Report on Vincristine-Producing Endophytic Fungus Nigrospora zimmermanii from Leaves of Catharanthus roseus
Abstract
1. Introduction
2. Materials and Methods
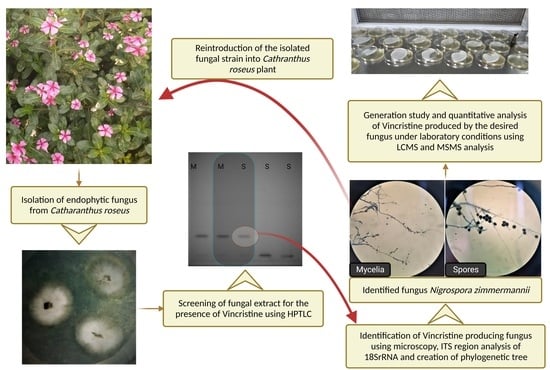
2.1. Endophytic Microbe Isolation
2.2. HPTLC Conditions for Qualitative and Quantitative Analysis
2.3. UPLC-MS Analysis Conditions
2.4. Microbe Identification
2.4.1. PCR Polymerase
2.4.2. Sequencing and PCR Conditions
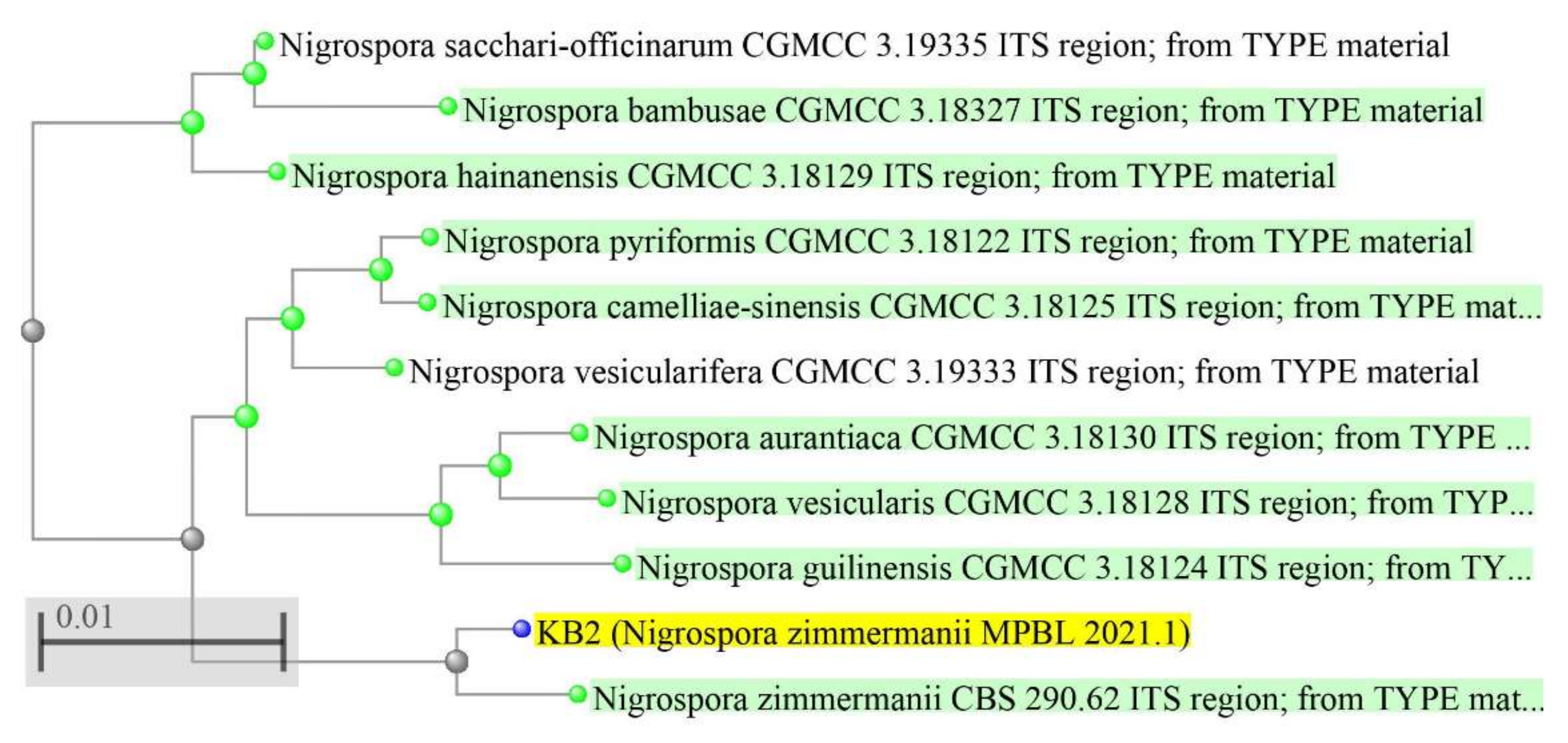
2.4.3. Phylogenetic Tree
2.5. Generation Study
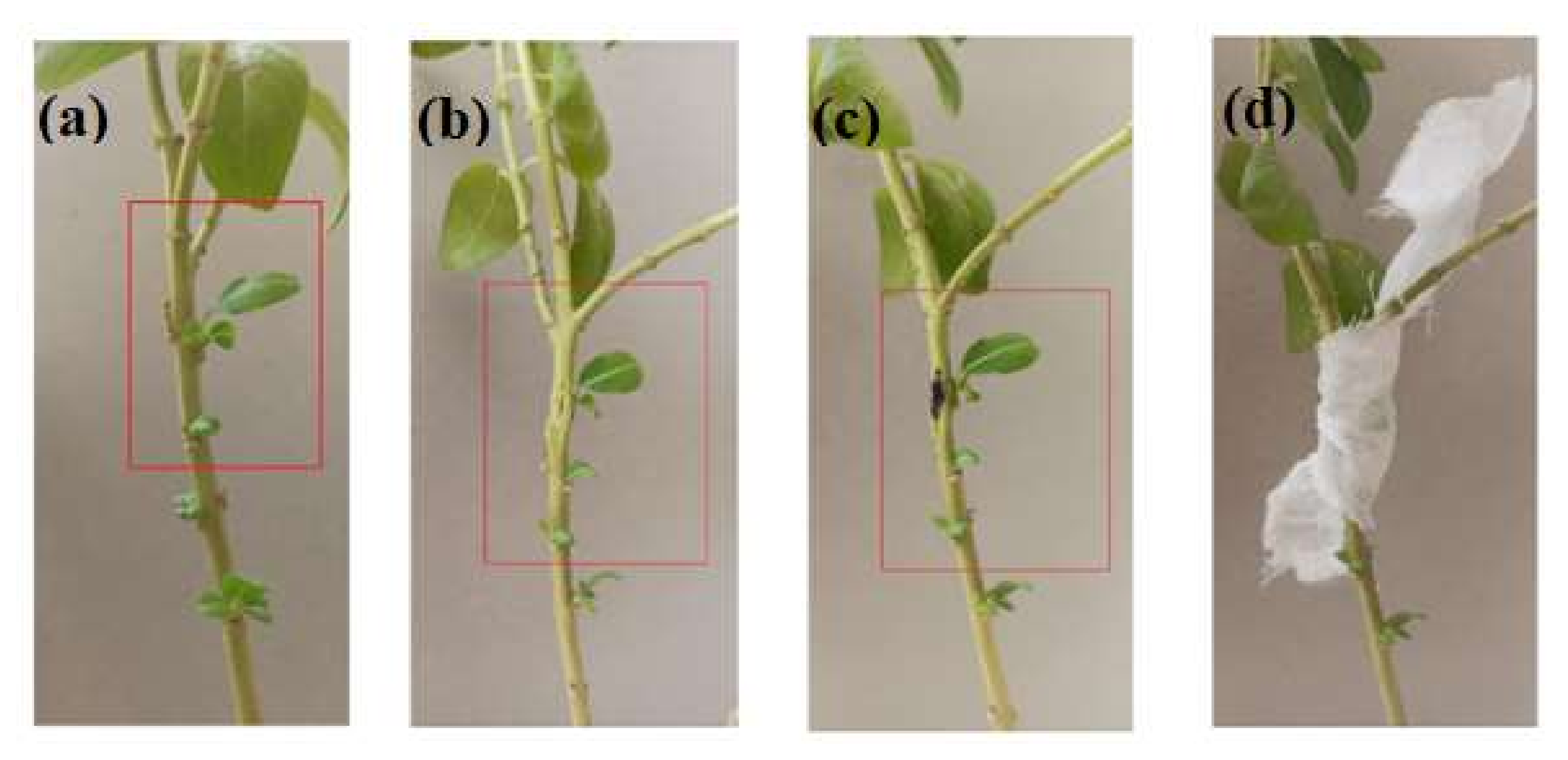
2.6. Colonization Study
2.7. Phenotypic Identification
3. Results
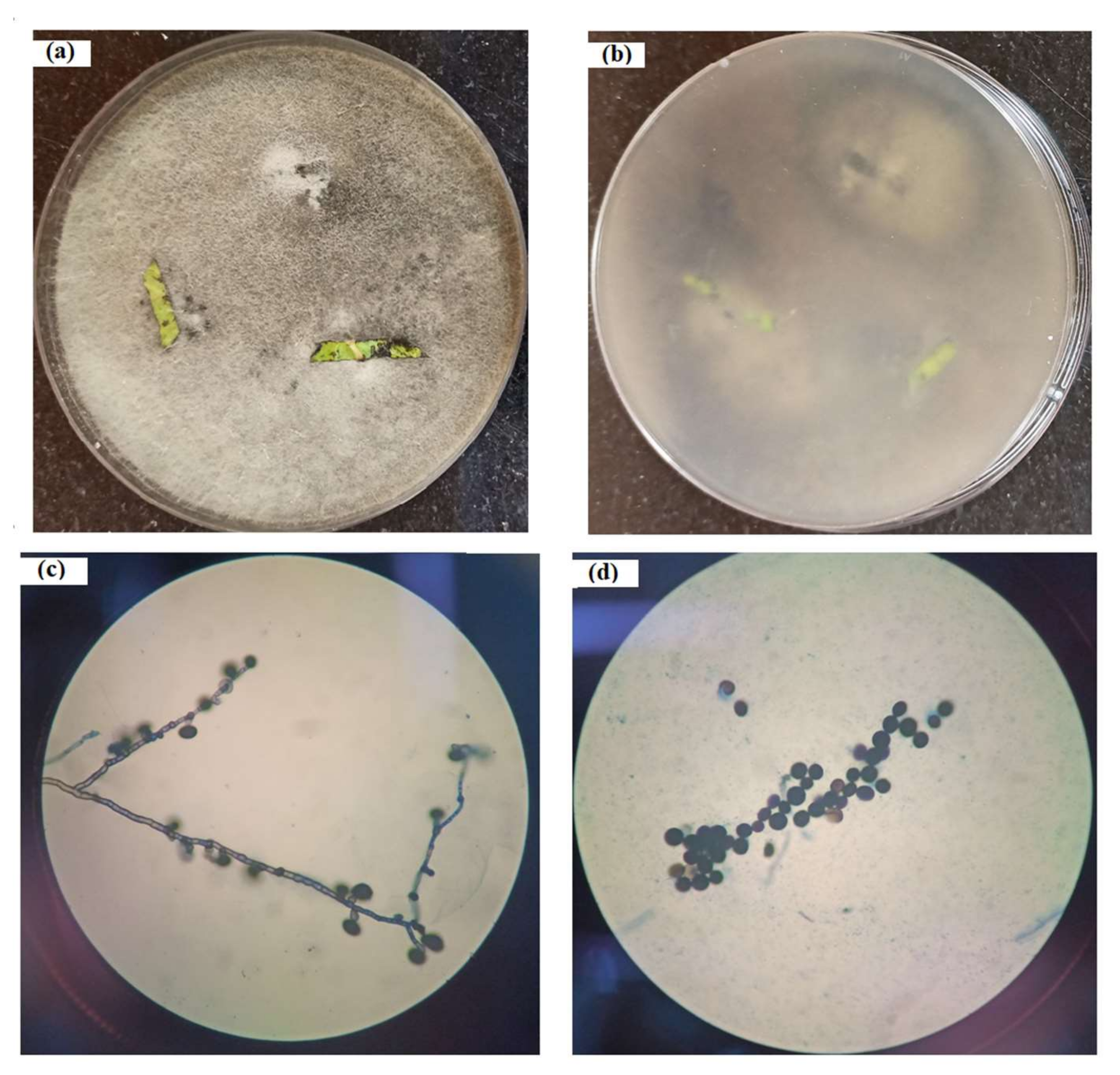
3.1. Microbe Isolation
3.2. Colonization and Generation Study
3.3. Microbe Identification
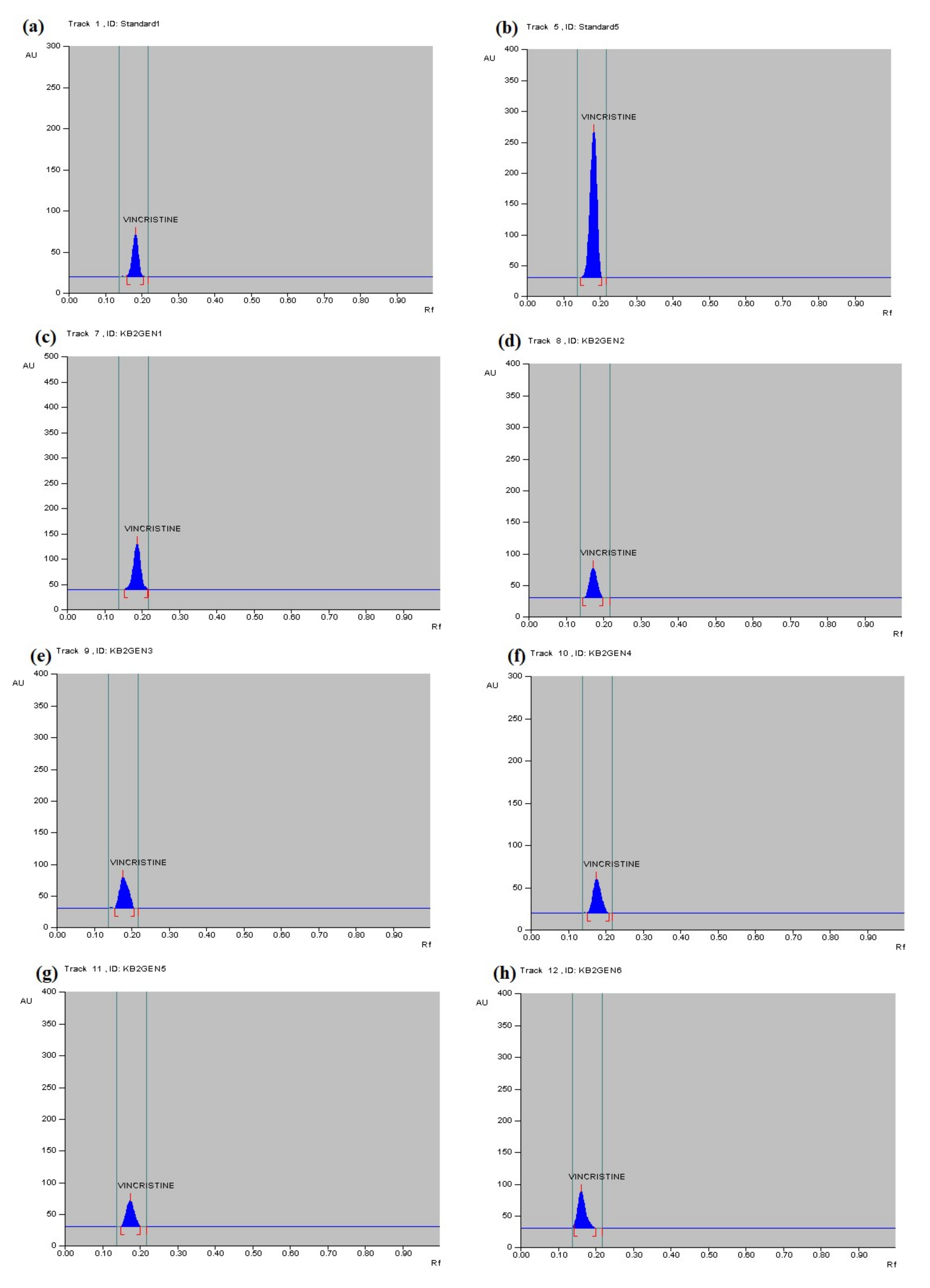
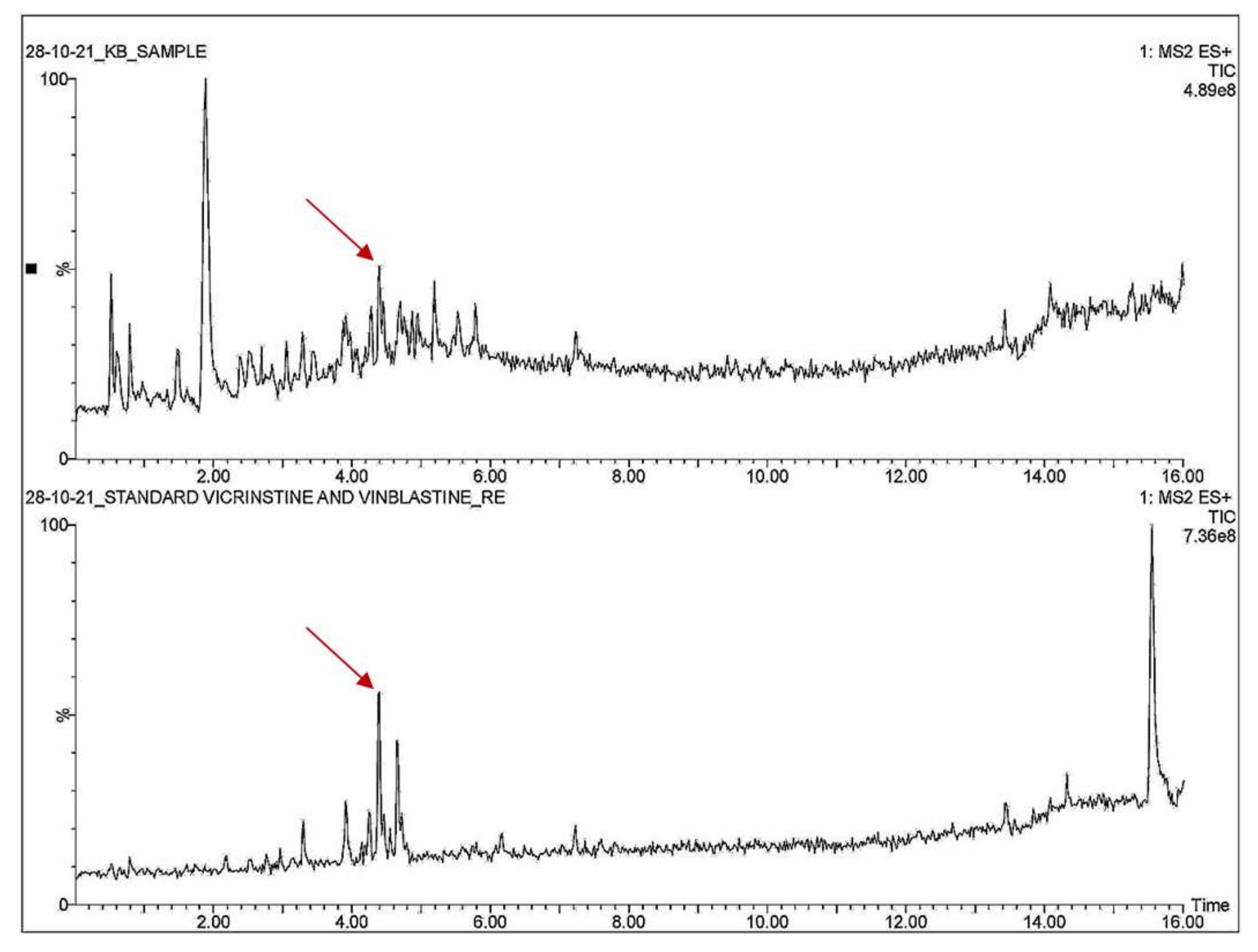
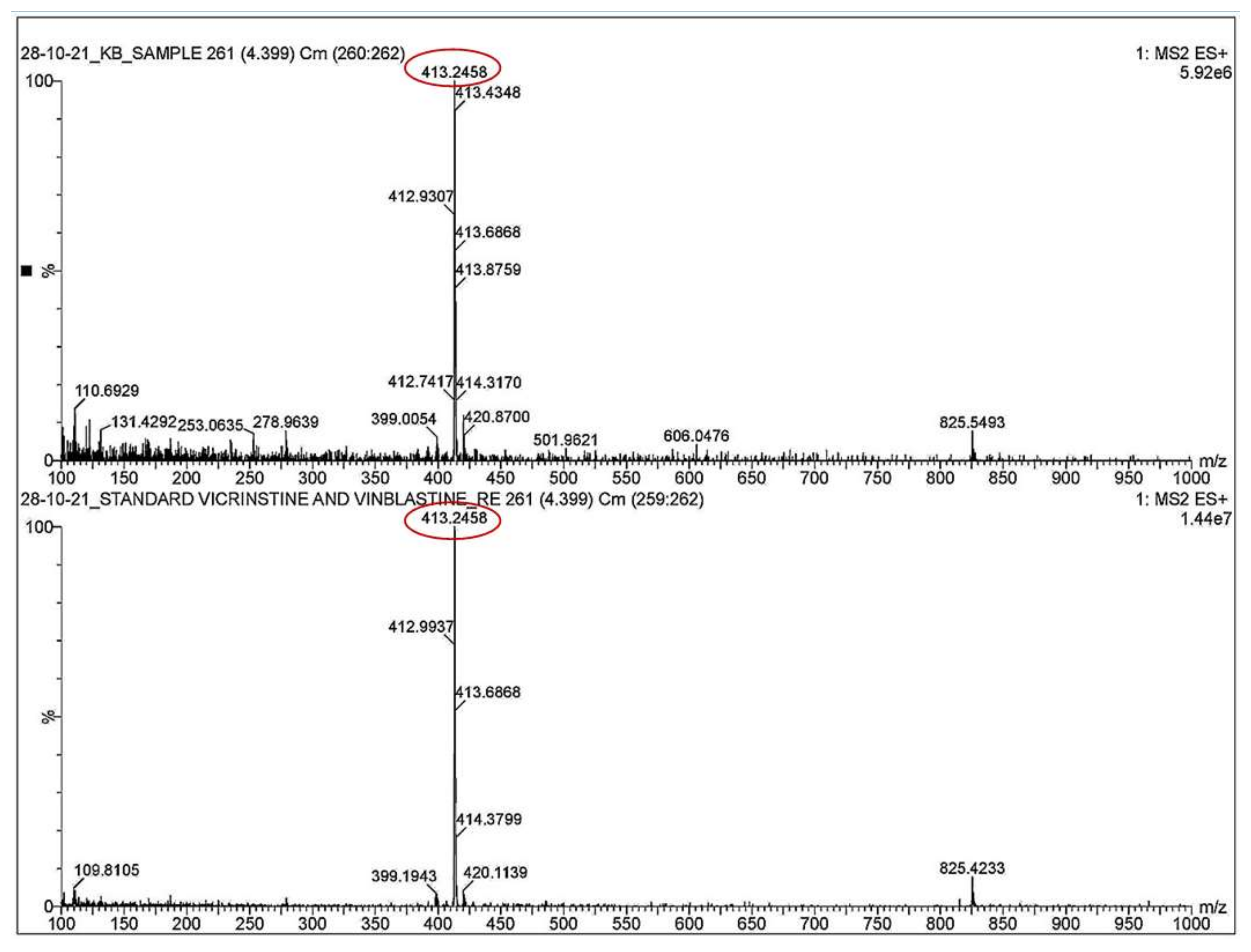
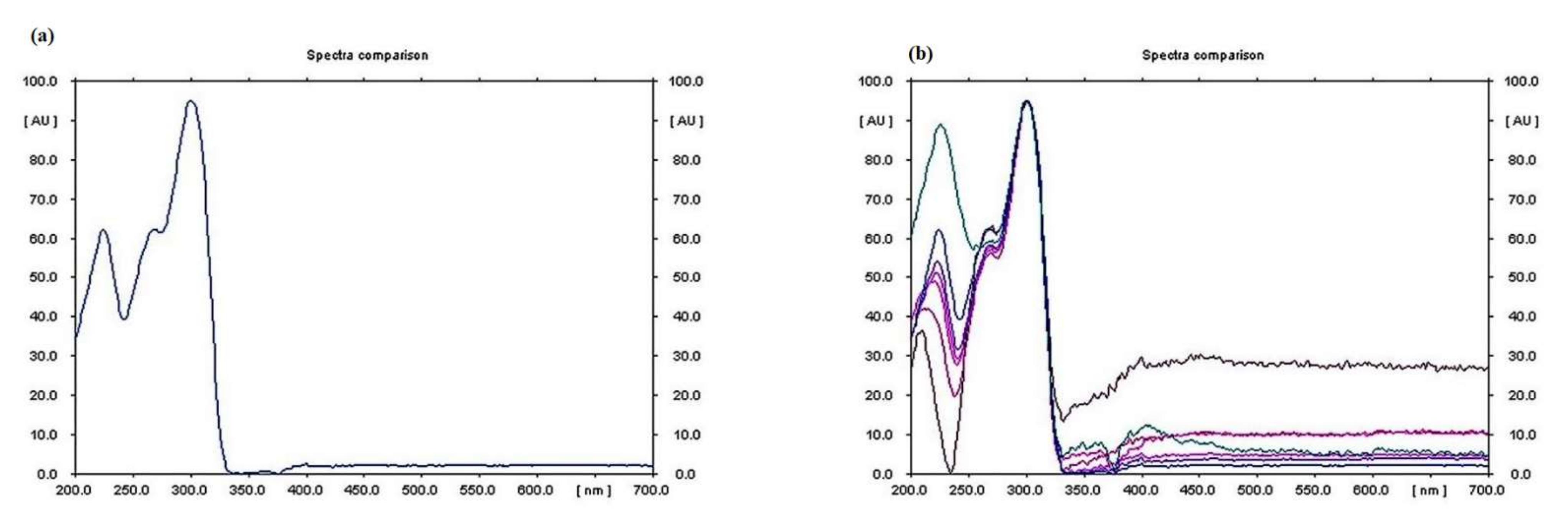
