Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria, Information Sources and Search Parameters
2.3. Study Selection
2.4. Data Collection Process and Data Items
2.5. Synthesis of Results
2.6. Risk of Bias
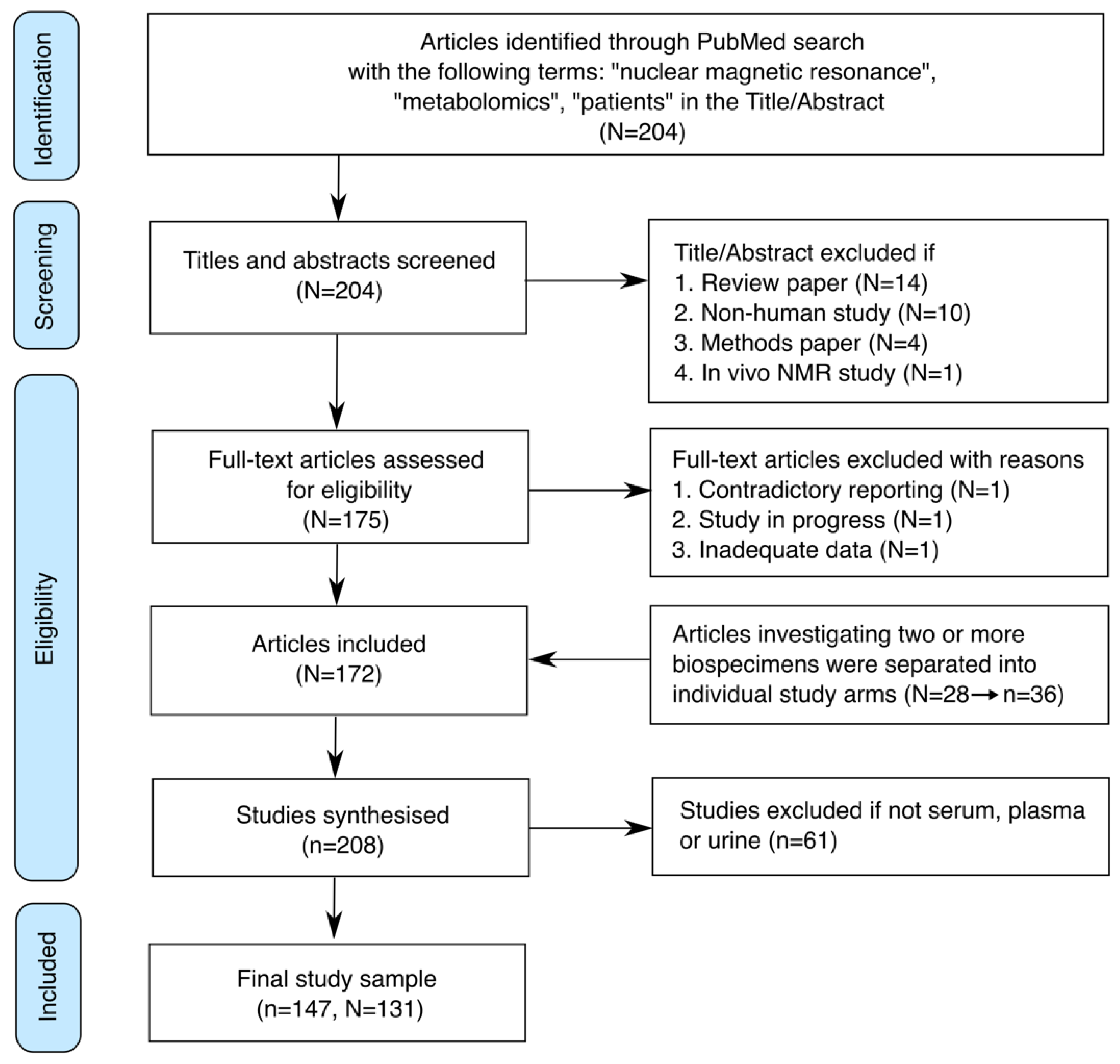
3. Results
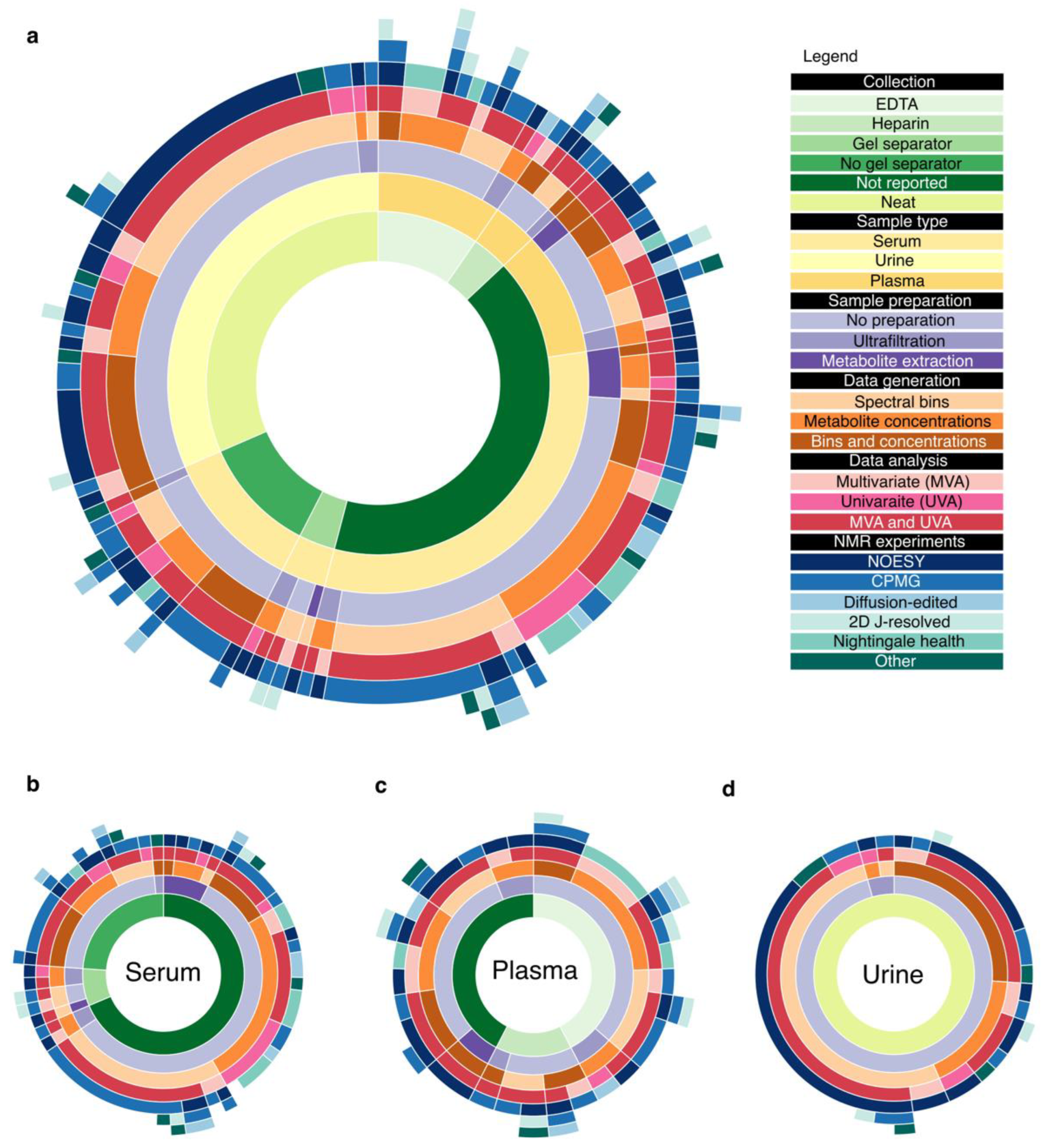
3.1. Study Selection and Data Collected
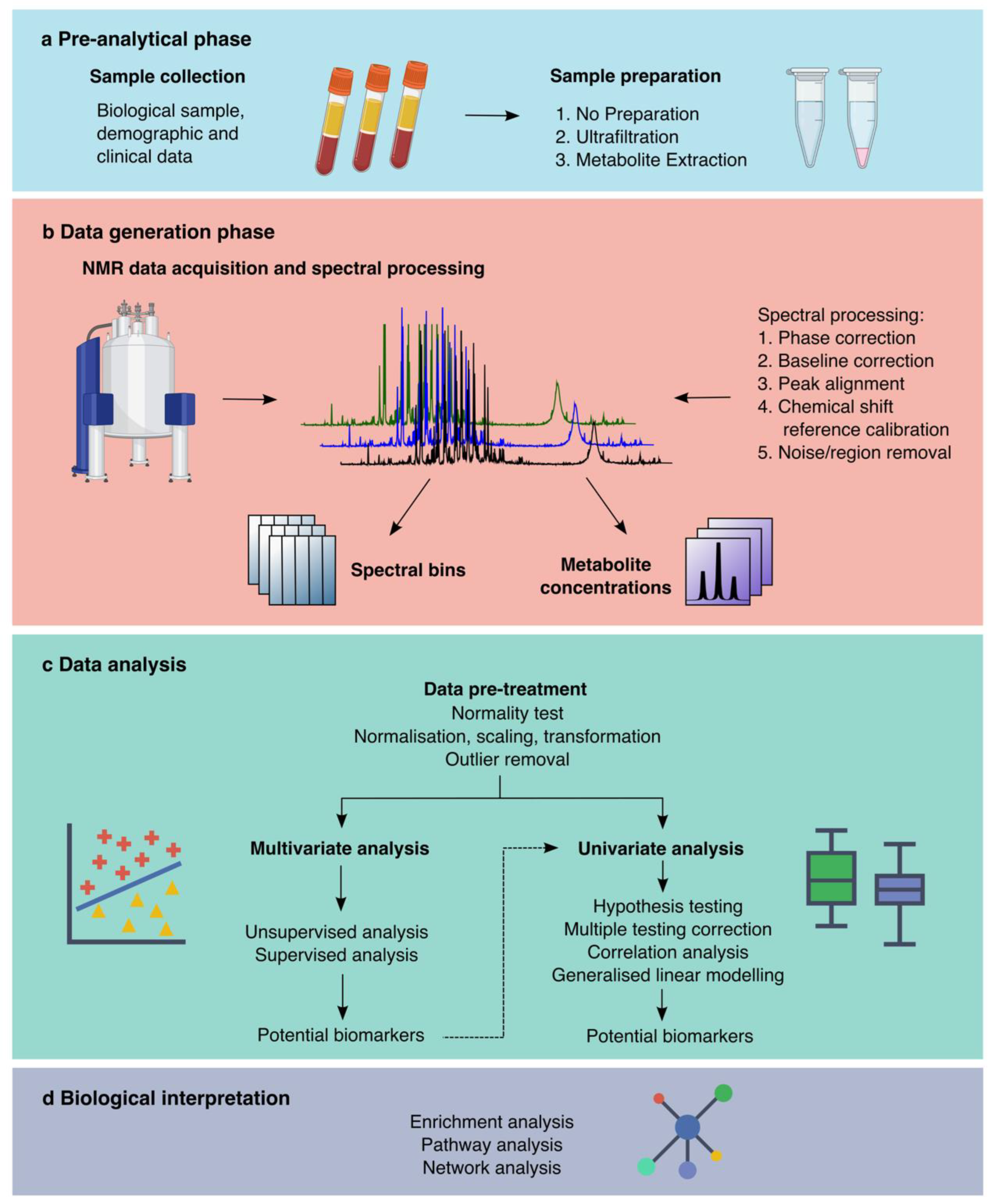
3.2. Pre-Analytical Phase
3.3. Blood Collection
3.4. Urine Collection
3.5. Sample Preparation
3.6. Data Generation Phase
3.6.1. NMR Introduction
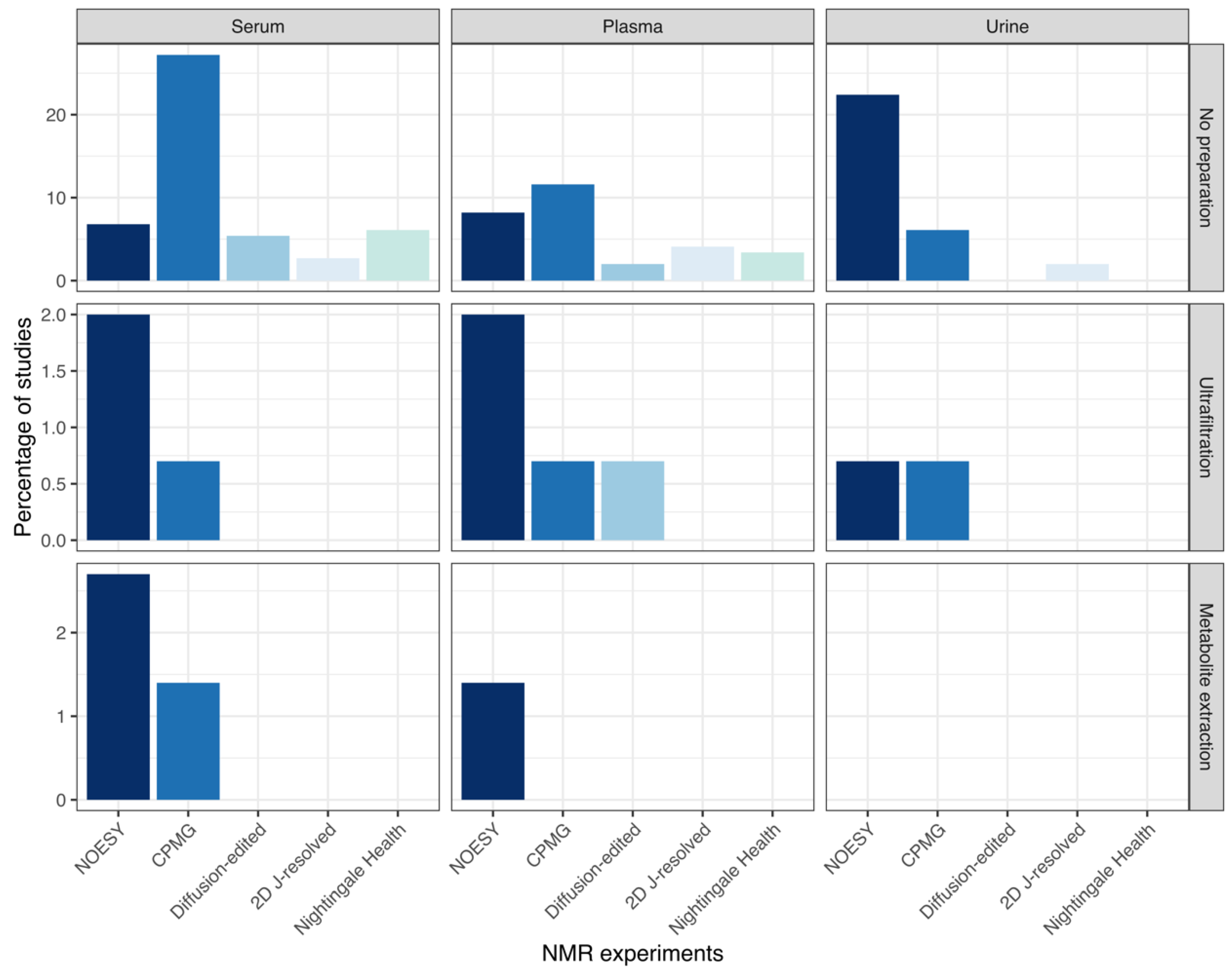
3.6.2. NMR Experiments
3.6.3. Spectral Binning
3.6.4. Metabolite Profiling
3.7. Data Analysis Phase
3.7.1. Data Pre-Treatment
3.7.2. Multivariate Analyses
3.7.3. Univariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, R.; Gonzalez-Dominguez, A.; Sayago, A.; Fernandez-Recamales, A. Recommendations and Best Practices for Standardizing the Pre-Analytical Processing of Blood and Urine Samples in Metabolomics. Metabolites 2020, 10, 229. [Google Scholar] [CrossRef]
- Dona, A.C.; Jimenez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Lu, W.; Su, X.; Klein, M.S.; Lewis, I.A.; Fiehn, O.; Rabinowitz, J.D. Metabolite Measurement: Pitfalls to Avoid and Practices to Follow. Annu. Rev. Biochem. 2017, 86, 277–304. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Members, M.S.I.B.; Sansone, S.A.; Fan, T.; Goodacre, R.; Griffin, J.L.; Hardy, N.W.; Kaddurah-Daouk, R.; Kristal, B.S.; Lindon, J.; Mendes, P.; et al. The metabolomics standards initiative. Nat. Biotechnol. 2007, 25, 846–848. [Google Scholar] [CrossRef]
