S.; Fernandez Do Porto, D.A.; Ulloa, R.M. Methylobacterium sp. 2A is a plant growth-promoting rhizobacteria that has the potential to improve potato crop yield under adverse conditions. Front. Plant Sci. 2020, 11, 71. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Chiquito-Contreras, C.J.; Murillo-Amador, B.; Vidal-Hernández, L.; Quiñones-Aguilar, E.E.; Chiquito-Contreras, R.G. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, C.; Ding, N.; Lin, Y.; Guo, B. Ameliorative effects of inoculation with the plant growth-promoting rhizobacterium Pseudomonas sp. DW1 on growth of eggplant (Solanum melongena L.) seedlings under salt stress. Agric. Water Manag. 2010, 97, 1994–2000. [Google Scholar] [CrossRef]
- Szymańska, S.; Dąbrowska, G.B.; Tyburski, J.; Niedojadło, K.; Piernik, A.; Hrynkiewicz, K. Boosting the Brassica napus L. tolerance to salinity by the halotolerant strain Pseudomonas stutzeri ISE12. Environ. Exp. Bot. 2019, 163, 55–68. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Soil Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B.; Yanni, Y.G.; Rolfe, B.G. Rhizobial inoculation influences seedling vigor and yield of rice. Agron. J. 2000, 92, 880–886. [Google Scholar] [CrossRef]
- Turhan, E.; Kiran, S.; Ates, Ç.; Ates, O.; Kusvuran, S.; Ellialtioglu, S.S. Ameliorative effects of inoculation with Serratia marcescens and grafting on growth of eggplant seedlings under salt stress. J. Plant Nutr. 2020, 43, 594–603. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Samac, D.A.; Kinkel, L.L. Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 2001, 235, 35–44. [Google Scholar] [CrossRef]
- Mauricio-Castillo, J.A.; Salas-Muñoz, S.; Reveles-Torres, L.R.; Salas-Luevano, M.A.; Salazar-Badillo, F.B. Could Alternaria solani IA300 be a plant growth-promoting fungus? Eur. J. Plant Pathol. 2020, 157, 413–419. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; Franco, D.G.; Chaves, P.O.; Giannesi, G.C.; Masui, D.C.; Ruller, R.; Corrêa, B.O.; da Silva Brasil, M.; Zanoelo, F.F. Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere 2021, 18, 100332. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kim, Y.H.; Kang, S.M.; Lee, J.H.; Lee, I.J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011, 46, 440–447. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Garstecka, Z.; Trejgell, A.; Dąbrowski, H.P.; Konieczna, W.; Szyp-Borowska, I. The impact of forest fungi on promoting growth and development of Brassica napus L. Agronomy 2021, 11, 2475. [Google Scholar] [CrossRef]
- Velásquez, A.; Vega-Celedón, P.; Fiaschi, G.; Agnolucci, M.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza 2020, 30, 161–170. [Google Scholar] [CrossRef]
- Saldajeno, M.G.B.; Hyakumachi, M. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae stimulate plant growth and reduce severity of anthracnose and damping-off diseases in cucumber (Cucumis sativus) seedlings. Ann. Appl. Biol. 2011, 159, 28–40. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I.J. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Estaún, V.; Camprubí, A.; Calvet, C.; Pinochet, J. Nursery and field response of olive trees inoculated with two arbuscular mycorrhizal fungi, Glomus intraradices and Glomus mosseae. J. Am. Soc. Hortic. Sci. 2003, 128, 767–775. [Google Scholar] [CrossRef]
- Senthil Kumar, C.M.; Jacob, T.K.; Devasahayam, S.; Thomas, S.; Geethu, C. Multifarious plant growth promotion by an entomopathogenic fungus Lecanicillium psalliotae. Microbiol. Res. 2018, 207, 153–160. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of indoleacetic acid, gibberellic acid and ACC-deaminase by Mortierella strains promote winter wheat seedlings growth under different conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef]
- Rozpądek, P.; Domka, A.; Ważny, R.; Nosek, M.; Jędrzejczyk, R.; Tokarz, K.; Turnau, K. How does the endophytic fungus Mucor sp. improve Arabidopsis arenosa vegetation in the degraded environment of a mine dump? Environ. Exp. Bot. 2018, 147, 31–42. [Google Scholar] [CrossRef]
- Vessey, J.K.; Heisinger, K.G. Effect of Penicillium bilaii inoculation and phosphorus fertilisation on root and shoot parameters of field-grown pea. Can. J. Microbiol. 2011, 81, 361–366. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Gupta, V.V.S.R.; Harvey, P.R.; Ryder, M.H. The effect of Penicillium fungi on plant growth and phosphorus mobilization in neutral to alkaline soils from southern Australia. Can. J. Microbiol. 2007, 53, 106–115. [Google Scholar] [CrossRef]
- Elsharkawy, M.M. Induced systemic resistance against Cucumber mosaic virus by Phoma sp. GS8-2 stimulates transcription of pathogenesis-related genes in Arabidopsis. Pest. Manag. Sci. 2019, 75, 859–866. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; El-Khateeb, N.M.M. Antifungal activity and resistance induction against Sclerotium cepivorum by plant growth-promoting fungi in onion plants. Egypt. J. Biol. Pest Control 2019, 29, 68. [Google Scholar] [CrossRef]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 2020, e9005. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T. The influence of fungi of the Trichoderma genus on the flowering of Freesia refracta Klatt ‘Argentea’ in winter. Hortic. Sci. 2020, 47, 203–210. [Google Scholar] [CrossRef]
- Vinayarani, G.; Prakash, H.S. Fungal endophytes of turmeric (Curcuma longa L.) and their biocontrol potential against pathogens Pythium aphanidermatum and Rhizoctonia solani. World J. Microbiol. Biotechnol. 2018, 34, 49. [Google Scholar] [CrossRef]
- Nuangmek, W.; Aiduang, W.; Kumla, J.; Lumyong, S.; Suwannarach, N. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front. Microbiol. 2021, 12, 410. [Google Scholar] [CrossRef]
- Znajewska, Z.; Dąbrowska, G.; Narbutt, O. Trichoderma viride strains stimulating the growth and development of winter rapeseed (Brassica napus L.). Prog. Plant Prot. 2018, 58, 264–269. [Google Scholar]
- Singh, D.P.; Prabha, R.; Yandigeri, M.S.; Arora, D.K. Cyanobacteria-mediated phenylpropanoids and phytohormones in rice (Oryza sativa) enhance plant growth and stress tolerance. Anton. Leeuw. Int. J. 2011, 100, 557–568. [Google Scholar] [CrossRef]
- Prasanna, R.; Chaudhary, V.; Gupta, V.; Babu, S.; Kumar, A.; Singh, R.; Shivay, Y.S.; Nain, L. Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur. J. Plant Pathol. 2013, 136, 337–353. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89. [Google Scholar] [CrossRef]
- Babu, S.; Prasanna, R.; Bidyarani, N.; Singh, R. Analysing the colonisation of inoculated cyanobacteria in wheat plants using biochemical and molecular tools. J. Appl. Phycol. 2015, 27, 327–338. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, E.J.; Hong, J.K.; Jeun, Y.C. Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. Plant Pathol. J. 2018, 34, 113. [Google Scholar] [CrossRef]
- Mohamed Taha, T.; Youssef, M.A. Improvement of growth parameters of Zea mays and properties of soil inoculated with two Chlorella species. Rep. Opin. 2015, 7, 22–27. [Google Scholar]
- Agwa, O.K.; Ogugbue, C.J.; Williams, E.E. Growth promotion of Telfairia occidentalis by application of Chlorella vulgaris (bioinoculant) colonized seeds and soil under tropical field conditions. Am. J. Plant Sci. 2018, 9, 403–415. [Google Scholar] [CrossRef]
- Kuang, X.; Gu, J.D.; Tie, B.Q.; Yao, B.; Shao, J. Interactive effects of cadmium and Microcystis aeruginosa (cyanobacterium) on the growth, antioxidative responses and accumulation of cadmium and microcystins in rice seedlings. Ecotoxicology 2016, 25, 1588–1599. [Google Scholar] [CrossRef]
- Hussain, A.; Hamayun, M.; Shah, S.T. Root colonization and phytostimulation by phytohormones producing entophytic Nostoc sp. AH-12. Curr. Microbiol. 2013, 67, 624–630. [Google Scholar] [CrossRef]
- Maqubela, M.P.; Mnkeni, P.N.S.; Issa, O.M.; Pardo, M.T.; D’Acqui, L.P. Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant Soil 2009, 315, 79–92. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; lo Piero, A.R.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Hassani, S.B.; Aliniaeifard, S. Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. J. Plant Growth Regul. 2020, 39, 1009–1021. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Flores, A.C.; Luna, A.A.E.; Portugal, V.O. Yield and quality enhancement of marigold flowers by inoculation with Bacillus subtilis and Glomus fasciculatum. J. Sustain. Agric. 2007, 31, 21–31. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.W.; Boonlue, S. Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Carvalho, P.; Marques, G.; Ferreira, L.; Nunes, M.; Rocha, I.; Ma, Y.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Increased protein content of chickpea (Cicer arietinum L.) inoculated with arbuscular mycorrhizal fungi and nitrogen-fixing bacteria under water deficit conditions. J. Sci. Food Agric. 2017, 97, 4379–4385. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016, 192, 1–12. [Google Scholar] [CrossRef]
- Duc, N.H.; Mayer, Z.; Pék, Z.; Helyes, L.; Posta, K. Combined inoculation of arbuscular mycorrhizal fungi, Pseudomonas fluorescens and Trichoderma spp. for enhancing defense enzymes and yield of three pepper cultivars. Appl. Ecol. Environ. Res. 2017, 15, 1815–1829. [Google Scholar] [CrossRef]
