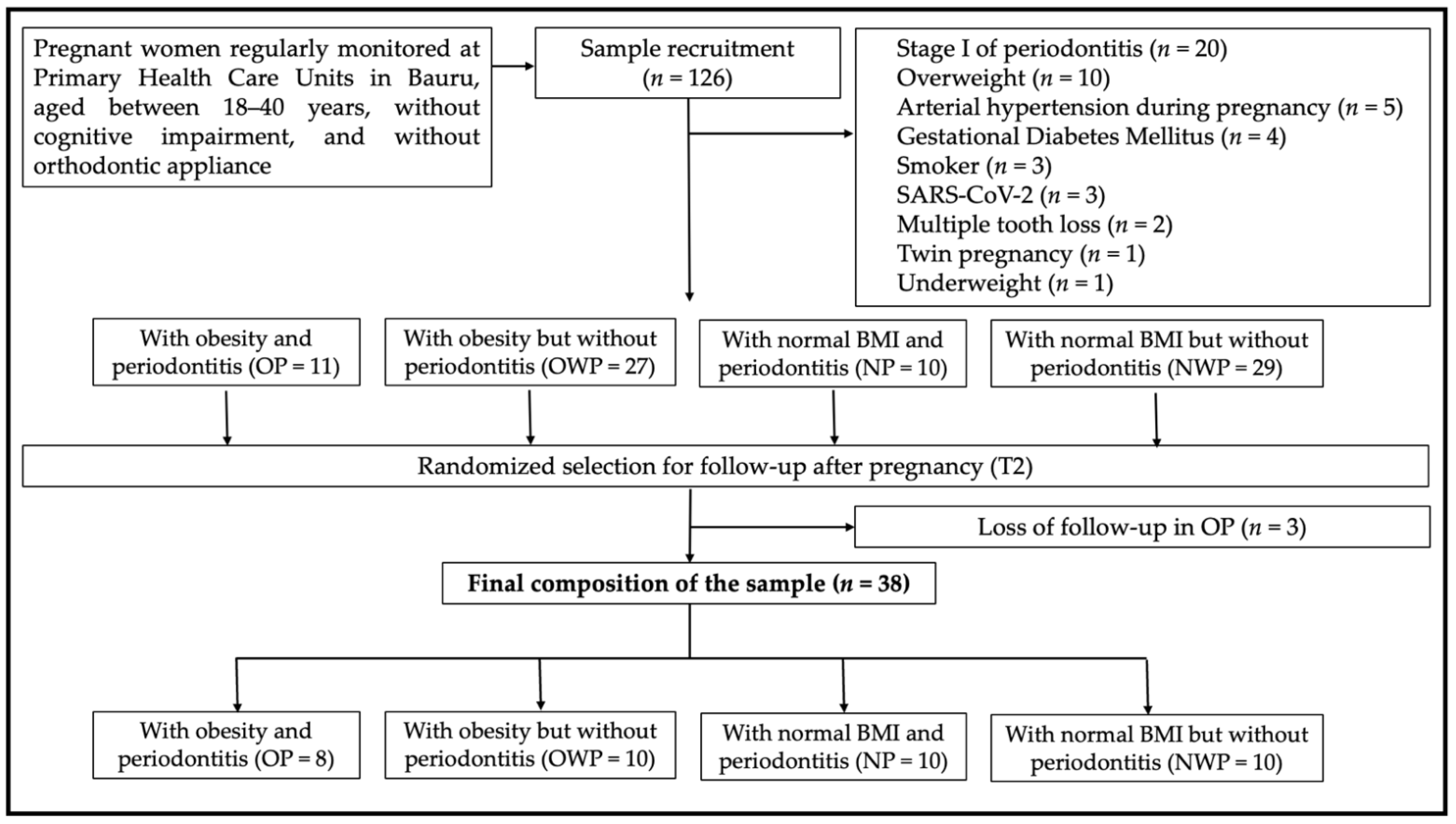
3. Results
Table 1 shows the systemic and periodontal parameters. Groups were homogeneous in terms of socioeconomic levels. Most individuals in all groups indicated that they had complete (total average of 50%) or incomplete (total average of 27.5%) secondary education. Regarding household monthly income, most of the sample indicated receiving up to one minimum wage (MW, approximately
$240.00) (17.5%), up to two MW (35%), or up to three MW (22.5%) monthly.
Groups were also homogeneous regarding unstimulated salivary flow, showing no changes in this parameter over time as well (
p = 0.140). The means of unstimulated salivary flow per group were 0.56, 0.55, 0.57, and 0.56 mL/min in T1 and 0.55, 0.53, 0.56, and 0.56 mL/min in T2 for OP, OWP, NP, and NWP, respectively. Most individuals from the periodontitis groups (five women from OP group and eight from NP group) were classified as stage II of periodontitis, but three pregnant women from OP and two from NP group were classified as stage III (
Table 1).