- Spicer, R.A.; Salek, R.; Steinbeck, C. Compliance with minimum information guidelines in public metabolomics repositories. Sci. Data 2017, 4, 170137. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2020. [Google Scholar]
- Kumar, U.; Sharma, S.; Durgappa, M.; Gupta, N.; Raj, R.; Kumar, A.; Sharma, P.N.; Krishna, V.P.; Kumar, R.V.; Guleria, A.; et al. Serum Metabolic Disturbances Associated with Acute-on-chronic Liver Failure in Patients with Underlying Alcoholic Liver Diseases: An Elaborative NMR-based Metabolomics Study. J. Pharm. Bioallied Sci. 2021, 13, 276–282. [Google Scholar] [CrossRef]
- Rocca, M.S.; Vignoli, A.; Tenori, L.; Ghezzi, M.; De Rocco Ponce, M.; Vatsellas, G.; Thanos, D.; Padrini, R.; Foresta, C.; De Toni, L. Evaluation of Serum/Urine Genomic and Metabolomic Profiles to Improve the Adherence to Sildenafil Therapy in Patients with Erectile Dysfunction. Front. Pharmacol. 2020, 11, 602369. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, J.L.; Comella-Del-Barrio, P.; Campos-Olivas, R.; Villar-Hernández, R.; Prat-Aymerich, C.; De Souza-Galvão, M.L.; Jiménez-Fuentes, M.A.; Ruiz-Manzano, J.; Stojanovic, Z.; González, A.; et al. Discovery and validation of an NMR-based metabolomic profile in urine as TB biomarker. Sci. Rep. 2020, 10, 22317. [Google Scholar] [CrossRef] [PubMed]
- Citterio, F.; Romano, F.; Meoni, G.; Iaderosa, G.; Grossi, S.; Sobrero, A.; Dego, F.; Corana, M.; Berta, G.N.; Tenori, L.; et al. Changes in the Salivary Metabolic Profile of Generalized Periodontitis Patients after Non-surgical Periodontal Therapy: A Metabolomic Analysis Using Nuclear Magnetic Resonance Spectroscopy. J. Clin. Med. 2020, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Q.; Xiang, J.; Zhang, H.; Sun, H.; Ruan, G.; Tang, Y. NMR-based plasma metabolomics of