- Pandey, A.; Yarzábal, L.A. Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments. Appl. Microbiol. Biotechnol. 2019, 103, 643–657. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. PNAS 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent. Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Middleton, H.; Yergeau, É.; Monard, C.; Combier, J.-P.; el Amrani, A. Rhizospheric plant–microbe interactions: miRNAs as a key mediator. Trends Plant Sci. 2021, 26, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, A.; Malusà, E.; Costa, C.; Pallottino, F.; Mocali, S.; Pinzari, F.; Canfora, L. Current methods, common practices, and perspectives in tracking and monitoring bioinoculants in soil. Front. Microbiol. 2021, 12, 698491. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, M.B.; Meera, M.S.; Hyakumachi, M. Sterile fungi from zoysiagrass rhizosphere as plant growth promoters in spring wheat. Can. J. Microbiol. 1994, 40, 637–644. [Google Scholar] [CrossRef]
- Dąbrowska, G.; Hrynkiewicz, K.; Mierek-Adamska, A.; Goc, A. The sensitivity of spring and winter varieties of oilseed rape to heavy metals and rhizobacteria. Oilseed Crop. 2012, 33, 201–220. [Google Scholar]
- Gnanamanickam, S.S.; Immanuel, J.E. Epiphytic bacteria, their ecology and functions. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 131–153. [Google Scholar]
- Kucharska, K.; Wachowska, U. The microbiome on the leaves of crop plants. Adv. Microbiol. 2014, 53, 352–359. [Google Scholar]
- Martins, G.; Lauga, B.; Miot-Sertier, C.; Mercier, A.; Lonvaud, A.; Soulas, M.-L.; Soulas, G.; Masneuf-Pomarède, I. Characterization of epiphytic bacterial communities from grapes, leaves, bark and soil of grapevine plants grown, and their relations. PLoS ONE 2013, 8, e73013. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Gong, T.; Xin, X. Phyllosphere microbiota: Community dynamics and its interaction with plant hosts. J. Integr. Plant Biol. 2021, 63, 297–304. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- You, Y.-H.; Yoon, H.; Kang, S.-M.; Shin, J.-H.; Choo, Y.-S.; Lee, I.-J.; Lee, J.-M.; Kim, J.-G. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Bergottini, V.M.; Otegui, M.B.; Sosa, D.A.; Zapata, P.D.; Mulot, M.; Rebord, M.; Zopfi, J.; Wiss, F.; Benrey, B.; Junier, P. Bio-inoculation of yerba mate seedlings (Ilex paraguariensis St. Hill.) with native plant growth-promoting rhizobacteria: A sustainable alternative to improve crop yield. Biol. Fertil. Soils 2015, 51, 749–755. [Google Scholar] [CrossRef]
- Poonguzhali, S.; Madhaiyan, M.; Sa, T. Isolation and identification of phosphate solubilizing bacteria from chinese cabbage and their effect on growth and phosphorus utilization of plants. J. Microbiol. Biotechnol. 2008, 18, 773–777. [Google Scholar] [PubMed]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Reed, M.L.E.; Glick, B.R. Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can. J. Microbiol. 2005, 51, 1061–1069. [Google Scholar] [CrossRef]
- Jousset, A.; Rochat, L.; Lanoue, A.; Bonkowski, M.; Keel, C.; Scheu, S. Plants respond to pathogen infection by enhancing the antifungal gene expression of root-associated bacteria. Mol. Plant-Microbe Interact. 2011, 24, 352–358. [Google Scholar] [CrossRef]
- Schirmböck, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, G.E.; Kubicek, C.P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef]
- Crowley, D.E. Microbial siderophores in the plant rhizosphere. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadia, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 169–198. [Google Scholar]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 466. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Ardanov, P.; Lyastchenko, S.; Karppinen, K.; Häggman, H.; Kozyrovska, N.; Pirttilä, A.M. Effects of Methylobacterium sp. on emergence, yield, and disease prevalence in three cultivars of potato (Solanum tuberosum L.) were associated with the shift in endophytic microbial community. Plant Soil 2016, 405, 299–310. [Google Scholar] [CrossRef]
- Lynch, J.M.; Leij, F. Rhizosphere. In eLS.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; Watt, M. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS ONE 2016, 11, e0164533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Zhang, T.L.; Wang, X.X.; Hua, K.; Zhao, L.; Han, Z.M. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int. J. Biol. Sci. 2013, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Lucas García, J.A.; Barbas, C.; Probanza, A.; Barrientos, M.L.; Gutierrez Mañero, F.J. Low molecular weight organic acids and fatty acids in root exudates of two Lupinus cultivars at flowering and fruiting stages. Phytochem. Anal. 2001, 12, 305–311. [Google Scholar] [CrossRef]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, F.M.; Santos, I.S.; da Cunha, M.; Carvalho, A.O.; Gomes, V.M. Antimicrobial proteins from cowpea root exudates: Inhibitory activity against Fusarium oxysporum and purification of a chitinase-like protein. Plant Soil 2005, 272, 223–232. [Google Scholar] [CrossRef]
- Schönwitz, R.; Ziegler, H. Exudation of water-soluble vitamins and of some carbohydrates by intact roots of maize seedlings (Zea mays L.) into a mineral nutrient solution. Z. Pflanzenphysiol. 1982, 107, 7–14. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Z.; Bian, J.; Bo, T.; Liu, Y. Chemotaxis response of Meloidogyne incognita to volatiles and organic acids from root exudates. Rhizosphere 2021, 17, 100320. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef]
- Bekkara, F.; Jay, M.; Viricel, M.R.; Rome, S. Distribution of phenolic compounds within seed and seedlings of two Vicia faba cvs differing in their seed tannin content, and study of their seed and root phenolic exudations. Plant Soil 1998, 203, 27–36. [Google Scholar] [CrossRef]
- Watt, M.; Evans, J.R. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiol. 1999, 120, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Tawaraya, K.; Horie, R.; Saito, A.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of shoot extracts, root extracts, and root exudates of rice plant under phosphorus deficiency. J. Plant Nutr. 2013, 36, 1138–1159. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014, 60, 679–694. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.R.; Borriss, R.; von Wirén, N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef]
- Wang, E.L.H.; Bergeson, G.B. Biochemical changes in root exudate and xylem sap of tomato plants infected with Meloidogyne incognita. J. Nematol. 1974, 6, 194. [Google Scholar]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa l. Cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Guttman, D.S.; McHardy, A.C.; Schulze-Lefert, P. Microbial genome-enabled insights into plant–microorganism interactions. Nat. Rev. Genet. 2014, 15, 797–813. [Google Scholar] [CrossRef]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Virk, A.L.; Lin, B.-J.; Kan, Z.-R.; Qi, J.-Y.; Dang, Y.P.; Lal, R.; Zhao, X.; Zhang, H.-L. Simultaneous effects of legume cultivation on carbon and nitrogen accumulation in soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 171, pp. 75–110. [Google Scholar]
- Stajković, O.; Delić, D.; Jošić, D.; Kuzmanović, D.; Rasulić, N.; Knežević-Vukčević, J. Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom. Biotechnol. Lett. 2011, 16, 5919–5926. [Google Scholar]
- Rana, A.; Kabi, S.R.; Verma, S.; Adak, A.; Pal, M.; Shivay, Y.S.; Prasanna, R.; Nain, L. Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro- and micronutrients in grains in rice–wheat cropping sequence. Cogent Food. Agric. 2015, 1, 1037379. [Google Scholar] [CrossRef]
- Morales, A.; Alvear, M.; Valenzuela, E.; Rubio, R.; Borie, F. Effect of inoculation with Penicillium albidum, a phosphate-solubilizing fungus, on the growth of Trifolium pratense cropped in a volcanic soil. J. Basic Microbiol. 2007, 47, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S.; Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Aledan, T.R.; Ali, A.K.; Lu, G.; Zhang, X.; Wu, J.; Fu, C.; Li, M. Detecting the hormonal pathways in oilseed rape behind induced systemic resistance by Trichoderma harzianum TH12 to Sclerotinia sclerotiorum. PLoS ONE 2017, 12, e0168850. [Google Scholar] [CrossRef]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.-J.; Yuan, B.; Xu, P.-Y.; Xing, K.; Wang, J.; Jiang, J.-H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Rafique, H.M.; Khan, M.Y.; Asghar, H.N.; Ahmad Zahir, Z.; Nadeem, S.M.; Sohaib, M.; Alotaibi, F.; Al-Barakah, F.N.I. Converging alfalfa (Medicago sativa L.) and petroleum hydrocarbon acclimated ACC-deaminase containing bacteria for phytoremediation of petroleum hydrocarbon contaminated soil. Int. J. Phytoremediation 2022. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Gobbato, E.; Wang, E.; Higgins, G.; Bano, S.A.; Henry, C.; Schultze, M.; Oldroyd, G.E.D. RAM1 and RAM2 function and expression during arbuscular mycorrhizal symbiosis and Aphanomyces euteiches colonization. Plant Signal. Behav. 2013, 8, e26049. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, N.D.; Chowdhary, P.K.; Haines, D.C.; González, J.E. L-canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, L.V.; Azarova, T.S.; Leonova-Erko, E.I.; Shaposhnikov, A.I.; Makarova, N.M.; Tikhonovich, I.A. Root exudates of tomato plants and their effect on the growth and antifungal activity of Pseudomonas strains. Microbiology 2003, 72, 37–41. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Jiménez Bremont, J.; Marina, M.; de la Luz Guerrero-González, M.; Rossi, F.; Sánchez-Rangel, D.; Rodríguez-Kessler, M.; Ruiz, O.; Gárriz, A. Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front. Plant Sci. 2014, 5, 95. [Google Scholar] [CrossRef]
