Abstract
Lingtou Dancong oolong tea is a famous Chinese oolong tea due to its special honey-like aroma. However, little is known about its specific aroma profile and key contributors. Furthermore, whether the aroma characteristics of Lingtou Dancong oolong tea are affected by the environmental conditions at different altitudes is unknown. In this study, the aromas in Lingtou Dancong oolong tea were extracted and analyzed by stir-bar sorptive extraction (SBSE) combined with gas chromatography-olfactometry (GC-O) and GC-mass spectrometry (GC-MS), and the aroma profiles of tea plants grown at different altitudes were compared. We detected 59 odor compounds in Lingtou Dancong oolong tea. Eight compounds with honey and floral odors were identified as key components on the basis of GC-O, GC-MS, odor activity value, and flavor dilution analyses. Differences in the contents of precursor geranyl diphosphate and transcript levels of structural genes were found to be responsible for the differential accumulation of linalool and hotrienol among plants grown at different altitudes. This is the first report on the aroma characteristics and key contributors of Lingtou Dancong oolong tea and their differences, as affected by altitude. These results provide details of the chemical basis of the aroma quality of Lingtou Dancong oolong tea.
1. Introduction
Tea is one of the most popular non-alcoholic beverages in the world and its popularity is closely related to its delicious taste and pleasant aroma [1]. Different processing methods contribute to taste and aroma. Tea can be divided into six categories, according to its quality characteristics and processing methods: green tea, white tea, yellow tea, oolong tea, black tea, and dark tea. Among them, oolong is semi-fermented tea, and therefore has both the fragrance of green tea and the mellow taste of black tea. It is also famous for its rich and varied floral fragrance. Dancong tea, which is mainly produced in the Guangdong province, has a long history of cultivation in China. This type of tea includes Camellia sinensis cv. Lingtou Dancong, a national variety in China, whose core production area is the Fenghuang mountain. Oolong tea made from fresh leaves of C. sinensis cv. Lingtou Dancong is loved by consumers because of its unique honey-like flavor. It has been approved as a product with high economic value and a national geographical indication.
The aroma of oolong tea is affected by many factors before and after harvesting. At the pre-harvest stage, the genotype of the tea variety plays an important role in the aroma of tea products, and the aroma of Dancong tea produced from fresh leaves differs widely among varieties and strains [1,2,3]. The growing environment of tea plants is another crucial factor in aroma formation. The ecological environment as a whole (i.e., the combination of light, temperature, and soil conditions) at different altitudes affects the types and contents of aroma compounds in Dancong tea [4]. The turnover process of oolong tea also affects its quality. However, few studies have explored the unique aroma characteristics of tea products made from the Lingtou Dancong cultivar (also known as Baiye Dancong), and whether this special aroma is affected by environmental factors [2].
Aroma compounds can be extracted using a variety of methods, including solvent extraction and headspace collection, and detected using methods such as gas chromatography-mass spectrometry (GC-MS) and GC-olfactometry (GC-O). These detection methods can be supplemented by statistical analyses and various calculations to analyze aroma compounds in detail [5]. More than 700 aroma compounds have been identified in tea leaves and tea soups, but the aroma characteristics of tea leaves are determined by a few key contributing aroma compounds. Many studies have shown that the human-perceivable odor is related to the threshold and concentration of aroma compounds, so the information obtained by a detector cannot completely reflect the real aroma [5]. Therefore, GC-O/MS is a promising method for fragrance analyses.
In this study, the aroma components in leaves of Lingtou Dancong oolong tea plants grown at different altitudes were comprehensively analyzed by GC-O/MS. The aroma components of oolong tea grown at different altitudes were identified and quantified, and the reasons for differences in their types and contents among these materials were explored. The overall aim of this study was to determine which compounds are responsible for the honey-like aroma of Lingtou Dancong oolong tea and to provide specific guidance for cultivating tea under the most suitable environmental conditions.
2. Materials and Methods
2.1. Materials and Treatments
The oolong tea was made from C. sinensis cv. Lingtou Dancong picked from Danhu village, Fenghuang town, Chaoan district (810 m), Qipan village, Fenghuang town, Chaoan district (560 m), and Xiaxiao village, Fubin town, Raoping distinct (250 m) in Chaozhou, Guangdong, China. All the materials were harvested in April 2020 and were evaluated using the stir-bar sorptive extraction (SBSE) approach combined with a gas chromatography-olfactometry/mass spectrometry (GC-O/MS) technique. The picking standard was one bud, two or three leaves, and the stem. The picked fresh leaves were made into oolong tea [1]. The processing method was as follows: the freshly plucked tea leaves were exposed to sunlight, outside, for 25–40 min, and then indoor-withered at 30 °C until the humidity of the leaves fell to 70%. The tea leaves were turned over 5–7 times every 1.5–2 h, rolled and dried at 120–130 °C for 10 min. Finally, the tea leaves were dried at 85–90 °C for 3 h.
The fresh leaves of C. sinensis cv. Lingtou Dancong were picked from plants cultivated at different altitudes in Chaozhou, Guangdong, China. The picking altitudes were 730 m (high altitude), 380 m (medium altitude), and 110 m (low altitude). All samples were harvested in October 2021, and the picking standard was one bud, two or three leaves, and the stem or clip leaf. Wounding stress from the oolong tea manufacturing process was the key factor responsible for the aroma formation [1]. Therefore, a portion of the picked leaves was subjected to a single wounding treatment (standing for 3 h) and another portion was subjected to a continuous wounding treatment (shaking for 3 h at 300 rpm). The temperature of both the single wounding treatment and continuous wounding treatment were 26 °C and the humidity were 70%. All samples were quickly frozen in liquid nitrogen and then stored at −80 °C until further analysis.
