Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Test Solutions
2.3. Time Inhibition Curves
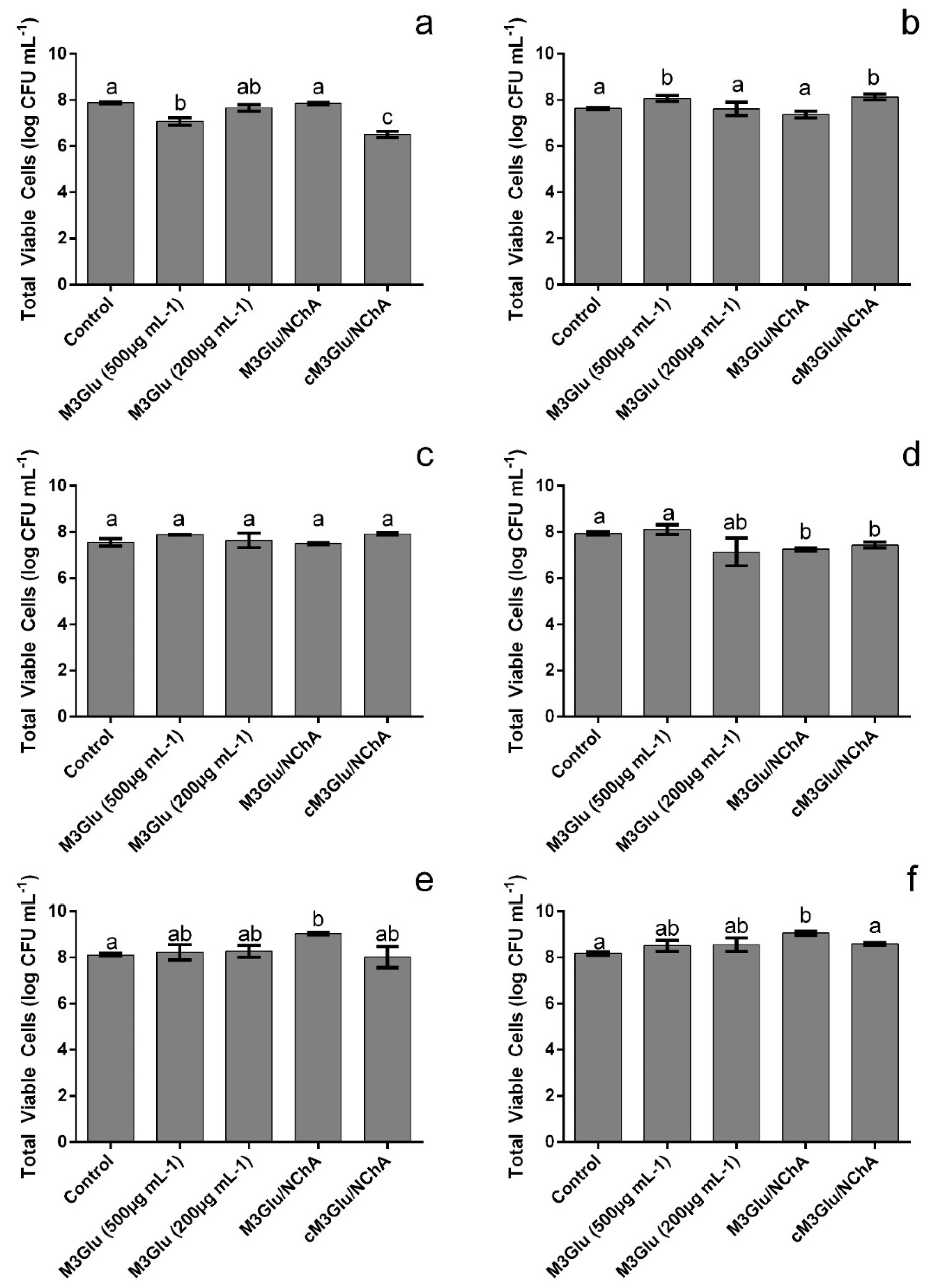
2.4. Total Planktonic Viable Cell Determination
2.5. Antibiofilm Activity
2.5.1. Biofilm-Entrapped Viable Cells
2.5.2. Biofilm Biomass
2.5.3. Biofilm Metabolic Activity
2.6. Impact on Bacterial Adhesion—Adhesion to Polystyrene (PS)
2.7. Impact on Bacterial Adhesion—Adhesion to Polystyrene Pre-Treated with Rabbit Plasma
2.8. Statistical Analysis
3. Results
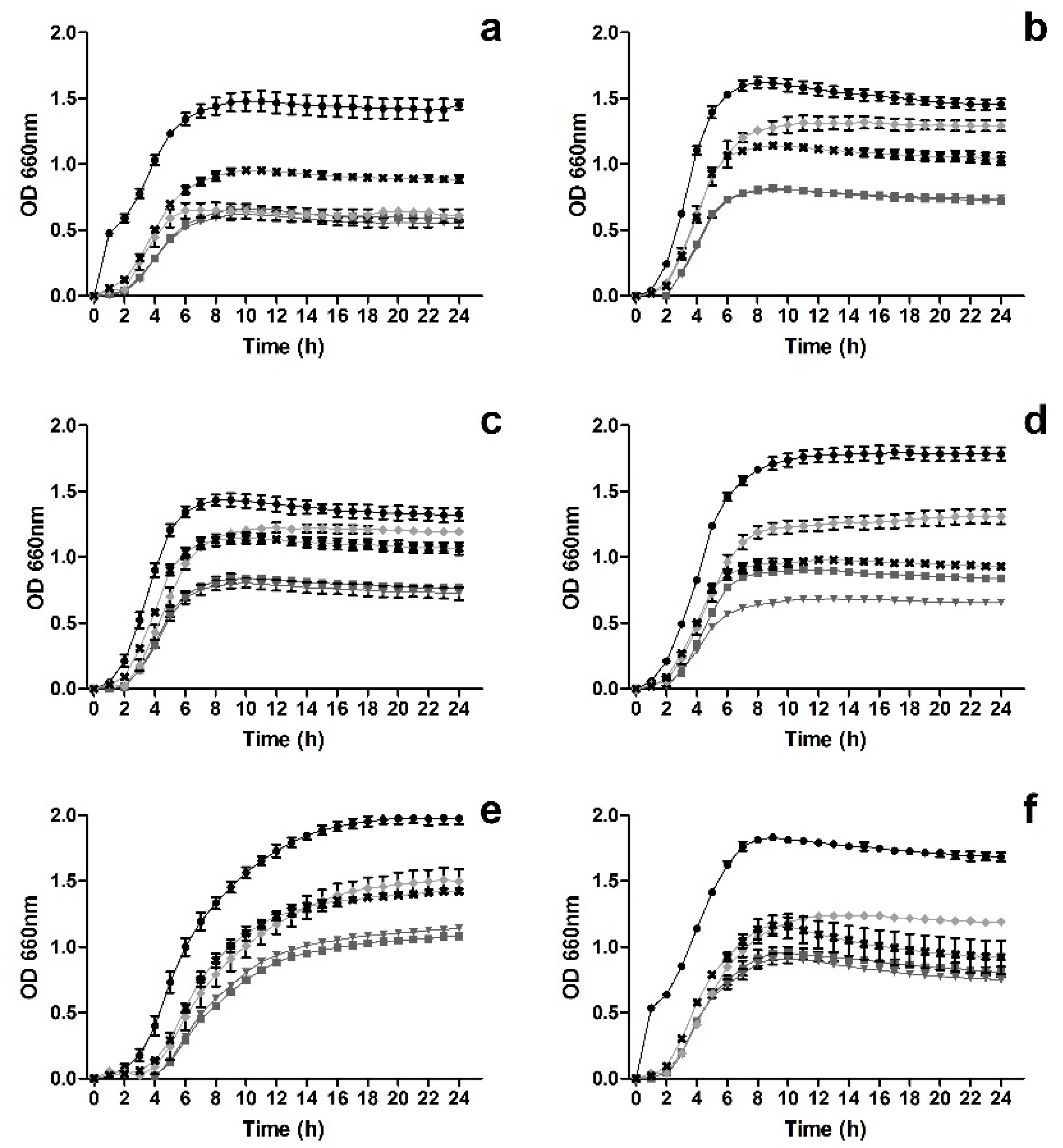
3.1. Impact upon Staphylococcus Growth
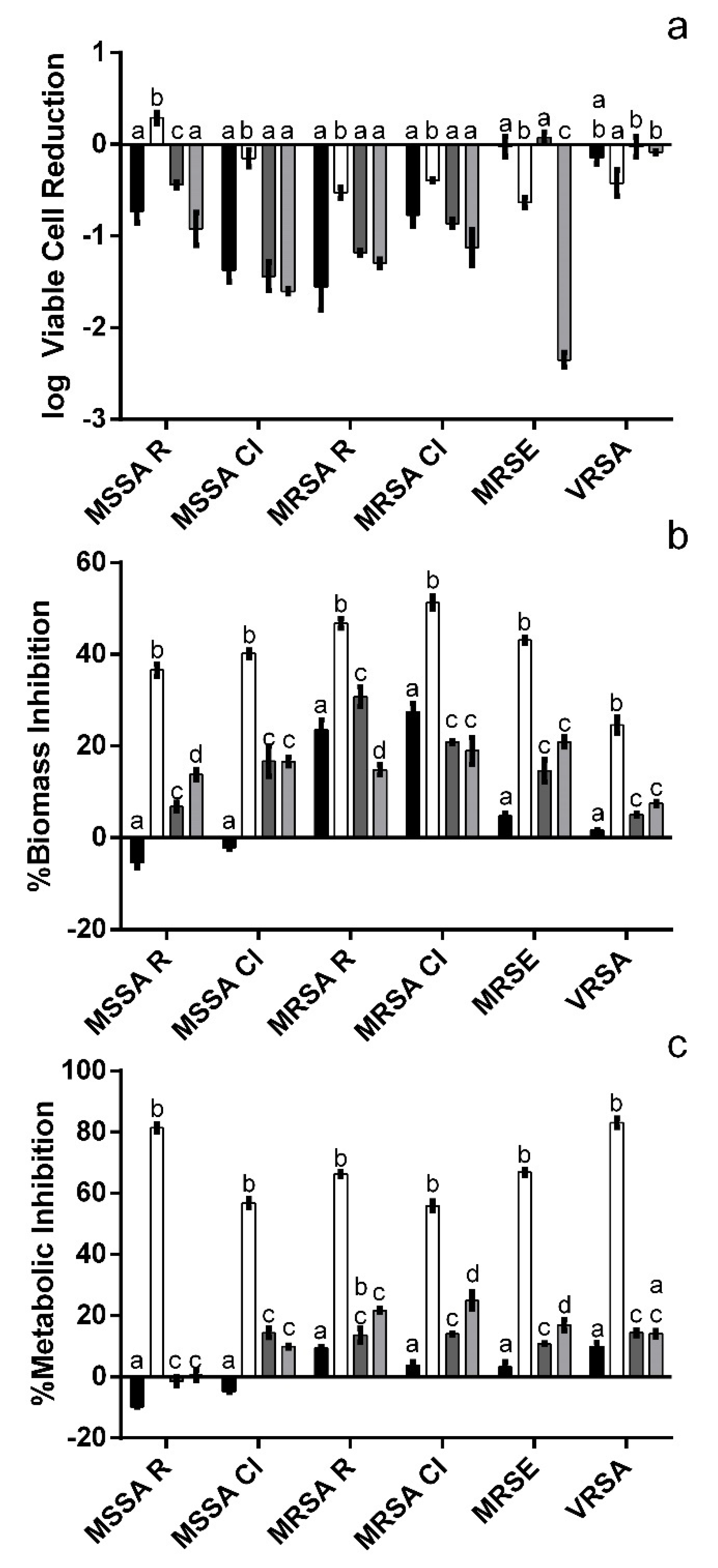
3.2. Impact upon Staphylococcus Biofilms
3.3. Impact upon Staphylococcus Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, S.; Costa, E.; Mendes, M.; Morais, R.; Calhau, C.; Pintado, M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernandez, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 3072–3083. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective effects of Anthocyanins against Amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins in Health and