- Liu, Z.; Beskrovnaya, P.; Melnyk, R.A.; Hossain, S.S.; Khorasani, S.; O’Sullivan, L.R.; Wiesmann, C.L.; Bush, J.; Richard, J.D.; Haney, C.H. A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. mBio 2018, 9, e00433-18. [Google Scholar] [CrossRef]
- Nelson, M.S.; Sadowsky, M.J. Secretion systems and signal exchange between nitrogen-fixing rhizobia and legumes. Front. Plant Sci. 2015, 6, 491. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Hugoni, M.; Luis, P.; Guyonnet, J.; el Zahar Haichar, F. Plant host habitat and root exudates shape fungal diversity. Mycorrhiza 2018, 28, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Dennis, P.G.; Forstner, C.; Raghavendra, A.K.H.; Mathesius, U.; Richardson, A.E.; Delhaize, E.; Gilliham, M.; Watt, M.; Ryan, P.R. Manipulating exudate composition from root apices shapes the microbiome throughout the root system. Plant Physiol. 2021, 187, 2279–2295. [Google Scholar] [CrossRef]
- Kudjordjie, E.N.; Sapkota, R.; Nicolaisen, M. Arabidopsis assemble distinct root-associated microbiomes through the synthesis of an array of defense metabolites. PLoS ONE 2021, 16, e0259171. [Google Scholar] [CrossRef]
- Qu, Q.; Li, Y.; Zhang, Z.; Cui, H.; Zhao, Q.; Liu, W.; Lu, T.; Qian, H. Effects of S-metolachlor on wheat (Triticum aestivum L.) seedling root exudates and the rhizosphere microbiome. J. Hazard. Mater. 2021, 411, 125137. [Google Scholar] [CrossRef]
- Weisskopf, L.; Abou-Mansour, E.; Fromin, N.; Tomasi, N.; Santelia, D.; Edelkott, I.; Neumann, G.; Aragno, M.; Tabacchi, R.; Martinoia, E. White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ. 2006, 29, 919–927. [Google Scholar] [CrossRef]
- EL Zahar Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Cai, T.; Cai, W.; Zhang, J.; Zheng, H.; Tsou, A.M.; Xiao, L.; Zhong, Z.; Zhu, J. Host legume-exuded antimetabolites optimize the symbiotic rhizosphere. Mol. Microbiol. 2009, 73, 507–517. [Google Scholar] [CrossRef]
- Mardani-Korrani, H.; Nakayasu, M.; Yamazaki, S.; Aoki, Y.; Kaida, R.; Motobayashi, T.; Kobayashi, M.; Ohkama-Ohtsu, N.; Oikawa, Y.; Sugiyama, A.; et al. L-canavanine, a root exudate from hairy vetch (Vicia villosa) drastically affecting the soil microbial community and metabolite pathways. Front. Microbiol. 2021, 12, 701796. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; van Bentum, S.; van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. PNAS 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Baune, M.; Kang, K.; Schenkeveld, W.D.C.; Kraemer, S.M.; Hayen, H.; Weber, G. Importance of oxidation products in coumarin-mediated Fe(hydr)oxide mineral dissolution. BioMetals 2020, 33, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Toljander, J.F.; Lindahl, B.D.; Paul, L.R.; Elfstrand, M.; Finlay, R.D. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol. Ecol. 2007, 61, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Quintana, N.; el Kassis, E.G.; Kim, H.K.; Choi, Y.H.; Sugiyama, A.; Verpoorte, R.; Martinoia, E.; Manter, D.K.; Vivanco, J.M. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol. 2009, 151, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Volkov, V.; Schwenke, H. A quest for mechanisms of plant root exudation brings new results and models, 300 years after hales. Plants 2020, 10, 38. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef]
- Dietz, S.; von Bülow, J.; Beitz, E.; Nehls, U. The aquaporin gene family of the ectomycorrhizal fungus Laccaria bicolor: Lessons for symbiotic functions. N. Phytol. 2011, 190, 927–940. [Google Scholar] [CrossRef]
- Hwang, J.H.; Ellingson, S.R.; Roberts, D.M. Ammonia permeability of the soybean nodulin 26 channel. FEBS Lett. 2010, 584, 4339–4343. [Google Scholar] [CrossRef]
- Masalkar, P.; Wallace, I.S.; Hwang, J.H.; Roberts, D.M. Interaction of cytosolic glutamine synthetase of soybean root nodules with the C-terminal domain of the symbiosome membrane nodulin 26 aquaglyceroporin. J. Biol. Chem. 2010, 285, 23880–23888. [Google Scholar] [CrossRef] [PubMed]
- Janczak, K.; Dąbrowska, G.; Znajewska, Z.; Hrynkiewicz, K. Effect of bacterial inoculation on the growth of miscanthus and bacterial and fungal density in the polymer-containing soil. Part 2. Non-biodegradable polymers. Chem. Ind. 2014, 93, 2222–2225. [Google Scholar]
- Janczak, K.; Dąbrowska, G.; Znajewska, Z.; Hrynkiewicz, K. Effect of bacterial inoculation on the growth of miscanthus and bacterial and fungal density in the polymer-containing soil. Part 1. Biodegradable polymers. Chem. Ind. 2014, 93, 2218–2221. [Google Scholar]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytoremediation 2012, 14, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 2011, 159, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Louvel, B.; Cébron, A.; Leyval, C. Root exudates affect phenanthrene biodegradation, bacterial community and functional gene expression in sand microcosms. Int. Biodeterior. Biodegrad. 2011, 65, 947–953. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Feng, G.; Du, L.; Zeng, D. Biodegradation of diuron by an endophytic fungus Neurospora intermedia DP8-1 isolated from sugarcane and its potential for remediating diuron-contaminated soils. PLoS ONE 2017, 12, e0182556. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; El-Ghany Tm, A.; Masmali, I.A. Fungal biodegradation of organophosphorus insecticides and their impact on soil microbial population. J. Plant Pathol. Microbiol. 2016, 7, 1000349. [Google Scholar]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5120. [Google Scholar] [CrossRef]
- Garbeva, P.; Silby, M.W.; Raaijmakers, J.M.; Levy, S.B.; de Boer, W. Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competitors. ISME J. 2011, 5, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Arendt, K.R.; Hockett, K.L.; Araldi-Brondolo, S.J.; Baltrus, D.A.; Arnold, A.E. Isolation of endohyphal bacteria from foliar Ascomycota and in vitro establishment of their symbiotic associations. Appl. Environ. Microbiol. 2016, 82, 2943–2949. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Gunatilaka, M.K.; Wijeratne, K.; Gunatilaka, L.; Arnold, A.E. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 2013, 8, e73132. [Google Scholar] [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yan, B.; Zhao, H.; Liu, F.; Zhang, H.; Sheng, Q.; Miao, F.; Liang, Z. Core microbiome of medicinal plant Salvia miltiorrhiza seed: A rich reservoir of beneficial microbes for secondary metabolism? Int. J. Mol. Sci. 2018, 19, 672. [Google Scholar] [CrossRef]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves: Biogeography of phyllosphere bacterial communities. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef]
- Germida, J.; Siciliano, S. Taxonomic diversity of bacteria associated with the roots of modern, recent and ancient wheat cultivars. Biol. Fertil. Soils 2001, 33, 410–415. [Google Scholar]
- Churchland, C.; Grayston, S.J. Specificity of plant-microbe interactions in the tree mycorrhizosphere biome and consequences for soil C cycling. Front. Microbiol. 2014, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, L.C.; Trappe, J.M.; Molina, R.; Spatafora, J.W. Biology of the ectomycorrhizal genus Rhizopogon. VI. Re-examination of infrageneric relationships inferred from phylogenetic analyses of ITS sequences. Mycologia 2002, 94, 607–619. [Google Scholar] [CrossRef]
- Young, J.P.W.; Johnston, A.W.B. The evolution of specificity in the legume-rhizobium symbiosis. Trends Ecol. Evol. 1989, 4, 341–349. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Cernava, T.; Berg, C.; Berg, G. Bog ecosystems as a playground for plant–microbe coevolution: Bryophytes and vascular plants harbour functionally adapted bacteria. Microbiome 2021, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Cobian, G.M.; Egan, C.P.; Amend, A.S. Plant–microbe specificity varies as a function of elevation. ISME J. 2019, 13, 2778–2788. [Google Scholar] [CrossRef]
- Salas-González, I.; Reyt, G.; Flis, P.; Custódio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Sessitsch, A. Plant–microbe partnerships in 2020. Microb. Biotechnol. 2016, 9, 635–640. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Wisniewski, M.; Schena, L.; Tack, A.J.M. Experimental evidence of microbial inheritance in plants and transmission routes from seed to phyllosphere and root. Environ. Microbiol. 2021, 23, 2199–2214. [Google Scholar] [CrossRef]
- Sánchez-López, A.S.; Pintelon, I.; Stevens, V.; Imperato, V.; Timmermans, J.P.; González-Chávez, C.; Carrillo-González, R.; van hamme, J.; Vangronsveld, J.; Thijs, S. Seed endophyte microbiome of Crotalaria pumila unpeeled: Identification of plant-beneficial methylobacteria. Int. J. Mol. Sci. 2018, 19, 291. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. N. Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.S.C.; Okura, V.K.; Armanhi, J.S.L.; Jorrín, B.; Lozano, N.; da Silva, M.J.; González-Guerrero, M.; de Araújo, L.M.; Verza, N.C.; Bagheri, H.C.; et al. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci. Rep. 2016, 6, 28774. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; McManus, P.S.; Handelsman, J. Unexpected diversity during community succession in the apple flower microbiome. mBio 2013, 4, e00602-12. [Google Scholar] [CrossRef] [PubMed]
- Wipf, H.M.L.; Bùi, T.N.; Coleman-Derr, D. Distinguishing between the impacts of heat and drought stress on the root microbiome of Sorghum bicolor. Phytobiomes J. 2021, 5, 166–176. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Anderson-Furgeson, J.; Estera, K.Y.; Pett-Ridge, J.; de Valpine, P.; Brodie, E.L.; Firestone, M.K. Climate and edaphic controllers influence rhizosphere community assembly for a wild annual grass. Ecology 2016, 97, 1307–1318. [Google Scholar] [CrossRef]