2.2. Extraction of Volatile Compounds
Volatile compounds were extracted by SBSE, as described elsewhere [6]. Each ground tea sample (1 g) was weighed into a headspace glass bottle (Agilent Technologies, Palo Alto, CA, USA). A polydimethylsiloxane twister and 10 mL distilled water, heated to 100 °C (Hangzhou Wahaha Group Co., Ltd., Hangzhou, China), were quickly added into the bottle, the bottle was tightly capped, and then the contents were stirred at 70 °C for 30 min at 1000 rpm. Finally, the volatiles were analyzed using a thermal desorption unit-cooled injection system (TDU-CIS) and GC-MS. The parameters for TDU-CIS, GC-MS, and GC-O were as described in a previous study [7]. The temperature program for the TDU was as follows: initial temperature of 30 °C (maintained for 1 min), increased to 240 °C in 2.4 min, and maintained for 5 min. The temperature program for the CIS-4 (Gerstel) GC inlet was −100 °C for 1 min, increased to 280 °C at 12 °C/s, and then held for 3 min. An Agilent 7890B/5977B GC equipped with an HP-5MS column (30 m × 250 μm × 0.25 μm, Agilent) was used to detect and quantify odor compounds using the parameters described previously, with some changes [6]. The temperature of quadrupole and mass spectrometer transmission line were set to 150 °C and 250 °C, respectively. The full scan range was m/z 40–450.
2.3. Identification and Quantification of Aroma Compounds in Lingtou Dancong Oolong Tea
The improved standard addition method was used to identify odorants [8]. A series of mixed standards was added to the prepared blank matrix of tea with the aroma compounds removed. The blank matrix was extracted and analyzed by the method described in Section 2.2. The peak area of odorants in samples was substituted into the standard curve to calculate their contents accurately. Methyl salicylate, geraniol, indole, and trans-β-ionone were ordered from Aladdin Corp. (Shanghai, China). α-Ionone, γ-nonanolactone, β-myrcene, 1-octen-3-ol, nonanal, butanal, 2-methyl-, dihydroactindiolide, jasmine lactone, γ-octanolactone, (E)-2-octenal and other chemicals were purchased from J&K Scientific Ltd. (Beijing, China) or Sigma-Aldrich (Shanghai, China).
The aroma compounds were analyzed using the Agilent ChemStation/LeCo Chroma TOF workstation and identified by comparison with data in the NIST2014 library, n-alkane retention indices (C8–C40), and authentic standards, by using tools in ODI software (Gerstel EL GMBH & Co., Mülheim An Der Ruhr, Germany), and on the basis of aroma standard descriptions (http://www.thegoodscentscompany.com/search3.php?qOdor=20126-76-5&submit.x=9&submit.y=9, accessed on 26 August 2022). Accurate quantification was performed by authentic standards. For available standards, the authentic standard was used for quantification. Some standards were not available, so the quantitative analysis was carried out by standards with similar structures, which are listed in Table 1.

Table 1.
Qualitative and quantitative analysis of aroma of Lingtou Dancong tea product from plants grown at different altitudes.
2.4. GC-O Analysis
Aroma compounds were analyzed using a GC-MS instrument equipped with an ODP-3 olfactory detection port (ODP; Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany), using the parameters described elsewhere [6]. The GC-O analysis group consisted of six healthy assessors with extensive evaluation experience [7]. All assessors were well trained for GC-O, having received at least 30 h of training with standard samples and tea samples. The assessors were trained to identify and determine the strength of the standards, as well as to be professionally trained in the sensory evaluation of tea. The sensory assessors described the odor properties of the compounds, and also the odor intensity. The aroma intensity was scored on a scale of 1 to 4, where “1” indicated the weakest aroma intensity, “2” indicated moderate aroma intensity, “3” indicated strong aroma intensity, and “4” indicated the strongest aroma intensity [9]. The flavor dilution (FD) analysis was completed by three highly trained assessors. The FD factor of the compound was determined by adjusting the split ratio (16:1; 32:1; 64:1; 128:1; 256:1; 512:1) to dilute the aroma extract [7]. The dilution multiple corresponding to the lowest concentration that the reviewers could smell was the FD value (16, 32, 64, 128, 256, 512) of that compound.
2.5. Calculation of Odor Activity Value
The odor activity value (OAV) describes the contribution of individual aroma components to the overall aroma; it is the ratio of the content of a compound to its threshold in water, and OAV > 1 is considered to indicate a significant contribution [7]. The contents of the volatiles were determined by GC-MS and information about thresholds was obtained from the literature (Table S1).
2.6. Extraction of Linalool from Fresh Leaves
Linalool was extracted and analyzed by GC-MS using the parameters described in our previous study [10]. A 300-mg (fresh weight) portion of powdered sample was extracted overnight with shaking at room temperature with dichloromethane (2.7 mL), containing ethyl decanoate (0.5 nmol) as the internal standard. The resulting liquid was dried and concentrated by anhydrous Na2SO4 under a nitrogen stream, and then analyzed using a QP2010SE (Shimadzu Corporation, Kyoto, Japan) GC-MS instrument, equipped with a Supelcowax-10 column (30 m × 250 μm × 0.25 μm, Supelco Inc., Bellefonte, PA, USA). Linalool was identified by comparison with the authentic standard.
2.7. Extraction of Geranyl Diphosphate from Fresh Leaves
Geranyl diphosphate (GPP) was extracted from fresh leaves and analyzed as described in our previous study [10]. A 200-mg portion of powdered fresh sample was mixed with 2 mL GPP buffer (50 mM Tris/HCl, pH 8.0, 20 mM DTT, 20 mM MgCl2, and 5% glycerin) and 20 μL 1 M sodium molybdate, then the mixture was vortexed, ultrasonically extracted in ice-cold water, and centrifuged (6000× g, 4 °C, 10 min). The supernatant was extracted with hexane to remove volatiles. The upper phase was collected and hydrolyzed using 2 mL 1 M H2SO4 at 37 °C for 30 min to release linalool. The mixture was kept on ice for 15 min, before adding 100 ng [2H3]linalool and 100 mg NaCl. The solution was extracted with hexane: ethyl acetate (1:1) and then concentrated by drying before GC-MS analysis. The analysis parameters were as described in Section 2.6, with some changes. The full-scan mode was used to detect linalool and [2H3]linalool, and the linalool content was calculated using data collected in the SIM mode (m/z = 71, 74, 93, 96).