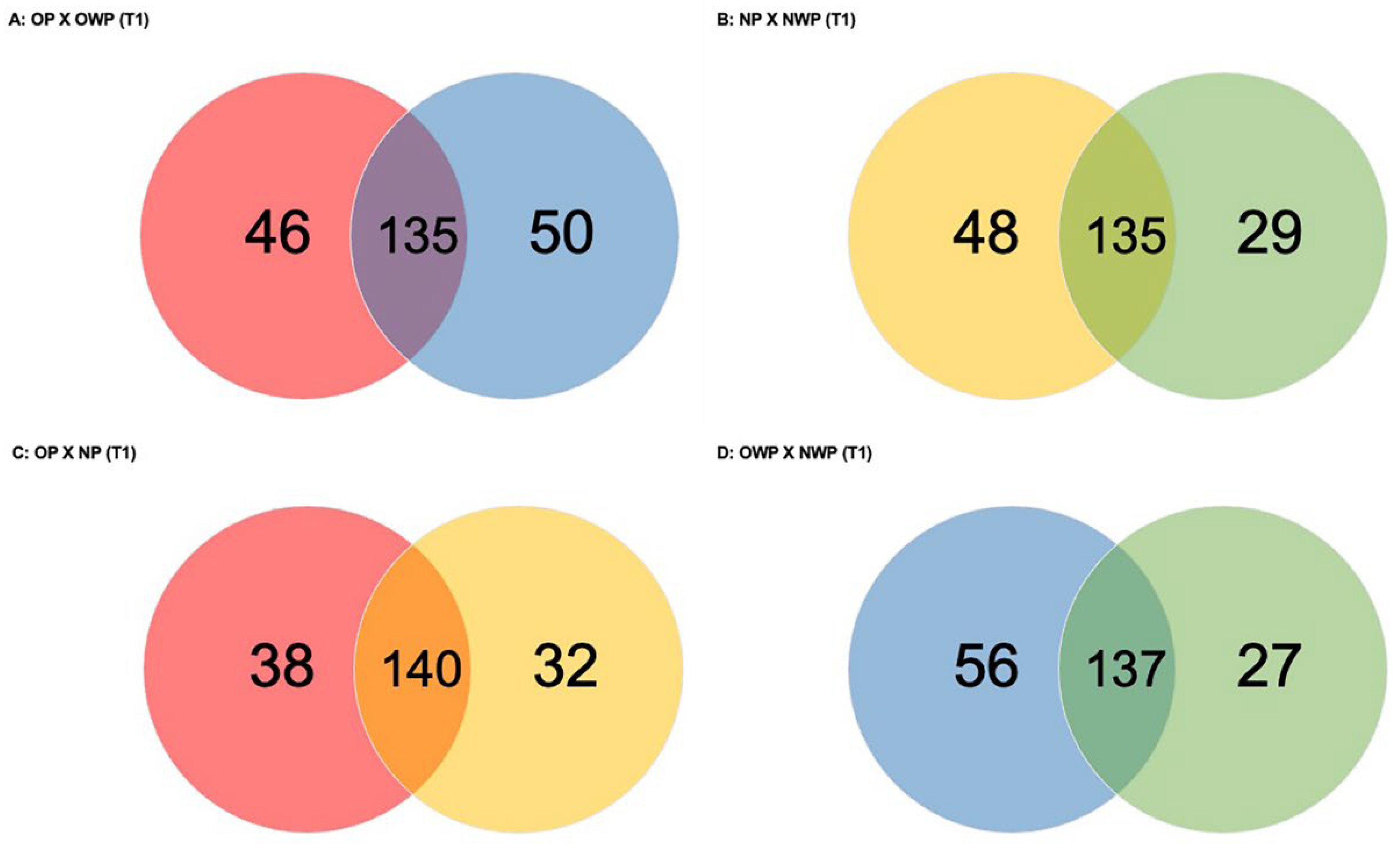
Considering the comparison between OP and OWP in T1 (
Figure 2A), a total of 231 proteins was found (46 and 50 proteins were exclusively identified in OP and OWP, respectively). Groups had 135 proteins in common: 65 were up-regulated in OP (i.e.,
Protein S100-A8,
Histatin-3,
Submaxillary gland androgen-regulated protein 3B—up-regulated more than 2-fold;
Matrix metalloproteinase-9—up-regulated more than 6-fold; 5 isoforms of
Immunoglobulin, 5 isoforms of
Alpha-amylase, 7 isoforms of
Hemoglobin–
Hemoglobin subunit alpha and
beta were up-regulated more than 10-fold; and
Statherin—up-regulated more than 29-fold); and 38 proteins were down-regulated in OP (
Lysozyme C,
Protein S100-A9,
Mucin-7, 5 isoforms of
Cystatin,
Neutrophil defensin 1 and
3,
Basic salivary proline-rich protein 1 and
2—down-regulated more than 4-fold,
Lactotransferrin—down-regulated more than 5-fold, and
WAP four-disulfide core domain protein 2—down-regulated more than 12-fold).
In the comparison between NP and NWP in T1 (
Figure 2B), a total of 212 proteins was found (48 and 29 proteins were exclusively identified in NP and NWP, respectively). Groups had 135 proteins in common: 58 were up-regulated in NP (i.e.,
Cystatin-B, Submaxillary gland androgen-regulated protein 3B, 6 isoforms of
Hemoglobin, 10 isoforms of
Immunoglobulin,
Albumin, and
Serotransferrin—up-regulated more than 2-fold,
Protein S100-A8—up-regulated more than 3-fold,
Alpha-2-macroglobulin—up-regulated more than 4-fold,
Protein S100-A9 and
Deleted in malignant brain tumors 1 protein—up-regulated more than 5-fold); and 41 proteins were down-regulated in NP (i.e.,
Mucin-7, 5 isoforms of
Cystatin,
Lactotransferrin, 7 isoforms of
proline-rich protein—
Salivary acidic proline-rich phosphoprotein 1/2 was down-regulated more than 5-fold,
BPI fold-containing family B member 1—down-regulated 5-fold and
Carbonic anhydrase 6—down-regulated 10-fold).
In the comparison between OP and NP in T1 (
Figure 2C), a total of 210 proteins was found (38 and 32 were exclusively identified in OP and NP, respectively). Groups had 140 proteins in common: 73 were up-regulated in OP (i.e.,
Protein S100-A8 and
A9,
Matrix metalloproteinase-9, 13 isoforms of
Immunoglobulin, 8 isoforms of
proline-rich protein,
Mucin-2—up-regulated more than 2-fold,
Cystatin-B and
Serotransferrin — up-regulated more than 3-fold, 6 isoforms of
Hemoglobin—with
Haptoglobin and
Haptoglobin-related protein up-regulated more than 9-fold,
Hemoglobin subunit gamma-2—up-regulated 19-fold,
Hemoglobin subunit gamma-1 and
subunit epsilon—up-regulated more than 20-fold, and
Hemoglobin subunit alpha—up-regulated more than 36-fold); and 31 proteins were down-regulated in OP (i.e.,
Lactotransferrin, Albumin, Neutrophil defensin 3,
Lysozyme C,
Carbonic anhydrase 6,
Proline-rich protein 4—down-regulated 3-fold,
Mucin-7—down-regulated 4-fold,
Prolactin-inducible protein—down-regulated 5-fold, and 4 isoforms of
Cystatin—
Cystatin-SA and
Cystatin-D were down-regulated more than 3-fold and 5-fold, respectively).
In the comparison between OWP and NWP in T1 (
Figure 2D), a total of 220 proteins was found (56 and 27 were exclusively identified in OWP and NWP, respectively). Groups had 137 proteins in common: 65 were up-regulated in OWP (i.e.,
Albumin, 12 isoforms of
Immunoglobulin,
Lactotransferrin,
Lysozyme C,
Platin-2,
Protein S100-A9,
Neutrophil defensin 1 and
3,
Cystatin-B—up-regulated 4-fold,
Serotransferrin—up-regulated more than 6-fold,
Complement C3—up-regulated 7-fold,
Myeloblastin—up-regulated 8-fold, 7 isoforms of
Hemoglobin–Hemoglobin subunit delta was up-regulated more than 7-fold,
subunit gamma-2 was up-regulated more than 8-fold, and
subunits gamma-1 and
epsilon were up-regulated more than 9-fold, and
WAP four-disulfide core domain protein 2—up-regulated more than 15-fold); and 32 proteins were down-regulated in OWP (i.e., 5 isoforms of
Cystatin,
Histatin-3—down-regulated 2-fold,
Proline-rich protein 4—down-regulated 4-fold,
Carbonic anhydrase 6—down-regulated 6-fold,
Mucin-7—down-regulated 7-fold,
Salivary acidic proline-rich phosphoprotein ½ and
BPI fold-containing family B member 1—both down-regulated 10-fold
, and
Statherin—down-regulated 50-fold in OWP).