- Gargouri, M.; Karray, F.; Chebaane, A.; Mhiri, N.; Partida-Martínez, L.P.; Sayadi, S.; Mliki, A. Increasing aridity shapes beta diversity and the network dynamics of the belowground fungal microbiome associated with Opuntia ficus-indica. Sci. Total Environ. 2021, 773, 145008. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.; Li, Q.; Xu, W.; Wang, J.; Hu, J.; Zhang, Z. Soil nutrient levels determine the variation of bacterial communities in the rhizosphere of rice under different conditions of climate and genotype. Appl. Soil Ecol. 2021, 167, 104025. [Google Scholar] [CrossRef]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Gontia-Mishra, I.; Sapre, S.; Deshmukh, R.; Sikdar, S.; Tiwari, S. Microbe-mediated drought tolerance in plants: Current developments and future challenges. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 351–379. [Google Scholar]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef]
- Wei, F.; Feng, H.; Zhang, D.; Feng, Z.; Zhao, L.; Zhang, Y.; Deakin, G.; Peng, J.; Zhu, H.; Xu, X. Composition of rhizosphere microbial communities associated with healthy and Verticillium wilt diseased cotton plants. Front. Microbiol. 2021, 12, 618169. [Google Scholar] [CrossRef]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The rhizosphere microbiome plays a role in the resistance to soil-borne pathogens and nutrient uptake of strawberry cultivars under field conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef]
- Kong, H.G.; Kim, B.K.; Song, G.C.; Lee, S.; Ryu, C.-M. Aboveground whitefly infestation-mediated reshaping of the root microbiota. Front. Microbiol. 2016, 7, 1314. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Neu, A.T.; Allen, E.E.; Roy, K. Defining and quantifying the core microbiome: Challenges and prospects. Proc. Natl. Acad. Sci. USA 2021, 118, e2104429118. [Google Scholar] [CrossRef]
- Lay, C.-Y.; Bell, T.H.; Hamel, C.; Harker, K.N.; Mohr, R.; Greer, C.W.; Yergeau, É.; St-Arnaud, M. Canola root–associated microbiomes in the canadian prairies. Front. Microbiol. 2018, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L.; Fester, R. Symbiosis as a source of evolutionary innovation: Speciation and morphogenesis. In Cambridge Mass; MIT Press: Cambridge, MA, USA, 1991; pp. 1–14. [Google Scholar]
- Teixeira, P.J.P.; Colaianni, N.R.; Fitzpatrick, C.R.; Dangl, J.L. Beyond pathogens: Microbiota interactions with the plant immune system. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019, 2019, 9106395. [Google Scholar] [CrossRef] [PubMed]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L.; Rossi, F.R.; Gonzalez, M.E.; Vignatti, P.; Gárriz, A. Gaining insight into plant responses to beneficial and pathogenic microorganisms using metabolomic and transcriptomic approaches. In Metabolic Engineering for Bioactive Compounds: Strategies and Processes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–140. [Google Scholar]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Jubic, L.M.; Saile, S.; Furzer, O.J.; el Kasmi, F.; Dangl, J.L. Help wanted: Helper NLRs and plant immune responses. Curr. Opin. Plant Biol. 2019, 50, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Block, A.; Li, G.; Fu, Z.Q.; Alfano, J.R. Phytopathogen type III effector weaponry and their plant targets. Curr. Opin. Plant Biol. 2008, 11, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J.; Nürnberger, T.; Joosten, M.H.A.J. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Teixeira, P.J.P.L.; Colaianni, N.R.; Law, T.F.; Conway, J.M.; Gilbert, S.; Li, H.; Salas-González, I.; Panda, D.; del Risco, N.M.; Finkel, O.M.; et al. Specific modulation of the root immune system by a community of commensal bacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2100678118. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Zhou, F.; Emonet, A.; Dénervaud Tendon, V.; Marhavy, P.; Wu, D.; Lahaye, T.; Geldner, N. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 2020, 180, 440–453.e18. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Sun, J.; Li, Y.; Chen, J. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Adhikari, P.; Pandey, A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 2019, 9, 2–9. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.Q. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.W.; Girlanda, M.; Grigoriev, I.V.; Martin, F.; et al. Fungal and plant gene expression in the Tulasnella calospora–Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. N. Phytol. 2017, 213, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. N. Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Gujar, P.D.; Bhavsar, K.P.; Khire, J.M. Effect of phytase from Aspergillus niger on plant growth and mineral assimilation in wheat (Triticum aestivum Linn.) and its potential for use as a soil amendment. J. Sci. Food Agric. 2013, 93, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Viterbo, A.; Chet, I. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 2006, 7, 249–258. [Google Scholar] [CrossRef]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef]
- Helber, N.; Wippel, K.; Sauer, N.; Schaarschmidt, S.; Hause, B.; Requena, N. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell 2011, 23, 3812–3823. [Google Scholar] [CrossRef]
- Viterbo, A.; Wiest, A.; Brotman, Y.; Chet, I.; Kenerley, C. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Mol. Plant Pathol. 2007, 8, 737–746. [Google Scholar] [CrossRef]
- Rubio, M.B.; Hermosa, R.; Reino, J.L.; Collado, I.G.; Monte, E. Thctf1 transcription factor of Trichoderma harzianum is involved in 6-pentyl-2H-pyran-2-one production and antifungal activity. Fungal Genet. Biol. 2009, 46, 17–27. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Brotman, Y.; Briff, E.; Viterbo, A.; Chet, I. Role of swollenin, an expansin-like protein from Trichoderma, in plant root colonization. Plant Physiol. 2008, 147, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.A.; Djonović, S.; Sukno, S.A.; Kenerley, C.M. Dimerization controls the activity of fungal elicitors that trigger systemic resistance in plants. JBC 2008, 283, 19804–19815. [Google Scholar] [CrossRef] [PubMed]
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889. [Google Scholar] [CrossRef]
- Nelkner, J.; Tejerizo, G.T.; Hassa, J.; Lin, T.W.; Witte, J.; Verwaaijen, B.; Winkler, A.; Bunk, B.; Spröer, C.; Overmann, J.; et al. Genetic potential of the biocontrol agent Pseudomonas brassicacearum (Formerly P. trivialis) 3Re2-7 unraveled by genome sequencing and mining, comparative genomics and transcriptomics. Genes 2019, 10, 601. [Google Scholar] [CrossRef]
- Glick, B.R.; Jacobson, C.B.; Schwarze, M.K.; Pasternak, J.J. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can. J. Microbiol. 2011, 40, 911–915. [Google Scholar] [CrossRef]
- Blaha, D.; Prigent-Combaret, C.; Mirza, M.S.; Moënne-Loccoz, Y. Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol. Ecol. 2006, 56, 455–470. [Google Scholar] [CrossRef]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef]
- Suryadi, Y.; Susilowati, D.N.; Lestari, P.; Priyatno, T.P.; Samudra, I.M.; Hikmawati, N.; Mubarik, D.N.R.; Mubarik, N.R. Characterization of bacterial isolates producing chitinase and glucanase for biocontrol of plant fungal pathogens. J. Agric. Technol. 2014, 10, 983–999. [Google Scholar]
- Gupta, C.P.; Kumar, B.; Dubey, R.C.; Maheshwari, D.K. Chitinase-mediated destructive antagonistic potential of Pseudomonas aeruginosa GRC1 against Sclerotinia sclerotiorum causing stem rot of peanut. BioControl 2006, 51, 821–835. [Google Scholar] [CrossRef]
- Ramette, A.; Frapolli, M.; Défago, G.; Moënne-Loccoz, Y. Phylogeny of HCN synthase-encoding hcnBC genes in biocontrol fluorescent pseudomonads and its relationship with host plant species and HCN synthesis ability. Mol. Plant Microbe. Interact. 2003, 16, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, W.; Hundeshagen, B.; Niederau, E. Demonstration of the indolepyruvate decarboxylase gene homologue in different auxin-producing species of the Enterobacteriaceae. Can. J. Microbiol. 1994, 40, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Ryu, R.J.; Patten, C.L. Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in Enterobacter cloacae UW5. J. Bacteriol. 2008, 190, 7200–7208. [Google Scholar] [CrossRef]
- Spaepen, S.; Versées, W.; Gocke, D.; Pohl, M.; Steyaert, J.; Vanderleyden, J. Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J. Bacteriol. 2007, 189, 7626. [Google Scholar] [CrossRef]
- Poza-Carrión, C.; Jiménez-Vicente, E.; Navarro-Rodríguez, M.; Echavarri-Erasun, C.; Rubio, L.M. Kinetics of nif gene expression in a nitrogen-fixing bacterium. J. Bacteriol. 2014, 196, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Satyanarayana, S.D.V.; Krishna, M.S.R.; Pavan Kumar, P.; Jeereddy, S. In silico structural homology modeling of nif A protein of rhizobial strains in selective legume plants. J. Genet. Eng. Biotechnol. 2018, 16, 731–737. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-ściseł, J. Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the Biolog GEN III MicroPlateTM Test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef]
- Sherathia, D.; Dey, R.; Thomas, M.; Dalsania, T.; Savsani, K.; Pal, K.K. Biochemical and molecular characterization of DAPG-producing plant growthpromoting rhizobacteria (PGPR) of groundnut (Arachis hypogaea L.). Legum. Res. Int. J. 2016, 39, 614–622. [Google Scholar]