2.8. Analysis of Gene Transcript Levels
The extraction of RNA and the reverse transcription to generate cDNA were as described in our previous study [10]. The primers used for qRT-PCR are shown in Table S2. The thermal cycling program was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 40 s on qTOWER384/G instrument (Analytik, Jena, Germany). The 2−ΔΔct method was used to calculate relative gene transcript levels. The reference genes were encoding Elongation Factor1 (CsEF-1α) and SAND family protein (CsSAND).
2.9. Statistical Analysis
Statistical analyses were performed using SPSS software (Ver25.0, SPSS Inc., Chicago, IL, USA). Differences among three or more groups were determined using one-way analysis of variance followed by Duncan’s multiple comparison tests. Differences were considered significant at p ≤ 0.05. The principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were conducted using SIMCA V14.1 (Umetrics AB, Umea, Sweden). An internal seven-fold cross-validation and 200 random permutation tests were conducted to verify the model.
3. Results
3.1. Differences in Aroma Composition of Lingtou Dancong Oolong Tea among Different Altitudes
We detected 59 volatile compounds common to the Lingtou Dancong oolong teas produced from plants grown at three different altitudes (Table 1). These compounds were classified into nine categories: aldehydes, alcohols, esters, ketones, terpenoids, oxides, nitrogen heterocyclic compounds, sulfur-containing compounds, and others (Table 1). The most abundant compounds were ketones (11), followed by aldehydes (10), alcohols (9), and esters (9) (Figure 1). Esters accounted for the largest proportion of the total volatiles content, and alcohols, aldehydes, ketones, and nitrogen heterocyclic compounds also accounted for relatively high proportions (Figure 2). The tea plants grown at different altitudes were ranked, from highest total aroma compound content to lowest, as follows: high altitude (26,227.13 μg/kg), low altitude (23,431.05 μg/kg), and then medium altitude (15,557.94 μg/kg) (Figure 2). Among the esters, those with the highest concentrations were cis-jasmine lactone (1974–8196 μg/kg) and δ-dodecalactone (2437–3410 μg/kg). Among the alcohols, those with relatively high concentrations were: hotrienol with a honey fragrance (1100–2664 μg/kg), linalool with a floral fragrance (421.8–701.3 μg/kg), and geraniol with a rose fragrance (733.4–910.7 μg/kg) [1,5]. The aldehyde butanal, 2-methyl- was present at relatively high concentrations (803.4–1441 μg/kg), as was the ketone (Z)-jasmone (392.9–1139 μg/kg). Among the nitrogenous compounds, indole (873.4–3882 μg/kg) showed the highest concentration.
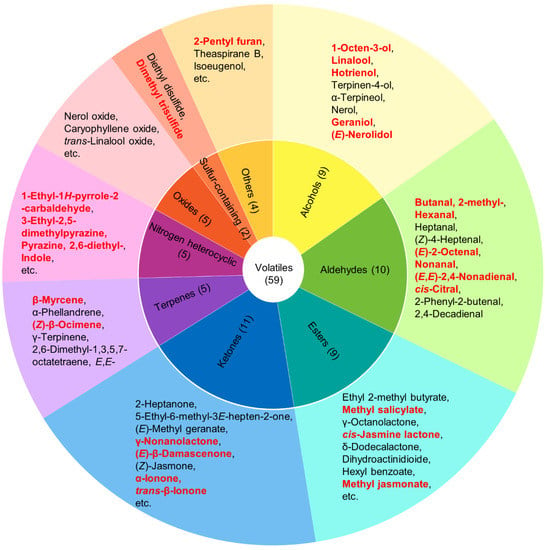
Figure 1.
Categories of aroma compounds and specific compounds in Lingtou Dancong oolong tea product. The number in brackets indicates the number of compounds in each category. Compounds with an odor activity value (OAV) higher than 1 are highlighted in red.
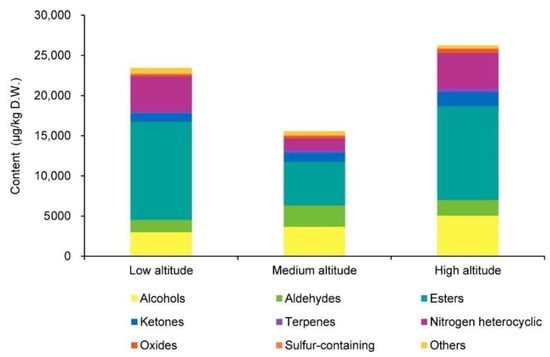
Figure 2.
Categories of aroma compounds and total aroma contents in Lingtou Dancong oolong tea product from different altitudes. High altitude, medium altitude and low altitude represent the Lingtou Dancong oolong teas from high, medium and low altitude, respectively. D.W. represents dry weight.
Aroma components are important indicators for evaluating tea quality, and multivariate chemometric approaches are often used to analyze the diversity of sensory characteristics and chemical components of tea samples. Raw data were subjected to unsupervised PCA analysis and supervised OPLA-DA analysis (Figure 3). In the PCA, the first principal component (PC1, X-axis) explained 34.3% of the variation in total aroma, and the second principal component (PC2, Y-axis) explained 22.8%. The samples from three different altitudes formed different clusters on the plot. The OPLS-DA model had R2X of 0.784, R2Y of 0.99, Q2 of 0.963. Cross validation and permutation tests showed that the intercept between the blue regression line and the Y axis was less than 0, and all values of R2 and Q2 were lower than the original values, indicating that the model was not over-fitted and had good applicability and predictability (Figure S1). The compounds in the loading plots are referred to using serial numbers corresponding to the compounds in Table 1 and Figure 3C. The loading plot showed that hotrienol (23), nerol oxide (26), α-ionone (46), (Z)-jasmone (44) were present at higher levels in high-altitude tea than in medium- and low-altitude teas (Figure 3C). Compounds such as 5-ethyl-6-methyl-3E-hepten-2-one (25), (Z)-4-hepten (6), 1-octen-3-ol (10) were present at higher levels in the medium-altitude tea than in the high- and low-altitude teas. The contents of isoeugenol (47) and 1,2-dihydro-1,1,6-trimethyl naphthalene (41) were higher in low-altitude tea than in medium- and high-altitude teas. These compounds had relatively important effects on the aroma quality of tea made from plants grown at different altitudes.