adult B-cell acute lymphoblastic leukemia. Mol. Omics 2021, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Värri, M.; Niskanen, L.; Tuomainen, T.P.; Honkanen, R.; Kröger, H.; Tuppurainen, M.T. Metabolite Profiling of Osteoporosis and Atherosclerosis in Postmenopausal Women: A Cross-Sectional Study. Vasc. Health Risk Manag. 2020, 16, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Laera, L.; Fantechi, B.; Monte, F.D.; Benelli, M.; McCartney, A.; Leonardo, T.; Luchinat, C.; Pozzessere, D. Metabolomics to Assess Response to Immune Checkpoint Inhibitors in Patients with Non-Small-Cell Lung Cancer. Cancers 2020, 12, 3574. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Z.Z.; Sun, X.L.; Duan, H.J.; Tian, J.S.; Wang, J.Y.; Yang, H. Metabolomic analysis to detect urinary molecular changes associated with bipolar depression. Neurosci. Lett. 2021, 742, 135515. [Google Scholar] [CrossRef]
- Paris, D.; Palomba, L.; Mirra, V.; Borrelli, M.; Corcione, A.; Santamaria, F.; Maniscalco, M.; Motta, A. NMR Profiling of Exhaled Breath Condensate Defines Different Metabolic Phenotypes of Non-Cystic Fibrosis Bronchiectasis. Int. J. Mol. Sci. 2020, 21, 8600. [Google Scholar] [CrossRef]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear magnetic resonance and surface-assisted laser desorption/ionization mass spectrometry-based metabolome profiling of urine samples from kidney cancer patients. J. Pharm. Biomed. Anal. 2021, 193, 113752. [Google Scholar] [CrossRef]
- Chachaj, A.; Matkowski, R.; Gröbner, G.; Szuba, A.; Dudka, I. Metabolomics of Interstitial Fluid, Plasma and Urine in Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms. Diagnostics 2020, 10, 936. [Google Scholar] [CrossRef]