- Lynch, D.; O’Brien, J.; Welch, T.; Clarke, P.; Cuív, P.O.; Crosa, J.H.; O’Connell, M. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 2001, 183, 2576–2585. [Google Scholar] [CrossRef]
- Dong, H.; Beer, S.V. Riboflavin induces disease resistance in plants by activating a novel signal transduction pathway. Phytopathology 2000, 90, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.S.; Wu, H.J.; Zang, H.Y.; Wu, L.M.; Zhu, Q.Q.; Gao, X.W. Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 354. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant. 2020, 42, 42. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, R.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Voloshina, M.; Meena, M. Trichoderma viride—Mediated modulation of oxidative stress network in potato challenged with Alternaria solani. J. Plant Growth Regul. 2022, 1–18. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Singh, S.; Singh, H.B. Microbial consortium-induced changes in oxidative stress markers in pea plants challenged with Sclerotinia sclerotiorum. J. Plant Growth Regul. 2013, 32, 388–398. [Google Scholar] [CrossRef]
- Singh, S.P.; Gupta, R.; Gaur, R.; Srivastava, A.K. Streptomyces spp. alleviate Rhizoctonia solani-mediated oxidative stress in Solanum lycopersicon. Ann. Appl. Biol. 2016, 168, 232–242. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Mierek-Adamska, A.; Kotowicz, K.; Goc, A.; Boniecka, J.; Berdychowska, J.; Dąbrowska, G.B. Potential involvement of rapeseed (Brassica napus L.) metallothioneins in the hydrogen peroxide-induced regulation of seed vigour. J. Agron. Crop Sci. 2019, 205, 598–607. [Google Scholar] [CrossRef]
- Hassinen, V.H.; Tervahauta, A.I.; Schat, H.; Kärenlampi, S.O. Plant metallothioneins-metal chelators with ROS scavenging activity? Plant Biol. 2011, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Cassán, F.; Perrig, D.; Sgroy, V.; Masciarelli, O.; Penna, C.; Luna, V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur. J. Soil Biol. 2009, 45, 28–35. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef]
- Timmusk, S.; Nicander, B.; Granhall, U.; Tillberg, E. Cytokinin production by Paenibacillus polymyxa. Soil Biol. Biochem. 1999, 31, 1847–1852. [Google Scholar] [CrossRef]
- García de Salamone, I.E.; Hynes, R.K.; Nelson, L.M. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can. J. Microbiol. 2011, 47, 404–411. [Google Scholar] [CrossRef]
- Sorokan, A.; Veselova, S.; Benkovskaya, G.; Maksimov, I. Endophytic strain Bacillus subtilis 26D increases levels of phytohormones and repairs growth of potato plants after Colorado potato beetle damage. Plants 2021, 10, 923. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Gupta, S.; Kumar, M.; Yadav, S.; Chauhan, P.S.; Nautiyal, C.S. Southern blight disease of tomato control by 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing Paenibacillus lentimorbus B-30488. Plant Signal. Behav. 2016, 11, e1113363. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2013, 58, 427–433. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. ACC deaminase-producing rhizosphere bacteria modulate plant responses to flooding. J. Ecol. 2017, 105, 979–986. [Google Scholar] [CrossRef]
- Carlos, M.-H.J.; Stefani, P.-V.Y.; Janette, A.-M.; Melani, M.-S.S.; Gabriela, P.-O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016, 188–189, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant. Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Kafle, A.; Cope, K.; Raths, R.; Krishna Yakha, J.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef]
- Linu, M.S.; Stephen, J.; Jisha, M.S. Phosphate solubilizing Gluconacetobacter sp., Burkholderia sp. and their potential interaction with cowpea (Vigna unguiculata (L.) Walp.). Int. J. Agric. Res. 2009, 4, 79–87. [Google Scholar] [CrossRef]
- Selvakumar, G.; Mohan, M.; Kundu, S.; Gupta, A.D.; Joshi, P.; Nazim, S.; Gupta, H.S. Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo). Lett. Appl. Microbiol. 2008, 46, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Sindhu, S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Javaid, A.; Shoaib, A.; Khan, I.H. Effect of soil amendment with Chenopodium album dry biomass and two Trichoderma species on growth of chickpea var. Noor 2009 in Sclerotium rolfsii contaminated soil. Egypt. J. Biol. Pest Control 2020, 30, 102. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Chen, W.; Huang, Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol. J. 2017, 34, 873–880. [Google Scholar] [CrossRef]
- Basak, B.B.; Biswas, D.R. Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 2009, 317, 235–255. [Google Scholar] [CrossRef]
- Raji, M.; Thangavelu, M. Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch. Microbiol. 2021, 203, 3147–3161. [Google Scholar] [CrossRef]
- Bityutskii, N.; Yakkonen, K.; Petrova, A.; Nadporozhskaya, M. Xylem sap mineral analyses as a rapid method for estimation plant-availability of Fe, Zn and Mn in carbonate soils: A case study in cucumber. J. Soil Sci. Plant Nutr. 2017, 17, 279–290. [Google Scholar] [CrossRef][Green Version]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Rani, N.; Kaur, S.; Nitu, R.; Rajinder, K.; Sukhminderjit, K. Zinc solubilizing bacteria to augment soil fertility—A comprehensive review. Int. Microbiol. 2020, 8, 38–44. [Google Scholar]
- Li, R.-X.; Cai, F.; Pang, G.; Shen, Q.-R.; Li, R.; Chen, W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Prasanna, R.; Kar, A.; Singh, A.M.; Saxena, A.K. Prospecting endophytes from different Fe or Zn accumulating wheat genotypes for their influence as inoculants on plant growth, yield, and micronutrient content. Ann. Microbiol. 2018, 68, 815–833. [Google Scholar] [CrossRef]
- Öǧüt, M.; Er, F. Micronutrient composition of field-grown dry bean and wheat inoculated with Azospirillum and Trichoderma. J. Plant Nutr. Soil Sci. 2006, 169, 699–703. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Armada, E.; López-Castillo, O.M.; Cornejo, P.; Mora, M.L.; Azcón, R. Inoculation with selenobacteria and arbuscular mycorrhizal fungi to enhance selenium content in lettuce plants and improve tolerance against drought stress. J. Soil Sci. Plant Nutr. 2016, 16, 211–225. [Google Scholar] [CrossRef][Green Version]
- De Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.; Twyman, R.M.; Zhu, C.; Farré, G.; Serrano, J.C.; Portero-Otin, M.; Muñoz, P.; Sandmann, G.; Capell, T.; Christou, P. Biofortification of crops with nutrients: Factors affecting utilization and storage. Curr. Opin. Biotechnol. 2017, 44, 115–123. [Google Scholar] [CrossRef]
- Khush, G.S.; Lee, S.; Cho, J.I.; Jeon, J.S. Biofortification of crops for reducing malnutrition. Plant Biotechnol. Rep. 2012, 6, 195–202. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Bonaterra, A.; Montesinos, E. Mechanisms of antagonism of Pseudomonas fluorescens EPS62e against Erwinia amylovora, the casual agent of fire blight. Int. Microbiol. 2007, 10, 123132. [Google Scholar]
- Kinsella, K.; Schulthess, C.P.; Morris, T.F.; Stuart, J.D. Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol. Biochem. 2009, 41, 374–379. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kurze, S.; Bahl, H.; Dahl, R.; Berg, G. Biological control of fungal strawberry diseases by Serratia plymuthica HRO-C48. Plant Dis. 2001, 85, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant Pathol. 2007, 71, 201–209. [Google Scholar] [CrossRef]
- Egusa, M.; Matsui, H.; Urakami, T.; Okuda, S.; Ifuku, S.; Nakagami, H.; Kaminaka, H. Chitin nanofiber elucidates the elicitor activity of polymeric chitin in plants. Front. Plant Sci. 2015, 6, 1098. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Giangrande, C.; Amoresano, A.; Pucci, P.; Molinaro, A.; Bertini, L.; Caporale, C.; Caruso, C. Xanthomonas campestris lipooligosaccharides trigger innate immunity and oxidative burst in Arabidopsis. Plant Physiol. Biochem. 2014, 85, 51–62. [Google Scholar] [CrossRef][Green Version]
- Nowak, A.; Tyśkiewicz, R.; Wiater, A.; Jaroszuk-ściseł, J. (1→3)-α-D-glucooligosaccharides as elicitors influencing the activity of plant resistance pathways in wheat tissues. Agronomy 2022, 12, 1170. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Francis, J.; Bowrin, V.; Ramjegathesh, R.; Ramsubhag, A.; Jayaraman, J. Bio-efficacy of a chitosan based elicitor on Alternaria solani and Xanthomonas vesicatoria infections in tomato under tropical conditions. Ann. Appl. Biol. 2016, 169, 274–283. [Google Scholar] [CrossRef]
- Qiu, H.; Su, L.; Wang, H.; Zhang, Z. Chitosan elicitation of saponin accumulation in Psammosilene tunicoides hairy roots by modulating antioxidant activity, nitric oxide production and differential gene expression. Plant Physiol. Biochem. 2021, 166, 115–127. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Cashel, M.; Gallant, J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 1969, 221, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Boniecka, J.; Prusińska, J.; Dąbrowska, G.B.; Goc, A. Within and beyond the stringent response-RSH and (p)ppGpp in plants. Planta 2017, 246, 817–842. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.; Prusińska, J.; Goc, A. The stringent response—Bacterial mechanism of an adaptive stress response. Adv. Biochem. 2006, 52, 87–93. [Google Scholar]
- Berdychowska, J.; Boniecka, J.; Dąbrowska, G.B. The stringent response and its involvement in the reactions of bacterial cells to stress. Adv. Microbiol. 2019, 58, 127–142. [Google Scholar] [CrossRef]