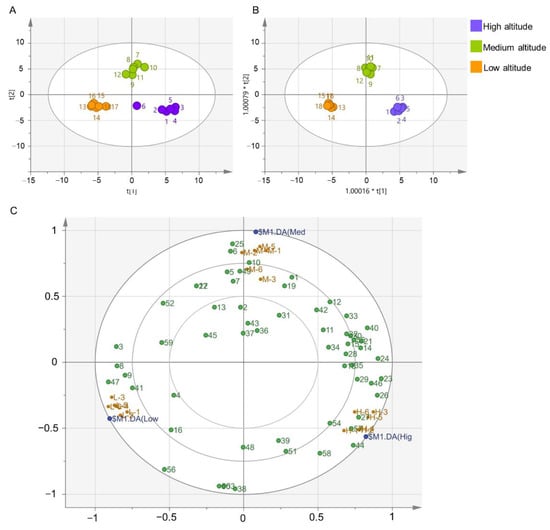
Figure 3.
Multivariate chemometric analysis of Lingtou Dancong oolong tea product from different altitudes. (A) PCA score plot; (B) OPLS-DA score plot; (C) Loading plot. The horizontal axis represents PC1, and the vertical axis represents PC2. Hig, high altitude, Med, medium altitude, Low, low altitude. Compounds numbered in (C) were correspond to Table 1. High altitude, medium altitude and low altitude represent the Lingtou Dancong oolong teas from high, medium and low altitude, respectively.
3.2. GC-O/MS Analysis to Determine Contribution of Odorants to Aroma of Lingtou Dancong Oolong Tea Made from Plants Grown at Different Altitudes
The contribution of volatiles to the aroma profile not only depends on their content but also on their threshold. GC-O/MS analysis was performed to further identify key contributors to the overall aroma profile of the various tea materials. The results of GC-O/MS are shown in Table 2, and detailed descriptions of each odorant and its intensity are summarized in Tables S3–S5. Key aroma compounds were identified on the basis of their OAV, where a higher OAV indicates a major contribution to aroma [6]. The odor thresholds of compounds were obtained from the literature (Table S1). As shown in Table 2, 25 compounds had OAV > 1 at all altitudes. Among these, 12 were floral or honey odor compounds (Table 2). Linalool, with the highest OAVs (OAV = 1917.27–3187.73), contributed the major floral fragrance; (E, E)-2,4-nonadienal (OAV = 453.50–713.33) contributed the major green flavor; and (E)-β-damascenone (OAV = 316–357.00) and cis-jasmine lactone (OAV = 282.00–1170.86) contributed the major honey fragrance. Some unpleasant flavors such as the onion-flavored dimethyl trisulfide (OAV = 827.00–1438.00, sulfurous) also contributed to the overall aroma. All OAV values were converted by setting the highest OAV (3187.73) to 5, indicating the highest contribution. A radar map constructed using the converted OAV scores (Figure 4A) showed that the overall aroma profile of Lingtou Dancong oolong tea mainly consisted of floral, honey, and green odors. The floral fragrance of tea was richer in medium- and high-altitude teas and the honey fragrance was richer in high- and low-altitude teas. The green odor was more obvious, and the unpleasant onion odor was weaker in medium- and high-altitude teas than in low-altitude teas (Figure 4A).

Table 2.
Analysis of aroma contribution of Lingtou Dancong Oolong tea product from plants grown at different altitudes.
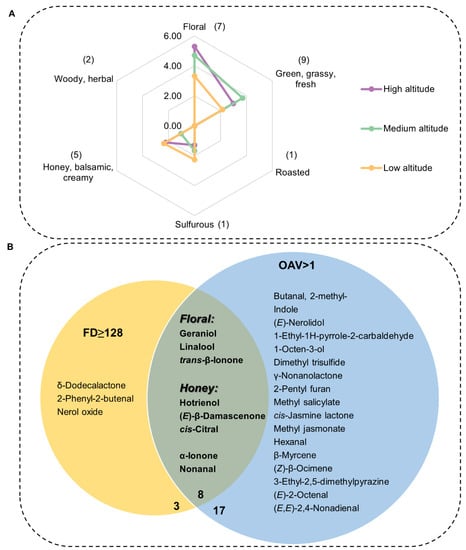
Figure 4.
Aroma profile and key contributors of Lingtou Dancong oolong tea. (A) Specific scores were obtained by conversion according to OAV, and the numbers in brackets indicated the number of compounds; (B) Most important aroma contributors in Lingtou Dancong oolong tea identified according to FD and OAV. FD, flavor dilution factor; OAV, odor activity value. The number bellowed the circle indicates the number of compounds. High altitude, medium altitude and low altitude represent the Lingtou Dancong oolong teas from high, medium and low altitude, respectively.
Although OAV is a recognized method for evaluating aroma contribution, GC-O/MS results can be affected by the tea matrix, and odor perception is very complex and can be affected by multiple factors [11]. Therefore, it is important to analyze aroma contributions from multiple perspectives. The FD factor of aroma compounds was determined in order to clarify their contributions to the aroma profile of Lingtou Dancong oolong tea. All of the detected odor compounds had an FD factor of >16. The highest FD factor (512) indicated the greatest contribution, and the compounds with this FD value were floral or honey odor compounds: i.e., hotrienol (honey), cis-citral (honey), D-β-damascenone (honey), and trans-β-ionone (floral). Eleven aroma compounds had FD factors higher than 128 at all three altitudes: three were floral aroma compounds, four were honey aroma compounds, three were green aroma compounds, and one was a woody aroma compound (Table 2). These results were consistent with the organoleptic aroma profile (Figure 4).
3.3. Contents of Linalool and Its Precursor GPP in Fresh Leaves from Tea Plants Grown at Different Altitudes
The contents of linalool and geraniol were higher in high- and medium-altitude teas than in low-altitude tea. The OAVs of linalool and geraniol were higher than 1 and their FD values were higher than 128, indicating that they were major contributors to the floral aroma. Linalool and geraniol are both generated by the terpene metabolic pathway and share a common precursor, GPP. To explore the reasons for the higher contents of linalool and geraniol in tea plants grown at high and medium altitudes, we determined the contents of linalool and GPP in fresh leaves. The results showed that the contents of both linalool and GPP were higher in fresh leaves from plants grown at high and medium altitudes than in those from plants grown at a low altitude (Figure 5A,B).