3.4. Qualitative Analysis of Metabolites of Endophytic Fungus
3.5. Quantitative Analysis of Metabolites of Endophytic Fungus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Phytochemicals derived from Catharanthus roseus and their health benefits. Technologies 2020, 8, 80. [Google Scholar] [CrossRef]
- Tiwari, P.; Nithya, R.; Mahalingam, G. Antidiabetic activity of endophytic fungi isolated from Ficus religiosa. Asian J. Pharm. Clin. Res. 2017, 10, 59. [Google Scholar] [CrossRef]
- Pandey, S.S.; Singh, S.; Babu, C.S.V.; Shanker, K.; Srivastava, N.K.; Shukla, A.K.; Kalra, A. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci. Rep. 2016, 6, 26583. [Google Scholar] [CrossRef] [PubMed]
- Ayob, F.W.; Simarani, K. Endophytic filamentous fungi from a Catharanthus roseus: Identification and its hydrolytic enzymes. Saudi Pharm. J. 2016, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole alkaloids from Catharanthus roseus: Bioproduction and their effect on human health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Hittelman, W.; Chambers, T. Cell cycle-dependent mechanisms underlie vincristine-induced death of primary acute lymphoblastic leukemia cells. Cancer Res. 2016, 76, 3553–3561. [Google Scholar] [CrossRef]
- Banasik, M.; Stedeford, T. Plants, Poisonous (Humans): Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 970–978. [Google Scholar]
- Mohammadgholi, A.; Rabbani-Chadegani, A.; Fallah, S. Mechanism of the interaction of plant alkaloid vincristine with DNA and chromatin: Spectroscopic study. DNA Cell Biol. 2013, 32, 228–235. [Google Scholar] [CrossRef]
- Chen, J.; He, H.; Li, S.; Shen, Q. An HPLC method for the pharmacokinetic study of vincristine sulfate-loaded PLGA-PEG nanoparticle formulations after injection to rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1967–1972. [Google Scholar] [CrossRef]