- Irving, S.E.; Corrigan, R.M.Y.R. Triggering the stringent response: Signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology 2018, 164, 268–276. [Google Scholar] [CrossRef]
- Irving, S.E.; Choudhury, N.R.; Corrigan, R.M. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat. Rev. Microbiol. 2021, 19, 256–271. [Google Scholar] [CrossRef]
- Sun, D.; Lee, G.; Lee, J.H.; Kim, H.Y.; Rhee, H.W.; Park, S.Y.; Kim, K.J.; Kim, Y.; Kim, B.Y.; Hong, J.I.; et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 2010, 17, 1188–1194. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef]
- Seyfzadeh, M.; Keener, J.; Nomura, M. spoT-dependent accumulation of guanosine tetraphosphate in response to fatty acid starvation in Escherichia coli. Proc. Natl. Acad. Sci. USA 1993, 90, 11004–11008. [Google Scholar] [CrossRef]
- Battesti, A.; Bouveret, E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 2006, 62, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Vinella, D.; Albrecht, C.; Cashel, M.; D’Ari, R. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 2005, 56, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Flardh, K.; Axberg, T.; Albertson, N.H.; Kjelleberg, S. Stringent control during carbon starvation of marine Vibrio sp. strain S14: Molecular cloning, nucleotide sequence, and deletion of the relA gene. J. Bacteriol. 1994, 176, 5949–5957. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Barton, G.; Pan, Z.; Buck, M.; Wigneshweraraj, S. Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat. Commun. 2014, 5, 4115. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, S.; Deochand, D.K.; Meariman, J.K.; Grove, A. The stringent response induced by phosphate limitation promotes purine salvage in Agrobacterium fabrum. Biochemistry 2017, 56, 5831–5843. [Google Scholar] [CrossRef] [PubMed]
- English, B.P.; Hauryliuk, V.; Sanamrad, A.; Tankov, S.; Dekker, N.H.; Elf, J. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc. Natl. Acad. Sci. USA 2011, 108, E365–E373. [Google Scholar] [CrossRef]
- Gallant, J.; Palmer, L.; Pao, C.C. Anomalous synthesis of ppGpp in growing cells. Cell 1977, 11, 181–185. [Google Scholar] [CrossRef]
- Thackray, P.D.; Moir, A. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J. Bacteriol. 2003, 185, 3491–3498. [Google Scholar] [CrossRef]
- Anderson, K.L.; Roux, C.M.; Olson, M.W.; Luong, T.T.; Lee, C.Y.; Olson, R.; Dunman, P.M. Characterizing the effects of inorganic acid and alkaline shock on the Staphylococcus aureus transcriptome and messenger RNA turnover. FEMS Immunol. Med. Microbiol. 2010, 60, 208–250. [Google Scholar] [CrossRef]
- Heizmann, P.; Howell, S.H. Synthesis of ppGpp and chloroplast ribosomal RNA in Chlamydomonas reinhardi. Biochim. Biophys. Acta 1978, 517, 115–124. [Google Scholar] [CrossRef]
- Van der Biezen, E.A. Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 3747–3752. [Google Scholar] [CrossRef] [PubMed]
- Givens, R.M.; Lin, M.-H.; Taylor, D.J.; Mechold, U.; Berry, J.O.; Hernandez, V.J. Inducible expression, enzymatic activity, and origin of higher plant homologues of bacterial RelA/SpoT stress proteins in Nicotiana tabacum. J. Biol. Chem. 2004, 279, 7495–7504. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Lee, M.W.; Qi, M.; Yang, Y. Identification of defense-related rice genes by suppression subtractive hybridization and differential screening. Mol. Plant Microbe Interact. 2001, 14, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.B.; Prusińska, J.; Goc, A. Identification of the RSH gene cDNA (RelA-SpoT homolog) involved in Pharbitis nil response to stress conditions. Zesz. Probl. Postępów Nauk. Rol. 2006, 509, 343–351. [Google Scholar]
- Yamada, A.; Tsutsumi, K.; Tanimoto, S.; Ozeki, Y. Plant RelA/SpoT homolog confers salt tolerance in Escherichia coli and Saccharomyces cerevisiae. Plant Cell Physiol. 2003, 44, 3–9. [Google Scholar] [CrossRef]
- Kim, T.-H.; Ok, S.H.; Kim, D.; Suh, S.-C.; Byun, M.O.; Shin, J.S. Molecular characterization of a biotic and abiotic stress resistance-related gene RelA/SpoT homologue (PepRSH) from pepper. Plant Sci. 2009, 176, 635–642. [Google Scholar] [CrossRef]
- Sato, M.; Takahashi, T.; Ochi, K.; Matsuura, H.; Nabeta, K.; Takahashi, K. Overexpression of RelA/SpoT homologs, PpRSH2a and PpRSH2b, induces the growth suppression of the moss Physcomitrella patens. Biosci. Biotechnol. Biochem. 2015, 79, 36–44. [Google Scholar] [CrossRef]
- Mizusawa, K.; Masuda, S.; Ohta, H. Expression profiling of four RelA/SpoT-like proteins, homologues of bacterial stringent factors, in Arabidopsis thaliana. Planta 2008, 228, 553–562. [Google Scholar] [CrossRef]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The RelA/SpoT Homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef]
- Shin, J.; Singal, B.; Grüber, A.; Wong, D.M.K.; Ragunathan, P.; Grüber, G. Atomic structure of the regulatory TGS domain of Rel protein from Mycobacterium tuberculosis and its interaction with deacylated tRNA. FEBS Lett. 2021, 595, 3006–3018. [Google Scholar] [CrossRef]
- Tozawa, Y.; Nozawa, A.; Kanno, T.; Narisawa, T.; Masuda, S.; Kasai, K.; Nanamiya, H. Calcium-activated (p)ppGpp synthetase in chloroplasts of land plants. J. Biol. Chem. 2007, 282, 35536–35545. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, D.; Goto, M.; Suzuki, S.; Masuda, S.; Iba, K.; Kusumi, K. Regulation of ppGpp synthesis and its impact on chloroplast biogenesis during early leaf development in rice. Plant Cell Physiol. 2022, 63, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Yamburenko, M.V.; Zubo, Y.O.; Börner, T. Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: Repression by guanosine-3′-5′-bisdiphosphate and activation by sigma factor 5. Plant J. 2015, 82, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Kanno, T.; Endo, Y.; Wakasa, K.; Tozawa, Y. Guanosine tetra- and pentaphosphate synthase activity in chloroplasts of a higher plant: Association with 70S ribosomes and inhibition by tetracycline. Nucleic Acids Res. 2004, 32, 5732–5741. [Google Scholar] [CrossRef]
- Imamura, S.; Nomura, Y.; Takemura, T.; Pancha, I.; Taki, K.; Toguchi, K.; Tozawa, Y.; Tanaka, K. The checkpoint kinase TOR (target of rapamycin) regulates expression of a nuclear-encoded chloroplast RelA-SpoT homolog (RSH) and modulates chloroplast ribosomal RNA synthesis in a unicellular red alga. Plant J. 2018, 94, 327–339. [Google Scholar] [CrossRef]
- Sugliani, M.; Abdelkefi, H.; Ke, H.; Bouveret, E.; Robaglia, C.; Caffarri, S.; Field, B. An ancient bacterial signaling pathway regulates chloroplast function to influence growth and development in Arabidopsis. Plant Cell 2016, 28, 661–679. [Google Scholar] [CrossRef]
- Abdelkefi, H.; Sugliani, M.; Ke, H.; Harchouni, S.; Soubigou-Taconnat, L.; Citerne, S.; Mouille, G.; Fakhfakh, H.; Robaglia, C.; Field, B. Guanosine tetraphosphate modulates salicylic acid signalling and the resistance of Arabidopsis thaliana to turnip mosaic virus. Mol. Plant Pathol. 2018, 19, 634–646. [Google Scholar] [CrossRef]
- Kahlau, S.; Bock, R. Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: Chromoplast gene expression largely serves the production of a single protein. Plant Cell 2008, 20, 856–874. [Google Scholar] [CrossRef]
- Maekawa, M.; Honoki, R.; Ihara, Y.; Sato, R.; Oikawa, A.; Kanno, Y.; Ohta, H.; Seo, M.; Saito, K.; Masuda, S. Impact of the plastidial stringent response in plant growth and stress responses. Nat. Plants 2015, 1, 15167. [Google Scholar] [CrossRef]
- Honoki, R.; Ono, S.; Oikawa, A.; Saito, K.; Masuda, S. Significance of accumulation of the alarmone (p)ppGpp in chloroplasts for controlling photosynthesis and metabolite balance during nitrogen starvation in Arabidopsis. Photosynth. Res. 2018, 135, 299–308. [Google Scholar] [CrossRef]
- Romand, S.; Abdelkefi, H.; Lecampion, C.; Belaroussi, M.; Dussenne, M.; Ksas, B.; Citerne, S.; Caius, J.; D’alessandro, S.; Fakhfakh, H.; et al. A guanosine tetraphosphate (ppGpp) mediated brake on photosynthesis is required for acclimation to nitrogen limitation in Arabidopsis. eLife 2022, 11, 75041. [Google Scholar] [CrossRef]
- Li, H.; Nian, J.; Fang, S.; Guo, M.; Huang, X.; Zhang, F.; Wang, Q.; Zhang, J.; Bai, J.; Dong, G.; et al. Regulation of nitrogen starvation responses by the alarmone (p)ppGpp in rice. J. Genet. Genom. 2022, 49, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kasai, K.; Ochi, K. Identification of the bacterial alarmone guanosine 5′-diphosphate 3′-diphosphate (ppGpp) in plants. Proc. Natl. Acad. Sci. USA 2004, 101, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Mizusawa, K.; Narisawa, T.; Tozawa, Y.; Ohta, H.; Takamiya, K.I. The bacterial stringent response, conserved in chloroplasts, controls plant fertilization. Plant Cell Physiol. 2008, 49, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Suzuki, S.; Ito, D.; Tagawa, S.; Shiina, T.; Masuda, S. Plastidial (p)ppGpp synthesis by the Ca2+-dependent RelA–SpoT homolog regulates the adaptation of chloroplast gene expression to darkness in Arabidopsis. Plant Cell Physiol. 2021, 61, 2077–2086. [Google Scholar] [CrossRef]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Pałasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6, 22263. [Google Scholar] [CrossRef]
- Mi, L.; Hood, B.L.; Stewart, N.A.; Xiao, Z.; Govind, S.; Wang, X.; Conrads, T.P.; Veenstra, T.D.; Chung, F.-L. Identification of potential protein targets of isothiocyanates by proteomics. Chem. Res. Toxicol. 2011, 24, 1735–1743. [Google Scholar] [CrossRef]