Supplementary File S1 is composed by
Table S1A–D that are full tables with all the identified proteins for each group comparation (OP versus OWP; NP versus NWP; OP versus NP; and OWP versus NWP) in T1. These tables highlight the down- and up-regulated proteins, the unique proteins in each group for each comparation, and the proteins with similar expression compared to controls in each comparation. In these tables, scores (natural logs), ratio (fold change), log(e), standard deviation (SD), and
p-values are included. Scores (natural logs) were considered to analyze the initial (prior) probability that any given protein in the database is up- or down-rated. Fold change is the ratio of A/B condition; therefore, in this study it referred to the abundance of a given protein in one group divided by the abundance of another group (its respective control group). The False Discovery Rate (FDR) value applied was 4.
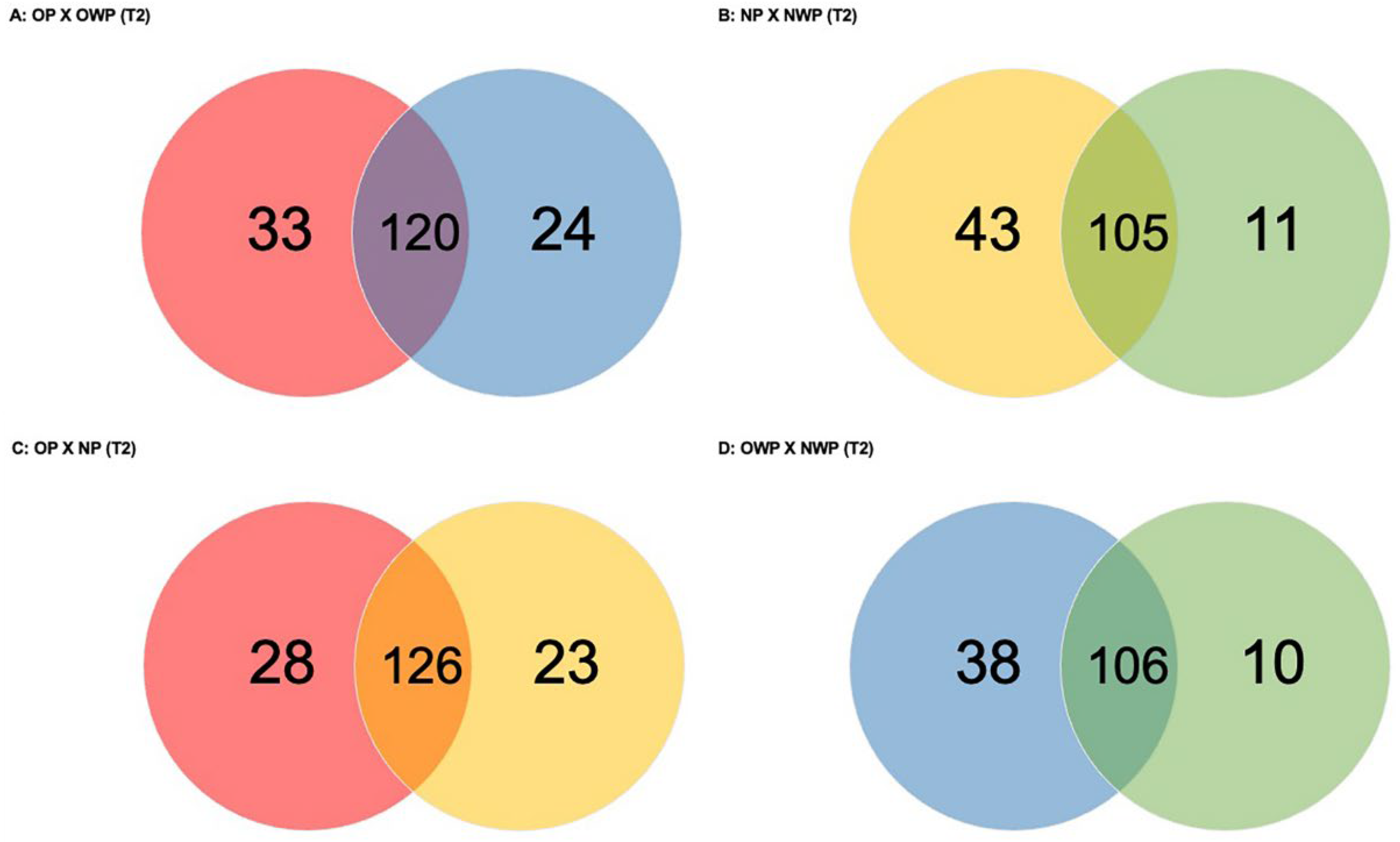
In the comparison between OP and OWP in T2 (
Figure 3A), a total of 177 proteins was found (33 and 24 were exclusively identified in OP and OWP, respectively). Groups had 120 proteins in common: 38 were up-regulated in OP (i.e.,
Hemoglobin subunits delta,
beta and
alpha,
Salivary acidic proline-rich phosphoprotein 1/2,
Statherin, and
Pyruvate kinase PKM were up-regulated approximately 19-fold, 18-fold, 8-fold, 6-fold, 5-fold, and 4-fold, respectively); and 34 were down-regulated in OP (i.e.,
Cystatin-D and
-B,
Hemoglobin subunits epsilon,
gamma-1 and
-2,
Mucin-7, and
Protein S100-A9 were down-regulated approximately 5-fold).
In the comparison between NP and NWP in T2 (
Figure 3B), a total of 159 proteins was found (43 and 11 were exclusively identified in NP and NWP, respectively). Groups had 105 proteins in common: 47 were up-regulated in NP (i.e.,
Alpha-1-antitrypsin,
Apolipoprotein A-I,
Hemoglobin subunits delta and
beta, and
Neutrophil defensin-3 and
-1 were up-regulated approximately 7-fold, 6-fold, 5-fold, 4-fold, 4-fold, and 4-fold, respectively); and 33 were down-regulated in NP (i.e.,
Basic salivary proline-rich protein 2,
Basic salivary proline-rich protein 1, and
Hemoglobin subunits gamma-1 and
-2 were down-regulated 15-fold, 9-fold, 7-fold, and 7-fold, respectively).
In the comparison between OP and NP in T2 (
Figure 3C), a total of 177 proteins was found (28 and 23 were exclusively identified in OP and NP, respectively). Groups had 126 proteins in common: 42 were up-regulated in OP (i.e.,
Fructose-bisphosphate aldolase A,
6-phosphogluconate dehydrogenase, decarboxylating,
Profilin-1, and
Fatty acid-binding protein 5 were up-regulated approximately 3-fold); and 54 were down-regulated in OP (i.e.,
LINE-1 type transposase domain-containing protein 1,
Mucin-7, and
Cystatin-D were down-regulated 20-fold, 14-fold, and 10-fold, respectively).