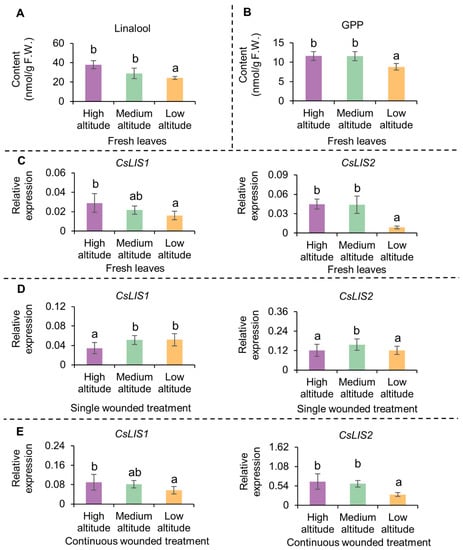
Figure 5.
Contents of linalool, GPP and expression level of CsLISs in fresh leaves and wounded leaves of Lingtou Dancong grown at different altitudes. (A) Contents of linalool in fresh leaves; (B) Contents of GPP in fresh leaves. GPP, geranyl diphosphate; (C) Expression levels of CsLIS1 and CsLIS2 in fresh leaves (CsEF-1α as reference gene). LIS, linalool synthase.; (D) Expression levels of CsLIS1 and CsLIS2 in leaves after single wounded treatment for 3 h (CsEF-1α as reference gene); (E) Expression levels of CsLIS1 and CsLIS2 in leaves after continuous wounded treatment for 3 h (CsEF-1α as reference gene). The contents of GPP were determined indirectly based on the peak area of linalool from GPP hydrolysis. F.W. represents fresh weight. Data are expressed as mean ± S. D. (n = 3). Means distinguished with different letters are significantly different from each other among high altitude, medium altitude and low altitude (p ≤ 0.05). High altitude, medium altitude and low altitude represents the C. sinensis cv. Lingtou Dancong leaves picked from high, medium and low altitude, respectively.
The transcript levels of the CsLISs encoding linalool synthases in fresh leaves and leaves after wounding treatment were also analyzed (Figure 2 and Figure S2). The transcript levels of CsLISs were higher in fresh leaves of plants grown at medium and high altitudes than in those of plants grown at a low altitude, and were increased by the continuous wounding treatment. After the 3 h continuous wounding treatment, the transcript levels of CsLISs were higher in fresh leaves from plants grown at medium and high altitudes than in fresh leaves of those grown at low altitudes.
4. Discussion
4.1. Differences in Aroma Composition of Lingtou Dancong Oolong Tea among Plants Grown at Different Altitudes
In the evaluation of oolong tea, sensory evaluation is widely used to score the aroma quality. However, this method is subjective. The combination of GC-MS with an organoleptic analysis can evaluate the aroma of oolong tea more objectively. The GC-O/MS system is useful for identifying the contribution of aroma-active compounds in a complex matrix. We found that the categories of odorants in Lingtou Dancong oolong tea products from different altitudes were the same, but the content and proportion of each category differed among materials from different altitudes. The contents of esters (mainly cis-jasmine lactone) and nitrogen heterocyclic compounds (mainly indole) were lower in medium-altitude teas than in high- or low-altitude teas (Figure 2 and Table 1), resulting in the lowest total aroma compound content in medium-altitude tea. As a result of cis-jasmine lactone’s (honey) low odor threshold, the low content of cis-jasmine lactone in the medium-altitude tea also directly led to the deficiency of a honey fragrance (Table 1 and Table 2, Figure 4). Comparing tea materials from all altitudes, the total proportions of alcohols, aldehydes, terpenes, and nitrogen heterocyclic compounds tended to first increase, and then decrease with elevation (Figure 2). Another study found that aldehydes accounted for the largest proportion of odor compounds in most oolong teas [5]. However, we found that esters accounted for the largest proportion in Lingtou Dancong oolong tea. For example, the contents of γ-nonanolactone and γ-octanolactone, which confer the coconut fragrance of green tea, were significantly higher in medium- and high-altitude teas than in the low-altitude tea [12,13]. Among the alcohols, linalool and geraniol share the same precursor, GPP, but they have different odors [14]. Linalool has a floral-orange fragrance, while geraniol has an obvious rose fragrance [15]. Among the tea products, those from high and medium altitudes had higher linalool and geraniol contents in the fresh leaves, and higher contents of their shared precursor GPP (Table 1 and Figure 5). Aldehydes in tea can be produced through lipid oxidation and Strecker degradation under thermal conditions [16]. For example, hexanal (OAV > 1) and (E, E)-2,4-nonadienal (OAV > 1) are generated by lipid oxidation, and butanal, 2-methyl- (OAV > 1) is produced by Strecker degradation. We found that the contents of all three of these compounds were higher in medium- and high-altitude teas than in low-altitude tea, and they were identified as the main contributors of the green grass odor in tea. Among the ketones, α-ionone (OAV > 1), which has a woody fragrance, was present at higher levels in high-altitude tea than in medium- and low-altitude teas. (E)-β-damascenone (OAV > 1) and trans-β-ionone (OAV > 1) are two representative odor compounds generated by carotenoid degradation, and both are essential contributors to aroma [5,17]. As reported in previous studies, indole is another important aroma substance in oolong tea. The accumulation of indole is caused by turning-over during oolong tea processing, and this contributes to the floral fragrance [18].