- Mwita, L.; Chan, W.Y.; Pretorius, T.; Lyantagaye, S.L.; Lapa, S.V.; Avdeeva, L.V.; Reva, O.N. Gene expression regulation in the plant growth promoting Bacillus atrophaeus UCMB-5137 stimulated by maize root exudates. Gene 2016, 590, 18–28. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Turkan, S.; Tylman-Mojżeszek, W.; Mierek-Adamska, A. In silico study of the RSH (Rela/spot homologs) gene family and expression analysis in response to PGPR bacteria and salinity in Brassica napus. Int. J. Mol. Sci. 2021, 22, 10666. [Google Scholar] [CrossRef]
- Chapelle, E.; Mendes, R.; Bakker, P.A.H.; Raaijmakers, J.M. Fungal invasion of the rhizosphere microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef]
- Manuel, J.; Berry, C.; Selin, C.; Fernando, W.G.D.; de Kievit, T.R. Repression of the antifungal activity of Pseudomonas sp. strain DF41 by the stringent response. Appl. Environ. Microbiol. 2011, 77, 5635–5642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manuel, J.; Selin, C.; Dilantha Fernando, W.G.; de Kievi, T. Stringent response mutants of Pseudomonas chlororaphis PA23 exhibit enhanced antifungal activity against Sclerotinia sclerotiorum in vitro. Microbiology 2012, 158, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; Manuel, J.; Fernando, W.G.D.; de Kievit, T. Expression of the Pseudomonas chlororaphis strain PA23 Rsm system is under control of GacA, RpoS, PsrA, quorum sensing and the stringent response. Biol. Control 2014, 69, 24–33. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yamada, K.; Haas, D. ppGpp controlled by the Gac/Rsm regulatory pathway sustains biocontrol activity in Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2012, 25, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Ochi, K. A rel mutation abolishes the enzyme induction needed for actinomycin synthesis by Streptomyces antibioticus. Agric. Biol. Chem. 2014, 51, 829–835. [Google Scholar]
- Ryu, Y.G.; Wook, J.; Jin, Y.K.; Jae, Y.K.; Sang, H.L.; Kye, J.L. Stringent factor regulates antibiotics production and morphological differentiation of Streptomyces clavuligerus. J. Microbiol. Biotechnol. 2004, 14, 1170–1175. [Google Scholar]
- Brechenmacher, L.; Lei, Z.; Libault, M.; Findley, S.; Sugawara, M.; Sadowsky, M.J.; Sumner, L.W.; Stacey, G. Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiol. 2010, 153, 1808–1822. [Google Scholar] [CrossRef]
- Murray, J.D. Invasion by invitation: Rhizobial infection in legumes. Mol. Plant Microbe Interact. 2011, 24, 631–639. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Pérez-Giménez, J.; Iturralde, E.T.; Torres Tejerizo, G.; Quelas, J.I.; Krol, E.; Borassi, C.; Becker, A.; Estevez, J.M.; Lodeiro, A.R. A stringent-response-defective Bradyrhizobium diazoefficiens strain does not activate the type 3 secretion system, elicits an early plant defense response, and circumvents NH4NO3 -induced inhibition of nodulation. Appl. Environ. Microbiol. 2021, 87, e02989-20. [Google Scholar] [CrossRef]
- Wippel, K.; Long, S.R. Symbiotic performance of Sinorhizobium meliloti lacking ppGpp depends on the Medicago host species. Mol. Plant Microbe Interact. 2019, 32, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.H.; Long, S.R. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 2002, 43, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Moris, M.; Braeken, K.; Schoeters, E.; Verreth, C.; Beullens, S.; Vanderleyden, J.; Michiels, J. Effective symbiosis between Rhizobium etli and Phaseolus vulgaris requires the alarmone ppGpp. J. Bacteriol. 2005, 187, 5460–5469. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Flores, A.; du Pont, G.; Huerta-Saquero, A.; Merchant-Larios, H.; Servín-González, L.; Durán, S. The stringent response is required for amino acid and nitrate utilization, nod factor regulation, nodulation, and nitrogen fixation in Rhizobium etli. J. Bacteriol. 2005, 187, 5075. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gao, T.; Yang, J.; Wang, E.; Liu, L.; Yuan, H. Water-soluble humic materials modulating metabolism and triggering stress defense in Sinorhizobium fredii. Microbiol. Spectr. 2021, 9, e00293-21. [Google Scholar] [CrossRef] [PubMed]
- Ancona, V.; Lee, J.H.; Chatnaparat, T.; Oh, J.; Hong, J.I.; Zhao, Y. The bacterial alarmone (p)ppGpp activates the type III secretion system in Erwinia amylovora. J. Bacteriol. 2015, 197, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Chatnaparat, T.; Li, Z.; Korban, S.S.; Zhao, Y. The bacterial alarmone (p)ppGpp is required for virulence and controls cell size and survival of Pseudomonas syringae on plants. Environ. Microbiol. 2015, 17, 4253–4270. [Google Scholar] [CrossRef]
- Chatnaparat, T.; Li, Z.; Korban, S.S.; Zhao, Y. The stringent response mediated by (p)ppGpp is required for virulence of Pseudomonas syringae pv. tomato and its survival on tomato. Mol. Plant Microbe Interact. 2015, 28, 776–789. [Google Scholar] [CrossRef]
- Bowden, S.D.; Hale, N.; Chung, J.C.S.; Hodgkinson, J.T.; Spring, D.R.; Welch, M. Surface swarming motility by Pectobacterium atrosepticum is a latent phenotype that requires O antigen and is regulated by quorum sensing. Microbiology 2013, 159, 2375–2385. [Google Scholar] [CrossRef]
- Petrova, O.; Gorshkov, V.; Sergeeva, I.; Daminova, A.; Ageeva, M.; Gogolev, Y. Alternative scenarios of starvation-induced adaptation in Pectobacterium atrosepticum. Res. Microbiol. 2016, 167, 254–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Teper, D.; Xu, J.; Wang, N. Stringent response regulators (p)ppGpp and DksA positively regulate virulence and host adaptation of Xanthomonas citri. Mol. Plant. Pathol. 2019, 20, 1550–1565. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak-Gębarowska, E. Mechanisms of biological control soil-borne plant pathogen by fungus from genus Trichoderma. Adv. Microbiol. 2006, 45, 261–273. [Google Scholar]
- Bustamante, D.E.; Calderon, M.S.; Leiva, S.; Mendoza, J.E.; Arce, M.; Oliva, M. Three new species of Trichoderma in the Harzianum and Longibrachiatum lineages from Peruvian cacao crop soils based on an integrative approach. Mycologia 2021, 113, 1056–1072. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Srivastava, S.N.; Singh, V.; Awasthi, S.K. Trichoderma induced improvement in growth, yield and quality of sugarcane. Sugar Tech 2006, 8, 166–169. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Rudresh, D.L.; Shivaprakash, M.K.; Prasad, R.D. Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer aritenium L.). Appl. Soil Ecol. 2005, 28, 139–146. [Google Scholar] [CrossRef]
- Khomari, S.; Golshan-Doust, S.; Seyed-Sharifi, R.; Davari, M. Improvement of soybean seedling growth under salinity stress by biopriming of high-vigour seeds with salt-tolerant isolate of Trichoderma harzianum. N. Z. J. Crop Hortic. Sci. 2018, 46, 117–132. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Alam, M.; Islam, M. Effect of Trichoderma on seed germination and seedling parameters of chili. J. Sci. Found. 2013, 8, 141–150. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Vergara, A.G.; López-Bucio, J. Trichoderma modulates stomatal aperture and leaf transpiration through an abscisic acid-dependent mechanism in Arabidopsis. J. Plant Growth Regul. 2015, 34, 425–432. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: A proteomic approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef]
- Mercl, F.; García-Sánchez, M.; Kulhánek, M.; Košnář, Z.; Száková, J.; Tlustoš, P. Improved phosphorus fertilisation efficiency of wood ash by fungal strains Penicillium sp. PK112 and Trichoderma harzianum OMG08 on acidic soil. Appl. Soil Ecol. 2020, 147, 103360. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.; Liu, Z.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 11152. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, X.; Wang, G.; Wu, B.; Xiao, Y. Medicago sativa and soil microbiome responses to Trichoderma as a biofertilizer in alkaline-saline soils. Appl. Soil Ecol. 2020, 153, 103573. [Google Scholar] [CrossRef]
- Adams, P.; De-Leij, F.A.A.M.; Lynch, J.M. Trichoderma harzianum Rifai 1295-22 mediates growth promotion of crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil. Microb. Ecol. 2007, 54, 306–313. [Google Scholar] [CrossRef]
- López Errasquín, E.; Vázquez, C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef]
- Rajendran, L.; Raja, P.; Jegadeeswari, V.; Santhi, V.P.; Selvaraj, N. Pseudomonas fluorescens and Trichoderma viride enriched bioconsortium for the management of Fusarium wilt in carnation and gerbera under protected cultivation. Indian Phytopathol. 2014, 67, 77–81. [Google Scholar]
- Ambuse, M.G.; Chatage, V.S.; Bhale, U.N. Influence of Trichoderma spp against Alternaria tenuissima incitining leaf spot of Rumex acetosa L. Biosci. Discov. 2012, 3, 259–262. [Google Scholar]
- De Padua, J.C.; de la Cruz, T.E.E. Isolation and characterization of nickel-tolerant Trichoderma strains from marine and terrestrial environments. J. Fungi 2021, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant growth promotion and biocontrol of Pythium ultimum by saline tolerant Trichoderma isolates under salinity stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Z.; Miao, F.-P.; Fang, S.-T.; Yin, X.-L.; Ji, N.-Y. Sulfurated diketopiperazines from an algicolous isolate of Trichoderma virens. Phytochem. Lett. 2018, 27, 101–104. [Google Scholar] [CrossRef]