- Quintero Escobar, M.; Costa, T.; Martins, L.G.; Costa, S.S.; vanHelvoort Lengert, A.; Boldrini, É.; da Silva, S.R.M.; Lopes, L.F.; Vidal, D.O.; Krepischi, A.C.V.; et al. Insights in Osteosarcoma by Proton Nuclear Magnetic Resonance Serum Metabonomics. Front. Oncol. 2020, 10, 506959. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ustun, I.; Ugur, Z.; Akyol, S.; Hu, W.T.; Fiandaca, M.S.; Mapstone, M.; Federoff, H.; Maddens, M.; Graham, S.F. A Community-Based Study Identifying Metabolic Biomarkers of Mild Cognitive Impairment and Alzheimer’s Disease Using Artificial Intelligence and Machine Learning. J. Alzheimers Dis. 2020, 78, 1381–1392. [Google Scholar] [CrossRef]
- Castaldo, G.; Pagano, I.; Grimaldi, M.; Marino, C.; Molettieri, P.; Santoro, A.; Stillitano, I.; Romano, R.; Montoro, P.; D’Ursi, A.M.; et al. Effect of Very-Low-Calorie Ketogenic Diet on Psoriasis Patients: A Nuclear Magnetic Resonance-Based Metabolomic Study. J. Proteome Res. 2021, 20, 1509–1521. [Google Scholar] [CrossRef]
- Fraser, D.D.; Slessarev, M.; Martin, C.M.; Daley, M.; Patel, M.A.; Miller, M.R.; Patterson, E.K.; O’Gorman, D.B.; Gill, S.E.; Wishart, D.S.; et al. Metabolomics Profiling of Critically Ill Coronavirus Disease 2019 Patients: Identification of Diagnostic and Prognostic Biomarkers. Crit. Care Explor. 2020, 2, e0272. [Google Scholar] [CrossRef]
- Sahni, S.; Pandya, A.R.; Hadden, W.J.; Nahm, C.B.; Maloney, S.; Cook, V.; Toft, J.A.; Wilkinson-White, L.; Gill, A.J.; Samra, J.S.; et al. A unique urinary metabolomic signature for the detection of pancreatic ductal adenocarcinoma. Int. J. Cancer 2021, 148, 1508–1518. [Google Scholar] [CrossRef]