We used OAV and FD analyses to evaluate whether a single odor compound makes a major contribution to fragrance. We detected eight odor compounds with FD ≥ 128 and OAV > 1 as the highest contributors, and they also had high sensory intensity (Figure 4B, Tables S3–S5). Among the eight fragrances, the contents of hotrienol (honey), linalool (floral), geraniol (floral), and α-ionone (woody, herbal) were positively correlated with altitude, while the trans-β-ionone (floral) content was highest in medium-altitude tea (Table 2). The cis-citral (honey) content was significantly higher in medium-and high-altitude teas than in low-altitude tea. Nonanal (green), which is a malt aroma, was present at significantly higher levels in medium-altitude teas than in low- or high-altitude teas [5]. The olfactory characteristics of these eight odor compounds were consistent with the stronger floral fragrance of high- and medium-altitude teas than low-altitude teas (Figure 4A). Hotrienol (honey), linalool (floral), and geraniol (floral) share the same terpene metabolic pathway. Altitude is negatively correlated with temperature, and cold stress can induce the release of linalool and geraniol [19]. Thus, environmental factors associated with altitude might affect the metabolism of aroma compounds in the terpene metabolic pathway, thereby affecting the aroma components and fragrance of tea. Overall, the honey and floral fragrances were prominent in high-altitude tea, and the green odor was weaker, meaning high-altitude tea had the best aroma quality.
Although all of the above odorants were screened based on the criteria of OAV > 1, there are many reasons why the OAV of a compound may be less than 1, such as a higher threshold or lower content of that compound. In addition, the tea matrix can affect the release of odorants [6]. Masking, synergistic, and additive effects among odorants can also affect perception [20]. Therefore, odorants with OAV < 1 can still contribute to overall aroma, but further research is required to explore their contributions and interactions in more detail.
4.2. Aroma Characteristics of Lingtou Dancong Oolong Tea
Lingtou Dancong, also known as Baiye Dancong, like Fenghuang Dancong, is a protected geographical indication product (PGI) in Guangdong province, China. The aroma of oolong tea from Guangdong province differs from those of other oolong teas because of the varieties used and the processing methods. Tea tasting experience and existing literature indicate that the floral and honey fragrances are key sensory characteristics of Dancong oolong tea [2,21]. Other Dancong oolong teas have a prominent floral fragrance, while Lingtou Dancong has a unique honey fragrance [2,21]. The literature indicates that linalool and linalool oxides are important substances for forming the honey fragrance [2,22]. However, linalool is a vital odor compound, not only in Lingtou Dancong oolong tea, but also in other oolong teas, green tea, black tea, and even dark tea with a higher degree of fermentation [7,23,24,25]. Therefore, linalool and its oxides are important and universal substances in tea, rather than the signature aroma of Lingtou Dancong oolong tea. The oolong tea “Oriental beauty” is widely known for its honey fragrance, which is largely conferred by hotrienol [26]. Hotrienol, which is present in honey and has a honey and fruit aroma, is a very unstable thermal product that can be converted from diols or linalool [27,28]. Studies have shown that the hotrienol content is higher in Lingtou Dancong oolong tea than in other Dancong oolong teas, and that hotrienol is not present in Rougui teas with different degrees of baking and fermentation [2,29,30]. In our study, we detected higher contents of both hotrienol and its precursor, linalool, in high- and medium-altitude teas than in low-altitude tea. This result suggests that hotrienol in tea might be converted from linalool, whose content might be related to altitude. (E)-β-Damascenone (OAV = 316–357) and cis-citral (OAV = 4.17–8.04) were also identified as key honey odorants with high OAVs, high sensory intensity (Tables S3–S5), and the highest FD (>512) among all odor compounds (Table 2). Notably, (E)-β-damascenone was not detected in any of the tea products made from Shuixian, Huangmeigui, and Zimudan (three varieties suitable for making oolong tea) [23]. cis-Citral was not detected in Dahongpao, Tieguanyin and Dongdingwulong teas [31]. Therefore, hotrienol, (E)-β-damascenone, and cis-citral may be the key components of the special honey aroma of Lingtou Dancong oolong tea. However, further aroma recombination experiments are required to test this hypothesis.
5. Conclusions
In this study, the SBSE method and GC-O/MS technique were used to evaluate the aroma components of Lingtou Dancong oolong tea made from plants grown at different altitudes. A total of 59 common aroma compounds were identified and quantified. The PCA and OPLS-DA confirmed differences in the aroma quality of tea from different altitudes. The categories of aroma compounds in teas from different altitudes were the same, but the contents and proportions were different. These differences, together with the thresholds of each aroma compound, affected the final aroma profile of the various teas. Using OAV (the information of threshold was referred to the literatures [5,6,12,31,32,33,34,35,36,37,38,39,40,41]) and FD analyses, the eight odor compounds making the largest contributions to aroma were screened, and most of them had honey and floral fragrances. Among them, hotrienol, (E)-β-damascenone, and cis-citral were the most noteworthy contributors to aroma (Figure 4B). All of them have a honey fragrance, and together they may confer the honey fragrance of Lingtou Dancong oolong tea. Their presence and contents may be related to the variety and/or the subsequent processing methods, but further analyses are required to explore these ideas in more detail. The contents of linalool (a key component of floral fragrance) and hotrienol (a key component of honey fragrance) varied with altitude. Its higher content at higher altitude was consistent with higher contents of its precursor, GPP, as well as higher transcript levels of CsLISs in the fresh leaves (Figure 6). The results of this study shed light on the aroma compounds that contribute to the fragrance of Lingtou Dancong oolong tea. Our results will be useful for devising strategies to control the contents of various aroma compounds in tea during cultivation and processing.
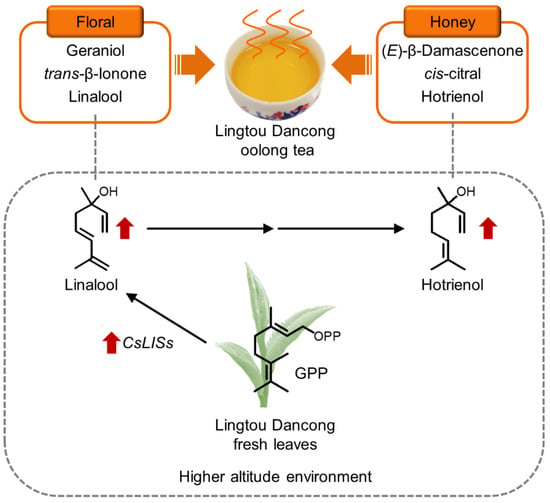
Figure 6.