- Touati, I.; Ruiz, N.; Thomas, O.; Druzhinina, I.S.; Atanasova, L.; Tabbene, O.; Elkahoui, S.; Benzekri, R.; Bouslama, L.; Pouchus, Y.F.; et al. Hyporientalin A, an anti-Candida peptaibol from a marine Trichoderma orientale. World J. Microbiol. Biotechnol. 2018, 34, 98. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Hu, Y.; Peng, Z.; Ren, S.; Xue, M.; Liu, Z.; Hou, J.; Xing, M.; Liu, T. A novel salt-tolerant strain Trichoderma atroviride HN082102.1 isolated from marine habitat alleviates salt stress and diminishes cucumber root rot caused by Fusarium oxysporum. BMC Microbiol. 2022, 22, 67. [Google Scholar] [CrossRef]
- Gal-Hemed, I.; Atanasova, L.; Komon-Zelazowska, M.; Druzhinina, I.S.; Viterbo, A.; Yarden, O. Marine isolates of Trichoderma spp. as potential halotolerant agents of biological control for arid-zone agriculture. Appl. Environ. Microbiol. 2011, 77, 5100–5109. [Google Scholar] [CrossRef]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M.; Mendoza-Mendoza, A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defence and scavenging oxidative stress secreted proteins. Front. Plant Sci. 2018, 9, 409. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Manfra, M.; de Nisco, M.; Tenore, G.; Troisi, J.; di Fiori, R.; Novellino, E. Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus × P. canescens). Plant Growth Regul. 2011, 65, 421–425. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [PubMed]
- Akhter, W.; Bhuiyan, M.K.A.; Sultana, F.; Hossain, M.M. Integrated effect of microbial antagonist, organic amendment and fungicide in controlling seedling mortality (Rhizoctonia solani) and improving yield in pea (Pisum sativum L.). Comptes Rendus. Biol. 2015, 338, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R.; Hanson, L.E.; Stipanovic, R.D.; Puckhaber, L.S. Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopatology 2000, 90, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.V.; Costa, M.D.N.; de Paula, R.G.; Ricci De Azevedo, R.; da Silva, F.L.; Noronha, E.F.; José Ulhoa, C.; Neves Monteiro, V.; Elena Cardoza, R.; Gutiérrez, S.; et al. The cerato-platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci. Rep. 2015, 5, 17998. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathology 2002, 92, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Hossain, N.; Sultana, F.; Mohammad, S.; Islam, N.; Khurshed, M.; Bhuiyan, A. Integrated management of Fusarium wilt of chickpea (Cicer arietinum L.) caused by Fusarium oxysporum f. sp. ciceris with microbial antagonist, botanical extract sp. ciceris with microbial antagonist, botanical extract. Afr. J. Biotechnol. 2016, 12, 4699–4706. [Google Scholar]
- Vujanovic, V.; Goh, Y.K. qPCR quantification of sphaerodes mycoparasitica biotrophic mycoparasite interaction with Fusarium graminearum: In vitro and in planta assays. Arch. Microbiol. 2012, 194, 707–717. [Google Scholar] [CrossRef]
- Gveroska, B.; Ziberoski, J. Trichoderma harzianum as a biocontrol agent against Alternaria alternata on tobacco. Appl. Technol. Innov. 2012, 7, 67–76. [Google Scholar] [CrossRef]
- Matroudi, S.; Zamani, M.; Motallebi, M. Antagonistic effects of three species of Trichoderma sp. on Sclerotinia sclerotiorum, the causal agent of canola stem rot. Egypt. Acad. J. Biol. Sci. 2010, 11, 37–44. [Google Scholar]
- De Meyer, G.; Bigirimana, J.; Elad, Y.; Höfte, M. Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur. J. Plant Pathol. 1998, 104, 279–286. [Google Scholar] [CrossRef]
- Herrera-Téllez, V.I.; Cruz-Olmedo, A.K.; Plasencia, J.; Gavilanes-Ruíz, M.; Arce-Cervantes, O.; Hernández-León, S.; Saucedo-García, M. The protective effect of Trichoderma asperellum on tomato plants against Fusarium oxysporum and Botrytis cinerea diseases involves inhibition of reactive oxygen species production. Int. J. Mol. Sci. 2019, 20, 2007. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Parra, J.A.; Pérez España, V.H.; Zavala-González, E.A.; Peralta-Gil, M.; Aparicio Burgos, J.E.; Romero-Cortes, T. Trichoderma asperellum strains as potential biological control agents against Fusarium verticillioides and Ustilago maydis in maize. Biocontrol. Sci. Technol. 2022, 32, 624–647. [Google Scholar] [CrossRef]
- Daniel, J.F.D.S.; Rodrigues Filho, E. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Degenkolb, T.; Berg, A.; Gams, W.; Schlegel, B.; Gräfe, U. The occurrence of peptaibols and structurally related peptaibiotics in fungi and their mass spectrometric identification via diagnostic fragment ions. J. Pept. Sci. 2003, 9, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, S.; Sun, M.-L.; Su, H.-N.; Li, H.-Y.; Liu, K.; Zhang, Y.-Z.; Chen, X.-L.; Cao, H.-Y.; Song, X.-Y. Antibacterial activity of peptaibols from Trichoderma longibrachiatum SMF2 against gram-negative Xanthomonas oryzae pv. oryzae, the causal agent of bacterial leaf blight on rice. Front. Microbiol. 2022, 13, 1034779. [Google Scholar]
- Zhao, P.; Ren, A.; Dong, P.; Sheng, Y.; Li, D. Antimicrobial peptaibols, trichokonins, inhibit mycelial growth and sporulation and induce cell apoptosis in the pathogenic fungus Botrytis cinerea. Appl. Biochem. Microbiol. 2018, 54, 396–403. [Google Scholar] [CrossRef]
- Otto, A.; Laub, A.; Wendt, L.; Porzel, A.; Schmidt, J.; Palfner, G.; Becerra, J.; Krüger, D.; Stadler, M.; Wessjohann, L.; et al. Chilenopeptins A and B, peptaibols from the Chilean Sepedonium aff. chalcipori KSH 883. J. Nat. Prod. 2016, 79, 929–938. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in peptaibol production of Trichoderma species during in vitro antagonistic interactions with fungal plant pathogens. Biomolecules 2020, 10, 730. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.D.; Dong, X.W.; Zhao, P.B.; Chen, L.L.; Song, X.Y.; Wang, X.J.; Chen, X.L.; Shi, M.; Zhang, Y.Z. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiol. Lett. 2010, 313, 120–126. [Google Scholar] [CrossRef][Green Version]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; et al. Structural diversity and bioactivities of peptaibol compounds from the Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Ran, W.; Shen, Q.; Yang, X. Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl. Microbiol. Biotechnol. 2011, 91, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.A.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Engelberth, J.; Koch, T.; Schüler, G.; Bachmann, N.; Rechtenbach, J.; Boland, W. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 2001, 125, 369–377. [Google Scholar] [CrossRef]
- Rotblat, B.; Enshell-Seijffers, D.; Gershoni, J.M.; Schuster, S.; Avni, A. Identification of an essential component of the elicitation active site of the EIX protein elicitor. Plant J. 2002, 32, 1049–1055. [Google Scholar] [CrossRef]
- Martinez, C.; Blanc, F.; le Claire, E.; Besnard, O.; Nicole, M.; Baccou, J.-C. Salicylic acid and ethylene pathways are differentially activated in melon cotyledons by active or heat-denatured cellulase from Trichoderma longibrachiatum. Plant Physiol. 2001, 127, 334–344. [Google Scholar] [CrossRef]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol. Plant Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ramírez, A.; Poveda, J.; Martín, I.; Hermosa, R.; Monte, E.; Nicolás, C. Salicylic acid prevents Trichoderma harzianum from entering the vascular system of roots. Mol. Plant Pathol. 2014, 15, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a new biocontrol gene in Trichoderma atroviride: The role of an ABC transporter membrane pump in the interaction with different plant-pathogenic fungi. Mol. Plant Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Masunaka, A.; Hyakumachi, M.; Takenaka, S. Plant growth-promoting fungus, Trichoderma koningi suppresses isoflavonoid phytoalexin vestitol production for colonization on/in the roots of Lotus japonicus. Microbes Environ. 2011, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Yang, H.; Shin, H.-J. Fungal and mushroom hydrophobins: A review. J. Mushroom 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Garstecka, Z.; Olewnik-Kruszkowska, E.; Szczepańska, G.; Ostrowski, M.; Mierek-Adamska, A. Comparative study of structural changes of polylactide and poly(ethylene terephthalate) in the presence of Trichoderma viride. Int. J. Mol. Sci. 2021, 22, 3491. [Google Scholar] [CrossRef]
- Viterbo, A.; Harel, M.; Chet, I. Isolation of two aspartyl proteases from Trichoderma asperellum expressed during colonization of cucumber roots. FEMS Microbiol. Lett. 2004, 238, 151–158. [Google Scholar]
- Saloheimo, M.; Paloheimo, M.; Hakola, S.; Pere, J.; Swanson, B.; Nyyssönen, E.; Bhatia, A.; Ward, M.; Penttilä, M. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur. J. Biochem. 2002, 269, 4202–4211. [Google Scholar] [CrossRef]
- Sánchez-Cruz, R.; Mehta, R.; Atriztán-Hernández, K.; Martínez-Villamil, O.; del Rayo Sánchez-Carbente, M.; Sánchez-Reyes, A.; Lira-Ruan, V.; González-Chávez, C.A.; Tabche-Barrera, M.L.; Bárcenas-Rodríguez, R.C.; et al. Effects on Capsicum annuum plants colonized with Trichoderma atroviride P. Karst strains genetically modified in taswo1, a gene coding for a protein with expansin-like activity. Plants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Guzmán-Gómez, A.; García-Juárez, P.; Contreras-Cornejo, H.A. Trichoderma atroviride promotes tomato development and alters the root exudation of carbohydrates, which stimulates fungal growth and the biocontrol of the phytopathogen Phytophthora cinnamomi in a tripartite interaction system. FEMS Microbiol. Ecol. 2018, 94, fiy137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).