Disease; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zhang, Y.; Lin, Y.; Huang, L.; Tekliye, M.; Rasheed, H.A.; Dong, M. Composition, antioxidant, and anti-biofilm activity of anthocyanin-rich aqueous extract from purple highland barley bran. LWT 2020, 125, 109181. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.; Mateus, N.; de Freitas, V.; Brás, N.; Gameiro, P.; Coelho, P.; et al. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Chambers, H.F. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 2001, 7, 178–182. [Google Scholar] [CrossRef]
- Livermore, D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 2000, 16 (Suppl. 1), 3–10. [Google Scholar] [CrossRef]
- Carbon, C. MRSA and MRSE: Is there an answer? Clin. Microbiol. Infect. 2000, 6 (Suppl. 2), 17–22. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanevello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef] [PubMed]
- M07-08; M07-08–Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute Guidelines: Wayne, PA, USA, 2009; pp. 3073–3099.
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Costa, M.R.; Pereira, M.F.; Pereira, J.O.; Soares, J.C.; Pintado, M.M. Aqueous extracts of Vaccinium corymbosum as inhibitors of Staphylococcus aureus. Food Control 2015, 51, 314–320. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Machado, I.; Graça, J.; Lopes, H.; Lopes, S.; Pereira, M.O. Antimicrobial Pressure of Ciprofloxacin and Gentamicin on Biofilm Development by an Endoscope-Isolated Pseudomonas aeruginosa. ISRN Biotechnol. 2013, 2013, 178646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Loosdrecht, M.C.M.; Norde, W.; Lyklema, J.; Zehnder, A.J.B. Hydrophobic and electrostatic parameters in bacterial adhesion. Aquat. Sci. 1990, 52, 103–114. [Google Scholar] [CrossRef]
- Chaignon, P.; Sadovskaya, I.; Ragunah, C.; Ramasubbu, N.; Kaplan, J.B.; Jabbouri, S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 2007, 75, 125–132. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Landini, P. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res. Microbiol. 2009, 160, 259–266. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, K.R.; Blum-Silva, C.H.; Souza, A.L.K.; Wulffschuch, M.; Reginatto, F.H.; Pereira, C.M.P.; Macedo, A.J.; Lencina, C.L. The Antibiofilm Effect of Blueberry Fruit Cultivars Against Staphylococcus epidermidis and Pseudomonas aeruginosa. J. Med. Food 2014, 17, 324–331. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.J.; Yaginuma, S.R.; Quadri, M.G.N.; Quadri, M.B. Evaluation of red cabbage anthocyanins after partial purification on clay. Braz. Arch. Biol. Technol. 2011, 54, 1349–1356. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Costa, E.M.; Machado, M.; Morais, R.; Calhau, C.; Pintado, M. Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites 2022, 12, 1062. https://doi.org/10.3390/metabo12111062
Silva S, Costa EM, Machado M, Morais R, Calhau C, Pintado M. Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites. 2022; 12(11):1062. https://doi.org/10.3390/metabo12111062
Chicago/Turabian StyleSilva, Sara, Eduardo M. Costa, Manuela Machado, Rui Morais, Conceição Calhau, and Manuela Pintado. 2022. "Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus" Metabolites 12, no. 11: 1062. https://doi.org/10.3390/metabo12111062
APA StyleSilva, S., Costa, E. M., Machado, M., Morais, R., Calhau, C., & Pintado, M. (2022). Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites, 12(11), 1062. https://doi.org/10.3390/metabo12111062