Aroma characteristics of Lingtou Dancong oolong tea and the effect of altitude on it. GPP, geranyl diphosphate; LIS, linalool synthase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo12111063/s1, Figure S1: OPLS-DA model validation; Figure S2: Expression level of CsLISs in fresh leaves and wounded leaves of Lingtou Dancong grown at different altitudes; Table S1: Threshold information; Table S2: The primers used for quantitative real time PCR (qRT-PCR) in the study; Table S3: Detailed information of Lingtou Dancong tea product at high altitude; Table S4: Detailed information of Lingtou Dancong tea product at medium altitude; Table S5: Detailed information of Lingtou Dancong tea product at low altitude.
Author Contributions
Data curation, M.W. and L.Z.; methodology, M.W.; writing original draft, M.W. and J.L.; investigation, M.W., J.L., C.L., J.Q., J.Y., X.Z. and Y.J.; resources, J.L., X.L. and J.T.; formal analysis, J.L., C.L., J.Q., J.Y., X.Z. and Y.J.; conceived and designed, supporting the project, J.L. and L.Z.; revising the manuscript, M.W. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the financial supports from the Basic Frontier Science Research Program of Chinese Academy of Sciences (ZDBS-LY-SM032), the Young Elite Scientists Sponsorship Program by China Association for Science and Technology (2020QNRC001), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2023KJ120), the Special Talents and Technical Support Program of Chinese Academy of Sciences (E225052A01), the Science and Technology Project of Shaoguang (200811124531655, 220322164531070), the Science and Technology Project of Qiangyuan (2020DZX023, 220804107510735), the Foundation of Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement, South China Botanical Garden, Chinese Academy of Sciences (KF202207), Industrial chain technology upgrading project of Jieyang (Jieyang Nonghan [2022]683), and by the Earmarked Fund for China Agriculture Research System (CARS-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeng, L.T.; Zhou, X.C.; Su, X.G.; Yang, Z.Y. Chinese oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Tech. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Chen, W.; Hu, D.; Miao, A.Q.; Qiu, G.J.; Qiao, X.Y.; Xia, H.L.; Ma, C.Y. Understanding the aroma diversity of Dancong tea (Camellia sinensis) from the floral and honey odors: Relationship between volatile compounds and sensory characteristics by chemometrics. Food Control 2022, 140, 109103. [Google Scholar] [CrossRef]
- Zhou, C.J.; Zhuang, D.H.; Guo, S.J.; Zhu, H.; Ma, R.J.; Wu, Q.H. Classification and identification of different aromatics in tea made from different cultivar of Fenghuang dancing. J. Tea Sci. 2014, 34, 609–616. (In Chinese) [Google Scholar]
- Tang, H.; Tang, J.C.; Cao, J.X.; Zhou, B.; Li, J.L.; Cai, J. Analysis of quality differences among Fenghuang dancong tea in different altitude ranges. Chin. Agric. Sci. Bull. 2015, 31, 143–151. (In Chinese) [Google Scholar]
- Zhai, X.T.; Zhang, L.; Granvogl, M.; Ho, Q.T.; Wan, X.C. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Zhang, Z.F.; Zeng, J.M.; Zhang, Y.; Wang, M.Q.; Peng, Q.H.; Lv, H.P.; Lin, Z. Assessment of the contribution of chiral odorants to aroma property of baked green teas using an efficient sequential stir bar sorptive extraction approach. Food Chem. 2021, 365, 130615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shao, C.Y.; Lv, H.P.; Zhang, Y.; Dai, W.D.; Guo, L.; Tan, J.F.; Peng, Q.H.; Lin, Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Jian, G.T.; Jia, Y.X.; Li, J.L.; Zhou, X.C.; Liao, Y.Y.; Dai, G.Y.; Zhou, Y.; Tang, J.C.; Zeng, L.T. Elucidation of the regular emission mechanism of volatile beta-ocimene with anti-insect function from tea plants (Camellia sinensis) exposed to herbivore attack. J. Agric. Food Chem. 2021, 69, 11204–11215. [Google Scholar] [CrossRef]
- Chen, X.H.; Sun, H.Y.; Qu, D.; Yan, F.; Jin, W.G.; Jiang, H.; Chen, C.; Zhang, Y.F.; Li, C.Y.; Xu, Z.M. Identification and characterization of key aroma compounds in Chinese high altitude and northernmost black tea (Camellia sinensis) using distillation extraction and sensory analysis methods. Flavour Frag. J. 2020, 35, 666–673. [Google Scholar] [CrossRef]
- Flaig, M.; Qi, S.; Wei, G.D.; Yang, X.G.; Schieberle, P. Characterization of the key odorants in a high-grade Chinese green tea beverage (Camellia sinensis; jingshan cha) by means of the sensomics approach and elucidation of odorant changes in tea leaves caused by the tea manufacturing process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef] [PubMed]
- Flaig, M.; Qi, S.C.; Wei, G.D.; Yang, X.G.; Schieberle, P. Characterisation of the key aroma compounds in a Longjing green tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. Eur. Food Res. Technol. 2020, 246, 2411–2425. [Google Scholar] [CrossRef]
- Zeng, L.T.; Watanabe, N.; Yang, Z.Y. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenarolis Handbook of Flavor Ingredients, 6th ed.; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Ho, C.T.; Zheng, X.; Li, S.M. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Lin, Q.; Ni, H.; Wu, L.; Weng, S.Y.; Li, L.J.; Chen, F. Analysis of aroma-active volatiles in an SDE extract of white tea. Food Sci. Nutr. 2021, 9, 605–615. [Google Scholar] [CrossRef]
- Zeng, L.T.; Zhou, Y.; Gui, J.D.; Fu, X.M.; Mei, X.; Zhen, Y.P.; Ye, T.X.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Wang, L.; Wang, J.M.; Jin, J.Y.; Zhang, N.; Lei, L.; Gao, T.; Jing, T.T.; Zhang, S.R.; Wu, Y.; et al. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J. Integr. Plant Biol. 2020, 62, 1461–1468. [Google Scholar] [CrossRef]