- Herrala, M.; Mikkonen, J.J.W.; Pesonen, P.; Lappalainen, R.; Tjäderhane, L.; Niemelä, R.K.; Seitsalo, H.; Salo, T.; Myllymaa, S.; Kullaa, A.M. Variability of salivary metabolite levels in patients with Sjögren’s syndrome. J. Oral Sci. 2020, 63, 22–26. [Google Scholar] [CrossRef]
- Haak, B.W.; Westendorp, W.F.; van Engelen, T.S.R.; Brands, X.; Brouwer, M.C.; Vermeij, J.D.; Hugenholtz, F.; Verhoeven, A.; Derks, R.J.; Giera, M.; et al. Disruptions of Anaerobic Gut Bacteria Are Associated with Stroke and Post-stroke Infection: A Prospective Case-Control Study. Transl. Stroke Res. 2021, 12, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.N.; Lee, H.; Park, J.W.; Kim, Y.H.; Park, S.; Kim, J.J. Screening for Early Gastric Cancer Using a Noninvasive Urine Metabolomics Approach. Cancers 2020, 12, 2904. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Kateb, F.; Caradeuc, C.; Marcellin, L.; Pocate-Cheriet, K.; Bourdon, M.; Chouzenoux, S.; Batteux, F.; Bertho, G.; et al. Endometriosis phenotypes are associated with specific serum metabolic profiles determined by proton-nuclear magnetic resonance. Reprod. Biomed. Online 2020, 41, 640–652. [Google Scholar] [CrossRef]
- Lins Neto MÁ, F.; Verdi, G.M.X.; Veras, A.O.; Veras, M.O.; Caetano, L.C.; Ursulino, J.S. Use of metabolomics to the diagnosis of inflammatory bowel disease. Arq. Gastroenterol. 2020, 57, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Pibiri, M.; Leoni, V.P.; Balsamo, A.; Tronci, L.; Arisci, N.; Mariotti, S.; Atzori, L. Analysis of metabolomics profile in hypothyroid patients before and after thyroid hormone replacement. J. Endocrinol. Investig. 