- Thirumaran, R.; Prendergast, G.; Gilman, P. Cytotoxic Chemotherapy in Clinical Treatment of Cancer. In Cancer Immunotherapy; Academic Press: Cambridge, MA, USA, 2007; pp. 101–116. [Google Scholar]
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Wang, M.; Liu, F.; Crous, P.W.; Cai, L. Phylogenetic reassessment of Nigrospora: Ubiquitous endophytes, plant and human pathogens. Persoonia 2017, 39, 118–142. [Google Scholar] [CrossRef] [PubMed]
- Ayob, F.W.; Simarani, K.; Zainal, A.N.; Mohamad, J. First report on a novel Nigrospora sphaerica isolated from Catharanthus roseus plant with anticarcinogenic properties. Microb. Biotechnol. 2017, 10, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Abid, K.; Sayeed, A.; Jalees, A.F.; Kishwarb, S. Simultaneous determination of Vincristine and Vinblastine in Vincarosea leaves by High Performance Thin Layer Chromatography. Int. J. Drug Dev. Res. 2013, 5, 341–348. [Google Scholar]
- Sayed, S.; El-Shehawi, A.; El Arnaouty, S.; Alotaibi, S.; Ahmed, M.; Ibrahim, R.; Elsehy, M. Molecular characterization of endophytic fungal communities associated with Vitis vinifera L. at Taif region of Saudi Arabia. J. Environ. Biol. 2021, 42, 177–185. [Google Scholar] [CrossRef]
- Bruno, W.J.; Socci, N.D.; Halpern, A.L. Weighted Neighbor Joining: A Likelihood-Based Approach to Distance-Based Phylogeny Reconstruction. Mol. Biol. Evol. 2000, 17, 189–197. [Google Scholar] [CrossRef]
- Wiley, E.O.; Brooks, D.R.; Siegel-Causey, D.; Funk, V.A. The CompleatCladist: A Primer of Phylogenetic Procedures; Natural History Museum; University of Kansas: Lawrence, KS, USA, 1991. [Google Scholar]
- Barrales-Cureño, H.J.; Reyes, C.R.; Garcia, I.L.; Jesús, A.G.; Ruiz, J.A.C.; Herrera, L.M.S.; Caballero, M.C.C.; Magallón, J.; Perez, J.; Montoya, J.M. Alkaloids of Pharmacological Importance in Catharanthus roseus; Intechopen: London, UK, 2019. [Google Scholar]