In the comparison between OWP and NWP in T2 (
Figure 3D), a total of 154 proteins was found (38 and 10 were exclusively identified in OWP and NWP, respectively). Groups had 106 proteins in common: 29 were up-regulated in OWP (i.e.,
BPI fold-containing family B member 1,
Mucin-7,
Cystatin-B, and
Thioredoxin were up-regulated approximately 7-fold, 6-fold, 6-fold, and 3-fold, respectively); and 44 were down-regulated in OWP (i.e.,
Histatin-3,
Submaxillary gland androgen-regulated protein 3B,
Putative Heat shock 70 kDa protein 7, and
Statherin were down-regulated 8-fold, 7-fold, 6-fold, and 5-fold, respectively).
Supplementary File S2 is composed by
Table S2A–D that are full tables with all the identified proteins for each group comparation (OP versus OWP; NP versus NWP; OP versus NP; and OWP versus NWP) in T2. These tables highlight the down- and up-regulated proteins, the unique proteins in each group for each comparation, and the proteins with similar expression compared to controls in each comparation. In these tables, scores (natural logs), ratio (fold change), log(e), standard deviation (SD), and
p-values are included.
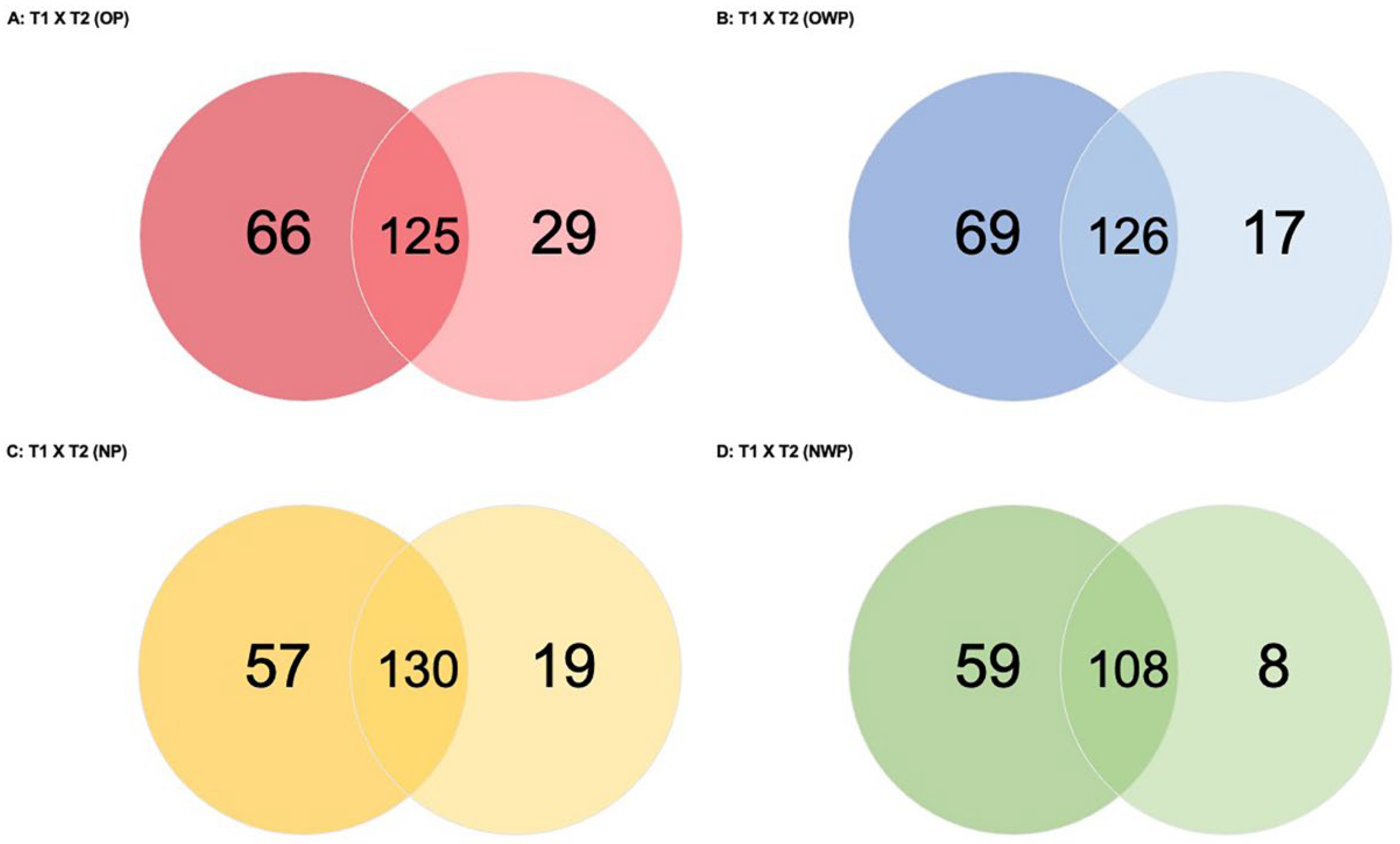
In the comparison between periods for OP (T1 versus T2—OP;
Figure 4A), a total of 220 proteins was found (66 and 29 were exclusively identified in T1 and T2, respectively). Periods had 125 proteins in common: 56 were up-regulated in T1 (i.e.,
Hemoglobin subunits gamma-1,
-2 and
subunit epsilon,
Pregnancy zone protein,
Protein S100-A9, and
Haptoglobin-related protein were up-regulated approximately 22-fold, 21-fold, 21-fold, 4-fold, 4-fold, and 4-fold, respectively); and 45 were down-regulated in T1 (i.e.,
Profilin-1,
Hemoglobin subunit delta,
beta, and
Fructose-bisphosphate aldolase A were down-regulated 9-fold, 7-fold, 4-fold, and 4-fold, respectively).
In the comparison between periods for OWP (T1 versus T2—OWP;
Figure 4B), a total of 212 proteins was found (69 and 17 were exclusively identified in T1 and T2, respectively). Periods had 126 proteins in common: 60 were up-regulated in T1 (i.e.,
Basic salivary proline-rich protein 1,
Lactotransferrin;
WAP four-disulfide core domain protein 2, and
Serotransferrin were up-regulated approximately 29-fold, 8-fold, 6-fold, and 4-fold, respectively); and 38 were down-regulated in T1 (i.e.,
Beta-2-microglobulin,
Protein S100-A9, and
Immunoglobulin mu heavy chain were down-regulated 6-fold, 3-fold, and 3-fold, respectively).