- Niu, Y.W.; Ma, Y.W.; Xiao, Z.B.; Zhu, J.C.; Xiong, W.; Chen, F. Characterization of the key aroma compounds of three kinds of chinese representative black tea and elucidation of the perceptual interactions of methyl salicylate and floral odorants. Molecules 2022, 27, 1631. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, W.W.; Chen, Y.; Zhang, X.; Wang, X.; Wang, F.H.; Qian, Y.Z.; Qiu, J. Identification of markers for tea authenticity assessment: Non-targeted metabolomics of highly similar oolong tea cultivars (Camellia sinensis var. sinensis). Food Control 2022, 142, 109223. [Google Scholar] [CrossRef]
- Ma, C.Y.; Li, J.X.; Chen, W.; Wang, W.W.; Qi, D.D.; Pang, S.; Miao, A.Q. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography-mass spectrometry combined with chemometrics. Food Res. Int. 2018, 108, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Schwab, W.; Ho, C.T.; Song, C.K.; Wan, X.C. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2021, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Xu, Y.J.; Wen, J.; An, K.J.; Wu, J.J.; Yu, Y.S.; Zou, B.; Guo, M.H. A comparative study of aromatic characterization of Yingde black tea infusions in different steeping temperatures. LWT-Food Sci. Technol. 2021, 143, 110860. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wu, X.J.; Zhang, Y.; He, Z.G.; Zhang, Y.; Zhang, X.M.; Li, Q.; Huang, J.A.; Liu, Z.H. Pu-erh tea unique aroma: Volatile components, evaluation methods and metabolic mechanism of key odor-active compounds. Trends Food Sci. Tech. 2022, 124, 25–37. [Google Scholar] [CrossRef]
- Ogura, M.; Terada, I.; Shirai, F.; Tokoro, K.; Chen, K.R.; Chen, C.L.; Lin, M.L.; Shimizu, B.; Kinoshita, T.; Sakata, K. Tracing aroma characteristics changes during processing of the famous Formosa oolong tea “Oriental Beauty”. In Proceedings of the 2004 International Conference on O-Cha (tea) Culture and Science, Shizuoka, Japan, 4–6 November 2005; pp. 240–242. [Google Scholar]
- Jerkovic, I.; Kus, P.M. Terpenes in honey: Occurrence, origin and their role as chemical biomarkers. RSC Adv. 2014, 4, 31710–31728. [Google Scholar] [CrossRef]
- Tsitsishvili, V.; Ramishvili, T.; Ivanova, I.; Kokiashvili, N.; Bukia, T.; Kurtsikidze, G. Linalool oxidation reaction with air under ultrasound and microwave irradiations. Bull. Georg. Natl. Acad. Sci. 2019, 13, 40–45. [Google Scholar]
- Yang, P.; Yu, M.G.; Song, H.L.; Xu, Y.Q.; Lin, Y.P.; Granvogl, M. Characterization of key aroma-active compounds in Rough and moderate fire rougui wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. J. Agric. Food Chem. 2022, 70, 267–278. [Google Scholar] [CrossRef]
- Liu, Z.B.; Chen, F.C.; Sun, J.Y.; Ni, L. Dynamic changes of volatile and phenolic components during the whole manufacturing process of Wuyi Rock tea (Rougui). Food Chem. 2022, 367, 130624. [Google Scholar] [CrossRef]
- Zhu, J.C.; Chen, F.; Wang, L.Y.; Niu, Y.W.; Yu, D.; Shu, C.; Chen, X.H. Comparison of aroma-active volatiles in oolong tea infusions using GC-Olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, D.J.; Hai, J.; Sun, H.Y.; Chen, Z.; Hua, Z.; Li, X.S.; Fei, Y.; Chen, C. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chang, Y.; Liu, B.; Chen, H.T.; Sun, B.G.; Zhang, N. Characterization of the key aroma-active compounds in Yongchuan Douchi (fermented soybean) by application of the sensomics approach. Molecules 2021, 26, 3048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Li, J.; Wang, J.; Sun, B.G.; Liu, Y.P.; Huang, M.Q. Characterization of aroma-active compounds in Jasminum sambac concrete by aroma extract dilution analysis and odour activity value. Flavour Fragr. J. 2021, 36, 197–206. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.W.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Deutsche Forschungsanstalt fur Lebensmittelchemie and Institut fur Lebensmittelchemie der Technischen Universitat Munchen: Garching, Germany, 1998. [Google Scholar]
- Qiu, S.; Chen, K.; Liu, C.; Wang, Y.X.; Chen, T.; Yan, G.L.; Li, J.M. Non-saccharomyces yeasts highly contribute to characterisation of flavour profiles in greengage fermentation. Food Res. Int. 2022, 157, 111391. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Gao, M.M.; Zhang, L.Q.; Qiao, L.; Li, J.X.; Du, L.P.; Zhang, H.L.; Wang, H. Characterization of the key aroma-active compounds in high-grade Dianhong tea using GC-MS and GC-O combined with sensory-directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Palomo, E.; Trujillo, M.; Ruiz, A.G.; González Viñas, M.A. Aroma profile of malbec red wines from la mancha region: Chemical and sensory characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef]
- Du, X.F.; Finn, C.E.; Qian, M.C. Volatile composition and odour-activity value of thornless ‘Black diamond’ and ‘Marion’ blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar] [CrossRef]
- Pang, X.L.; Yu, W.S.; Cao, C.D.; Yuan, X.X.; Qiu, J.; Kong, F.Y.; Wu, J.H. Comparison of potent odorants in raw and ripened Pu-Erh tea infusions based on odor activity value calculation and multivariate analysis: Understanding the role of pile fermentation. J. Agric. Food Chem. 2019, 67, 13139–13149. [Google Scholar] [CrossRef]
- Wang, B.; Meng, Q.; Xiao, L.; Li, R.L.; Peng, C.H.; Liao, X.L.; Yan, J.N.; Liu, H.L.; Xie, G.H.; Ho, Q.T.; et al. Characterization of aroma compounds of pu-erh ripen tea using solvent assisted flavor evaporation coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Sci. Hum. Well. 2022, 11, 618–626. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).