- Kumar, A.; Abnave, P.; Ahmad, A. Cultural, morphological and molecular characterization of vinca alkaloids producing endophytic fungus Fusarium solani isolated from Catharanthus roseus. Int. J. Botany 2013, 3, 1–12. [Google Scholar]
- Birat, K.; Siddiqi, T.O.; Mir, S.R.; Aslan, J.; Bansal, R.; Khan, W.; Dewangan, R.P.; Panda, B.P. Enhancement of vincristine under in vitro culture of Catharanthus roseus supplemented with Alternaria sesami endophytic fungal extract as a biotic elicitor. Int. Microbiol. 2022, 25, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ashoka, H.; Hegde, P.; Manasa, K.H.; Madihalli, C.; Pradeep, S.; Shettihalli, A.K. Isolation and detection of vinca alkaloids from endophytes isolated from Catharanthus roseus. Eur. J. Biomed.Pharm. Sci. 2017, 10, 675–683. [Google Scholar]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn. Rev. 2015, 9, 24–28. [Google Scholar] [PubMed]
- Sonawane, H. Endophytic fungi: A source of potential anticancer compounds. Stud. Fungi 2021, 6, 188. [Google Scholar]
| S. No. | Generation | Color of Colony | Vincristine Content Produced in Extracellular Fungal Methanolic Extract (µg/mL) |
|---|---|---|---|
| 1 | 1st | White | 5.344 |
| 2 | 2nd | Pale white to grey | 1.517 |
| 3 | 3rd | Grey | 2.647 |
| 4 | 4th | Grey | 1.066 |
| 5 | 5th | Brown | 1.150 |
| 6 | 6th | Dark brown | 2.506 |
| 7 | 7th | Black | Nil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birat, K.; Binsuwaidan, R.; Siddiqi, T.O.; Mir, S.R.; Alshammari, N.; Adnan, M.; Nazir, R.; Ejaz, B.; Malik, M.Q.; Dewangan, R.P.; et al. Report on Vincristine-Producing Endophytic Fungus Nigrospora zimmermanii from Leaves of Catharanthus roseus. Metabolites 2022, 12, 1119. https://doi.org/10.3390/metabo12111119