In the comparison between periods for NP (T1 versus T2—NP;
Figure 4C), a total of 206 proteins was found (57 and 19 were exclusively identified in T1 and T2, respectively). Periods had 130 proteins in common: 33 were up-regulated in T1 (i.e.,
Protein S100-A8,
Hemoglobin subunit epsilon, and
Glucose-6-phosphate isomerase were up-regulated approximately 8-fold, 7-fold, and 5-fold, respectively); and 59 were down-regulated in T1 (i.e.,
Beta-2-microglobulin,
Hemoglobin subunit zeta,
Hemoglobin subunit alpha, and
Haptoglobin were down-regulated approximately 13-fold, 12-fold, 7-fold, and 5-fold, respectively).
In the comparison between periods for NWP (T1 versus T2—NWP;
Figure 4D), a total of 175 proteins was found (59 and 8 were exclusively identified in T1 and T2, respectively). Periods had 108 proteins in common: 28 were up-regulated in T1 (i.e.,
BPI fold-containing family B member 1,
Statherin, and
Lactotransferrin were up-regulated approximately 11-fold, 4-fold, and 4-fold, respectively); and 47 were down-regulated in T1 (i.e.,
Hemoglobin subunits beta,
delta,
epsilon, and
gamma-2 were down-regulated approximately 20-fold).
Supplementary File S3 is composed by
Table S3A–D that are full tables with all the identified proteins for the comparations between T1 and T2 for each group (G1—T1 versus T2; G2—T1 versus T2; G3—T1 versus T2; and G4—T1 versus T2). These tables highlight the down- and up-regulated proteins, the unique proteins in each period for each comparation, and the proteins with similar expression compared to controls in each comparation. In these tables, scores (natural logs), ratio (fold change), log(e), standard deviation (SD), and
p-values are included.
The detailed functional analysis with the most significant terms according to the Biological Process, Immune System, Cellular Component, and Molecular Function by Gene Ontologies for group comparations in T1 can be found in
Supplementary Figure S1. In short, for the Biological Process, the categories with the highest percentages of genes among OP and OWP in T1 were humoral immune response (25.7%), negative regulation of endopeptidase activity (17.1%), and antibacterial humoral response (9.5%); while for the Immune System, they were antimicrobial humoral response (37.5%), humoral immune response mediated by circulating immunoglobulin (32.5%), and antimicrobial humoral immune response mediated by antimicrobial peptide (
Supplementary Figure S1A). Among NP and NWP in T1 for the Biological Process, they were humoral immune response (25.7%), retina homeostasis (20.8%), negative regulation of endopeptidase activity (13.9%), and antioxidant activity (10.9%), while for the Immune System, they were humoral immune response mediated by circulating immunoglobulin (44.1%), antimicrobial humoral response (35.3%), and mucosal immune response (8.8%) (
Supplementary Figure S1B). When OP was compared to NP in T1, the highest percentages of genes for the Biological Process were related to the defense response to bacterium (22.4%) and tissue homeostasis (21.4%), while for the Immune System they were antimicrobial humoral response (44.4%) and complement activation (37%) (
Supplementary Figure S1C).
In the same way, the detailed functional analysis with the most significant terms according to the Biological Process, Immune System, Cellular Component, and Molecular Function by Gene Ontologies for group comparations in T2 can be found in
Supplementary Figure S2. In short, for the Biological Process, the categories with the highest percentages of genes among OP and OWP in T2 were retina homeostasis (24.6%), antioxidant activity (15.4%), antimicrobial humoral response (12.3%), and cysteine-type endopeptidase inhibitor activity (12.3%), while for Immune System, they were antimicrobial humoral response (40%) and complement activation, classical pathway (35%) (
Supplementary Figure S2A). Among NP and NWP in T2, for the Biological Process, the categories with the highest percentages were defense response to bacterium (26.1%), humoral immune response (23.9%), and retina homeostasis (18.5%), while for Immune System, they were humoral immune response mediated by circulating immunoglobulin (62.5%) and antimicrobial humoral response (37.5%) (
Supplementary Figure S2B). When OP was compared to NP in T2, the highest percentages of genes for the Biological Process were related to the defense response to bacterium (22.5%), humoral immune response (20%), and retina homeostasis (15.8%), while for the Immune System, they were humoral immune response mediated by circulating immunoglobulin (46.7%) and antimicrobial humoral response (40%) (
Supplementary Figure S2C).
Finally, the detailed functional analysis with the most significant terms according to the Biological Process, Immune System, Cellular Component, and Molecular Function by Gene Ontologies for comparations between periods can be found in
Supplementary Figure S6. In short, when the Biological Process by GO was compared between T1 and T2 for OP, the main terms were defense response to bacterium (20.3%), humoral immune response (19.6%), and retina homeostasis (15.2%) (
Supplementary Figure S6A), while for the Immune System, they were humoral immune response mediated by circulating immunoglobulin (45.7%) and antimicrobial humoral response (37.1%). For the Biological Process compared between periods for NP, the following percentages for the same main terms were found: defense response to bacterium (21.1%), humoral immune response (19.5%), and retina homeostasis (14.6%), while for the Immune System, they were humoral immune response mediated by circulating immunoglobulin (57.7%) and antibacterial humoral response (26.9%) (
Supplementary Figure S3C).
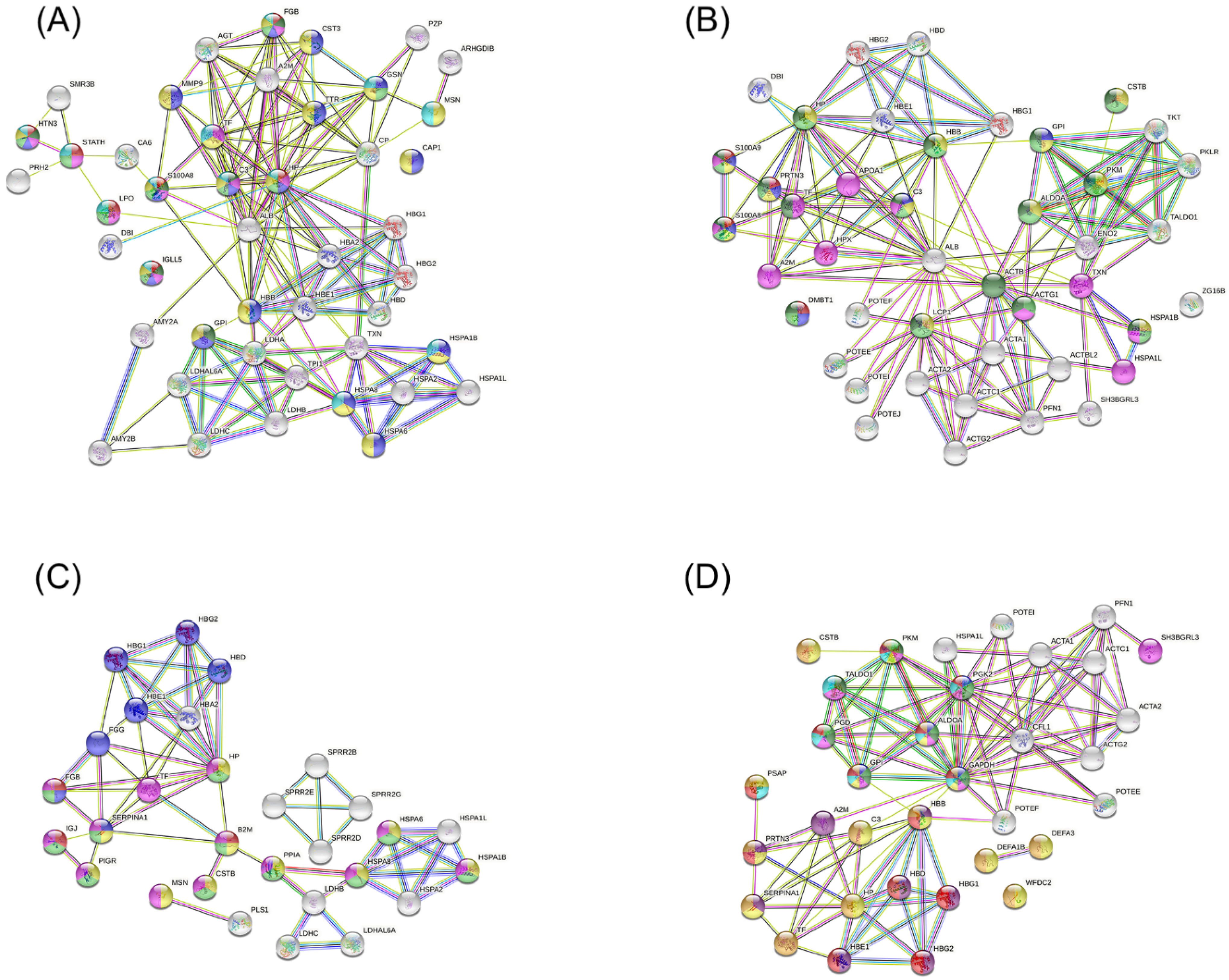
Figure 5 shows the interaction networks between up-regulated proteins identified in each group during pregnancy for the following comparations: OP versus OWP (
Figure 5A), NP versus NWP (
Figure 5B), OP versus NP (
Figure 5C), and OWP versus NWP (
Figure 5D). Regarding the comparations between OP and OWP, the proteins that had the greatest number of functions were
Fibrinogen beta chain (FGB),
Histatin-3 (HTN3),
S100-A8 (S100A8),
Complement C3 (C3),
Haptoglobin-related protein (HP), and
Immunoglobulin lambda like polypeptide 5 (IGLL5) (
Figure 5A). For the comparations between NP and NWP, they were
S100-A9 (S100A9),
S100-A8 (S100A8),
Myeloblastin (PRTN3), and
Complement C3 (C3) (
Figure 5B). For the comparation between OP and NP, the proteins with the greatest number of functions were
Beta-2-microglobulin (B2M),
Alpha-1-antitrypsin (SERPINA1), and
Fibrinogen beta chain (FGB) (
Figure 5C). For the comparations between OWP and NWP, they were
Pyruvate kinase (PKM),
Phosphoglycerate kinase 2 (PGK2),
6-phosphogluconate dehydrogenase, Decarboxylating (PGD),
Fructose-bisphosphate aldolase A (ALDOA),
Glucose-6-phosphate isomerase (GPI), and
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (
Figure 5D). The main functions of these proteins can be found in the legend of
Figure 5.
4. Discussion
With technological advances, many studies sought to identify disease biomarkers through body fluids. The analysis of saliva biomarkers has been widely performed because it is an easy, non-invasive, and, consequently, painless method. Before the detection of salivary biomarkers, the mapping of differentially expressed proteins in the saliva of the target population is necessary. Our results highlighted several differentially expressed proteins associated with obesity and periodontitis separately. Nonetheless, this study calls attention to the importance of those up- or down-regulated proteins when obesity and periodontitis are present in combination during pregnancy, such as Submaxillary gland androgen-regulated protein 3B, Protein S100-A8, Matrix metalloproteinase-9 (MMP9), Heat shock 70 kDa protein 2 and 6, Putative Heat shock 70 kDa protein 7, Heat shock 71 kDa protein, Haptoglobin, Plastin-1, Prolactin-inducible protein, and Alpha-defensins 1 and 3.
An important and recent report on the longitudinal changes in salivary proteomics across term pregnancy showed that most differentially regulated proteins that they found were associated with neutrophil degranulation, the regulation of Toll-like receptor by endogenous ligands, antimicrobial peptide function, platelet function regulation, and glucose metabolism [
24]. Among the proteins related to these mechanisms they cited
Heat shock protein cognate 71 kDa,
Protein S100-A8, Protein S100-A9, Cathepsin-D, MMP9,
Fructose-bisphosphate aldolase A, Complement C3, Lactotransferrin, Myeloblastin, Enolase-1, and
Galectin-3-binding protein [
24].
Higher abundances of MMP9,
S100 proteins (
S100-A6/
-A8/
-A9),
Complement C3,
profilin-1,
Alpha-2-macroglobulin,
Haptoglobin,
Submaxillary gland androgen-regulated protein,
Histatin-1,
Fatty acid-binding protein,
Thioredoxin, and
Albumin were previously linked to periodontitis [
6,
7,
9,
25,
26,
27,
28,
29,
30]. In contrast, lower levels of
Lactotransferrin,
Prolactin-inducible proteins,
Salivary acid proline-rich phosphoprotein 1/2, and
Cystatin (mainly
S,
SA, and
SN) were shown to be associated with periodontitis. Our findings are in line with these previous studies and the main highlights are discussed in detail below.
In this study, pregnant women with periodontitis (OP and NP groups) had higher levels of
Albumin when compared to their respective controls (OWP and NWP groups, respectively). We hypothesized
Albumin levels were influenced by gestational hormones since all groups showed an increase in albumin levels after delivery. The main function of
Albumin is the regulation of the colloidal osmotic pressure of blood and some hormones, acting as an ion transporter as well [
7]. It is believed that some periodontal microbes that trigger an inflammatory response, such as
T. denticola, increase the levels of salivary
Albumin. Also, these microbes use
Albumin, as well as
Immunoglobulins, as potential energy sources [
31].