Birat K, Binsuwaidan R, Siddiqi TO, Mir SR, Alshammari N, Adnan M, Nazir R, Ejaz B, Malik MQ, Dewangan RP, et al. Report on Vincristine-Producing Endophytic Fungus Nigrospora zimmermanii from Leaves of Catharanthus roseus. Metabolites. 2022; 12(11):1119. https://doi.org/10.3390/metabo12111119
Chicago/Turabian StyleBirat, Kanchan, Reem Binsuwaidan, Tariq Omar Siddiqi, Showkat Rasool Mir, Nawaf Alshammari, Mohd Adnan, Rahila Nazir, Bushra Ejaz, Moien Qadir Malik, Rikeshwer Prasad Dewangan, and et al. 2022. "Report on Vincristine-Producing Endophytic Fungus Nigrospora zimmermanii from Leaves of Catharanthus roseus" Metabolites 12, no. 11: 1119. https://doi.org/10.3390/metabo12111119
APA StyleBirat, K., Binsuwaidan, R., Siddiqi, T. O., Mir, S. R., Alshammari, N., Adnan, M., Nazir, R., Ejaz, B., Malik, M. Q., Dewangan, R. P., Ashraf, S. A., & Panda, B. P. (2022). Report on Vincristine-Producing Endophytic Fungus Nigrospora zimmermanii from Leaves of Catharanthus roseus. Metabolites, 12(11), 1119. https://doi.org/10.3390/metabo12111119