Submaxillary gland androgen-regulated protein 3B is a secreted endopeptidase that inhibits the cleavage of peptide bonds of non-terminal amino acids and has a role in the regulation of sensory perception of pain [
7]. Yet, previous evidence found that lipopolysaccharide of
P. gingivalis binds to
Submaxillary gland androgen-regulated protein 3B [
32], and probably it is related in promoting angiogenesis and establishing microvasculature, associating with periodontal diseases. In this study, OP and NP also had higher levels of
Submaxillary gland androgen-regulated protein 3B when compared to OWP and NWP, but the level was more than 2-fold higher in OP when compared to NP. Curiously, women with obesity (OP and OWP) showed a decrease in this protein after delivery, while eutrophic women (NP and NWP) had an increase.
Thioredoxin is related to cellular oxidant detoxification, cellular responses to a toxic substance, cell redox homeostasis, and the inhibition of caspase-3 activity that interferes with cellular apoptosis [
7]. In this study,
Thioredoxin was approximately 3-fold and 2-fold up-regulated in OP and NP, respectively, when compared to their controls (OWP and NWP), but with no difference for the comparation of OP versus NP.
In this study,
Protein S100-A8 was also up-regulated in the saliva of pregnant women with obesity and periodontitis, and after delivery the level of this protein increased even more in this group.
Protein S100-A8 and
S100-A9 were up-regulated approximately 4-fold and 5-fold, respectively, in NP when compared to NWP. These proteins are calcium- and zinc-binding proteins with functions involving proinflammatory, antimicrobial, oxidant-scavenging, and apoptosis-inducing activities. Their proinflammatory activity includes the recruitment of leukocytes, promotion of cytokine and chemokine production, and regulation of leukocyte adhesion and migration [
24,
33]. Previous evidence pointed out that these proteins are involved in neutrophil migration to inflammatory sites [
34], corroborating our findings. Nonetheless, our results suggest their up-expression was more significative in OP than in NP, therefore being possible biomarkers of the combination of obesity and periodontitis during pregnancy, and this should be evaluated in future studies.
MMP9 was separately associated with both obesity and periodontitis in this study, but it was even more expressed in the saliva of pregnant women with the combination of these outcomes, as a result of those two distinct inflammatory processes. In contrast, it was not present in pregnant women with normal BMI and without periodontitis. MMP9 is an enzyme known to degrade many components of the extracellular matrix, having a role both in the physiological tissue remodeling and in the pathological tissue destruction [
33]. Previous studies highlighted that MMP9 is one of the major collagen-degrading enzymes in saliva [
33,
35], which is associated with periodontitis. Interestingly, our report shows that pregnant women with obesity and periodontitis expressed higher levels of
Histatin-3 and
Metalloproteinase inhibitor 1 (MMP1), with the latter being a protein uniquely expressed in OP.
Histatin-3 is a salivary protein that exhibits antibacterial and antifungal activities, and it has the His3-(20–43)-peptide, which is a potent inhibitor of MMP2 and MMP9 [
36], similarly to MMP1. Therefore, we hypothesized that this is a physiological defense mechanism that occurs in OP to prevent pathological tissue destruction upon the high levels of MMP9.
An important result from this study that deserves attention is related to the expression of
Heat shock proteins (HSPs). In addition to being related to the immune response and interspecies interactions between organisms (
Figure 5), HSPs also actively participate in the metabolic and catabolic processes of the organism. HSP70 and HSP71 are examples of different classes of this protein. HSP70 has intracellular and extracellular activities, including cytoprotection and immune modulation response. Due to its protective role and inhibition of apoptosis, HSP70 protects cells from tissue destruction [
37]. Previous evidence showed a positive expression of HSPs in the basal layer of periodontal pockets, highlighting that there is an increase in the infiltration of mononuclear inflammatory cells below the basal layer of periodontal pockets [
37,
38,
39]. Therefore, periodontal bacteria stimulate the periodontal cells to increase the expression of HSPs, which, in turn, stimulate macrophages and other inflammatory cells to produce proinflammatory cytokines, a mechanism that is involved in the tissue destruction of periodontitis [
37,
38,
39].
In this study, Heat shock 70 kDa protein 1A, 1B and 1-like were more expressed in OP and NP when compared to their respective controls (OWP and NWP, respectively). However, when comparing OWP with NWP, these proteins were also more expressed in the former, showing their relationship with the inflammatory process resulting from obesity as well. Nevertheless, our findings call for attention to be paid to the greater expression of Heat shock 70 kDa protein 2 and 6, Putative Heat shock 70 kDa protein 7, and Heat shock 71 kDa protein, which seem to be proteins that mark the combination of obesity and periodontitis during pregnancy. Interestingly, Heat shock 70 kDa protein 6 and Putative Heat shock 70 kDa protein 7 were not found in the saliva samples of women from the OP group after delivery. Similarly, Heat shock 70 kDa protein 6, Putative Heat shock 70 kDa protein 7, Heat shock 70 kDa protein 2, and Heat shock cognate 71 kDa protein were present in the saliva samples of the NP group in T1 only. We suggest that future studies should be carried out to establish a deeper understanding of HSPs as potential biomarkers of periodontitis in pregnancy, being associated, or not, with obesity.
A classical study had already found higher levels of
Haptoglobin in periodontitis cases [
40]. That result was confirmed by Haigh and collaborators (2010), who indicated that this protein was associated with host defense [
27]. Similarly, our results showed that
Haptoglobin was an important up-regulated protein in the saliva of pregnant women with obesity and periodontitis. Despite this protein being more abundant in the saliva of NP in comparation to NWP, the level of this protein was even higher in individuals with both obesity and periodontitis.
Haptoglobin is a hemoglobin-binding acute-phase protein that possesses anti-inflammatory and antioxidative properties. This protein may act as a bacteriostatic agent and, indirectly, as an antioxidant due to its facility to bind free hemoglobin and promote its clearance by macrophages. Considering that Langerhans cells in the epithelium can also synthesize
Haptoglobin, its levels may be in increased expression in local or systemic inflammation [
41], justifying our results related to the higher levels of this protein in obese women with periodontitis.
A recent study showed an underrepresentation of cysteine endopeptidase inhibitor activity in healthy pregnant women and pregnant women with gingivitis (mediated by
type-2 cystatins, mainly
Cystatin-S,
-SA, and
-SN) [
42]. Previous evidence also showed lower levels of the
S-type salivary cystatins during gingivitis and periodontitis [
43,
44]. These results are in line with our study. When compared to OWP, pregnant women with obesity and periodontitis showed a down-regulation of
Cystatin-S,
-SA,
-SN,
-B, and
-D, while
Cystatin-C was up-regulated. When NP was compared to NWP,
Cystatin-S,
-SA,
-SN,
-C, and
-D were down-regulated, and
Cystatin-B was up-regulated. We hypothesize that as
Cystatin-C is related to hormonal influence, endotheliosis, and glomerular filtration rate [
45], its higher expression was expected in OP due to the physiological mechanisms related to obesity and pregnancy.
Cystatins are protease inhibitors abundantly found in saliva and they have an important role in inhibiting tissue-destructive proteases in inflammatory processes, such as lysosomal cathepsins B, H, and L in the oral cavity [
42]. Additionally, despite
cystatins not being able to inactivate the proteases from bacterial origin, they play an important role in inhibiting the growth of species associated with periodontal impairment, such as
P. gingivalis and
A. actinomycetemcomitans [
42], justifying our findings related to the low levels of
cystatins in OP and NP. In addition, to reinforce this hypothesis, our results showed that other important antimicrobial proteins, such as
BPI fold-containing family A member 2 and
Lipocalin-1 (or
Putative lipocalin 1-like protein 1) were less expressed in OP and NP, and, curiously, there was an increase in the levels of
Cystatin-C,
-S,
-SA,
-SN, and
Lipocalin-1 in OP after delivery, while
Cystatin-B and
Cystatin-C decreased even more. In the NP group, there was an increase in
Cystatin-SN and
Lipocalin-1 levels after delivery, but
Cystatin-SA,
-B,
-C, and
-D decreased.
Prolactin-inducible protein (PIP) is responsible for the negative regulation of the T cell apoptotic process, positive regulation of gene expression, and proteolysis. PIP binds to a variety of oral bacteria, suggesting it has a role in protecting the oral mucosa by inhibiting bacterial colonization and growth [
27]. Similarly,
Lactotransferrin is an iron-binding protein, and its antibacterial effect is achieved by competing for iron with bacteria, thereby inhibiting bacterial growth [
25]. In this study, both PIP and
Lactotransferrin were down-regulated in pregnant women with periodontitis (OP and NP) when compared to OWP and NWP (
Lactotransferrin was down-regulated 5-fold and 3-fold in OP and NP, respectively). When OP was compared to NP, PIP was more than 5-fold down-regulated in OP, showing its down-regulation is strongly associated with the combination of obesity and periodontitis during pregnancy.
Interestingly, this study showed that
Plastin-1 is a specific protein present in saliva of pregnant women with periodontitis (both OP and NP), but with higher expression when periodontitis is associated with obesity (approximately 4-fold), and this protein is present in these groups only during pregnancy. To the best of our understanding, there is no previous evidence associating
Plastin-1 with periodontitis. Baliban et al. (2012) found
Plastin-1 and
-2 in the investigated samples (health and periodontitis cases), but there was no intergroup difference, and the authors did not indicate the plausible mechanism of this protein [
46]. Previous evidence showed a positive correlation of
L-Plastin (or
Plastin-2) with the presence and severity of periodontitis, suggesting that L-Plastin-expressing cells are participating in local inflammatory responses, and the activation of
L-Plastin mediates leukocyte adhesion and migration, as well as osteoclast adhesion and bone resorption [
47]. Nevertheless, there is a gap in the literature regarding the role of
Plastin-1. Recently,
Plastin-1 was shown to be highly homologous to
Plastin-3, whose mutations are responsible for X-linked osteoporosis. In addition, it was revealed that
Plastin-1 promotes osteoblast differentiation by regulating intracellular Ca
2+ [
48]. Therefore, we hypothesize that the presence of
Plastin-1 in OP and NP only in T1 could be a compensatory mechanism in those pregnant women due to the high inflammatory process and bone loss, acting in bone homeostasis. Further investigations must pay attention to the role and the mechanism of
Plastin-1 in periodontitis individuals.
In this study, proteins related to glycolytic, carbohydrate catabolic, glucose metabolic, organophosphate metabolic, and carbohydrate derivative metabolic processes (such as
6-phosphogluconate dehydrogenase, Decarboxylating;
Pyruvate kinase PKM;
Glyceraldehyde-3-phosphate dehydrogenase;
Transaldolase;
Fructose-bisphosphate aldolase A;
Phosphoglycerate kinase 2; and
Glucose-6-phosphate isomerase) were more expressed in individuals with obesity (
Figure 5). In contrast, proteins related to the immune system, such as those associated with the antimicrobial humoral response and response to other organism (such as
BPI fold-containing family B member 1 and
2,
Secretory leucocyte peptidase inhibitor,
Mucin-7,
Histatin-3, and
Statherin) were down-regulated in individuals with obesity (
Figure 5). The literature is scarce regarding salivary proteomic analysis in association with obesity. Lamy et al. (2015) reported higher levels of
Zinc-α-2 glycoprotein in individuals with obesity, as well as a tendency for them to present higher levels of
Carbonic Anhydrase 6 (CA-VI) [
49]. Our findings are partially in accordance with that evidence. In this study, the CA-VI levels were decreased in individuals with obesity (OP and OWP) when compared to their controls (NP and NWP, respectively), while
Zinc-α-2 glycoprotein was positively associated with obesity when we compared OP versus NP since it was a specific protein found only in OP; however, there was no intergroup difference when OWP was compared to NWP.
Rangé et al. (2012) suggested an overexpression of
alpha-defensins in individuals with obesity and revealed that the down-expression of the
alpha-defensins in the saliva of periodontitis patients versus non-periodontitis patients seems to be independent of the obesity status [
9]. In this study,
Neutrophil defensin 1 and
3 were down-regulated in OP, even when they were compared to OWP and NP. We did not find intergroup differences for these proteins when we compared NP to NWP. Therefore, we suggest that
Neutrophil defensin 1 and
3 are further investigated as potential biomarkers of the combination between obesity and periodontitis during pregnancy.
This study has some limitations. Future evaluations must be carried out through the gestational trimesters and with more cut-time points after delivery to understand the cause and effect regarding the physiopathology of the disease during pregnancy. Moreover, in this study, individuals with stages II and III of periodontitis were included in the same sample group (for both OP and NP). Ideally, future longitudinal studies with larger samples should analyze specific proteins expressed in saliva considering the different stages of the periodontal diseases (including gingivitis as well), and of the BMI range (i.e., underweight, and overweight classifications), to ensure a better biological understanding regarding the progression of the diseases. Moreover, this study did not evaluate the nutritional/dietary profile of the sample. It is extremely important to understand how the dietary pattern would reflect in the salivary proteomic analysis of the population studied. For this, future transdisciplinary studies, involving well-defined protocols for characterizing the dietary pattern, should be conducted. Furthermore, this study did not access the bacterial profile of the sample, but metagenomic assessments would contribute to our understanding of the identified proteins and their association with outcomes, taking into account, for instance, qualitative and quantitative bacterial evaluations of saliva and gingival fluid. Finally, future studies using specific methods that isolate and quantify the proteins presented here are necessary to validate our results (i.e., enzyme-linked immunosorbent assay). Despite the limitations, this is the first study to investigate the biological mechanisms related to salivary proteome associated with obesity and periodontitis during pregnancy and after delivery using an individual label-free quantitative shotgun proteomic analysis.