2021, 44, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Breda, J.; Croitor Sava, A.; Himmelreich, U.; Somers, A.; Matthys, C.; Rocha Sousa, A.; Vandewalle, E.; Stalmans, I. Metabolomic profiling of aqueous humor from glaucoma patients—The metabolomics in surgical ophthalmological patients (MISO) study. Exp. Eye Res. 2020, 201, 108268. [Google Scholar] [CrossRef]
- Huhtala, M.S.; Tertti, K.; Rönnemaa, T. Serum lipids and their association with birth weight in metformin and insulin treated patients with gestational diabetes. Diabetes Res. Clin. Pract. 2020, 170, 108456. [Google Scholar] [CrossRef]
- Signoriello, E.; Iardino, P.; Casertano, S.; De Lucia, D.; Pucciarelli, A.; Puoti, G.; Chiosi, E.; Lus, G. 12-months prospective Pentraxin-3 and metabolomic evaluation in multiple sclerosis patients treated with glatiramer acetate. J. Neuroimmunol. 2020, 348, 577385. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; García-Flores, M.; Rubio-Briones, J.; López-Guerrero, J.A.; Pineda-Lucena, A.; Puchades-Carrasco, L. Targeted Metabolomics Analyses Reveal Specific Metabolic Alterations in High-Grade Prostate Cancer Patients. J. Proteome Res. 2020, 19, 4082–4092. [Google Scholar] [CrossRef]
- Wang, S.; Wen, S.; Guo, P.; Liu, H.; Feng, J.; Huang, H. Understanding metabolomic characteristics of pancreatic ductal adenocarcinoma by HR-MAS NMR detection of pancreatic tissues. J. Pharm. Biomed. Anal. 2020, 190, 113546. [Google Scholar] [CrossRef]
- Kimhofer, T.; Lodge, S.; Whiley, L.; Gray, N.; Loo, R.L.; Lawler, N.G.; Nitschke, P.; Bong, S.H.; Morrison, D.L.; Begum, S.; et al. Integrative Modeling of Quantitative Plasma Lipoprotein, Metabolic, and Amino Acid Data Reveals a Multiorgan Pathological Signature of SARS-CoV-2 Infection. J. Proteome Res. 2020, 19, 4442–4454. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaran, S.S.; Goyal, V.; Sharma, R.K.; Sinha, N.; Dwivedi, S.N.; Srivastava, A.K.; Jagannathan, N.R. Identification of potential urine biomarkers in idiopathic parkinson’s disease using NMR. Clin. Chim. Acta 2020, 510, 442–449. [Google Scholar] [CrossRef]
- Gupta, L.; Guleria, A.; Rawat, A.; Kumar, D.; Aggarwal, A. NMR-based clinical metabolomics revealed distinctive serum metabolic profiles in patients with spondyloarthritis. Magn. Reson. Chem. 2021, 59, 85–98. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Pérez-Álvarez, Á.I.; Gil-Serret, M.; Amigó, N.; Ulloa, C.; Benavente, L.; Ballina-García, F.J.; Suárez, A. GlycA Levels during the Earliest Stages of Rheumatoid Arthritis: Potential Use as a Biomarker of Subclinical Cardiovascular Disease. J. Clin. Med. 2020, 9, 2472. [Google Scholar] [CrossRef]
- Pauzi, F.A.; Sahathevan, S.; Khor, B.H.; Narayanan, S.S.; Zakaria, N.F.; Abas, F.; Karupaiah, T.; Daud, Z.A.M. Exploring Metabolic Signature of Protein Energy Wasting in Hemodialysis Patients. Metabolites 2020, 10, 291. [Google Scholar] [CrossRef]
- Hao, D.; Sengupta, A.; Ding, K.; Ubeydullah, E.R.; Krishnaiah, S.; Leighl, N.B.; Shepherd, F.A.; Seymour, L.; Weljie, A. Metabolites as Prognostic Markers for Metastatic Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Platinum-Doublet Chemotherapy. Cancers 2020, 12, 1926. [Google Scholar] [CrossRef]
- Prokić, I.; Lahousse, L.; de Vries, M.; Liu, J.; Kalaoja, M.; Vonk, J.M.; van der Plaat, D.A.; van Diemen, C.C.; van der Spek, A.; Zhernakova, A.; et al. A cross-omics integrative study of metabolic signatures of chronic obstructive pulmonary disease. BMC Pulm. Med. 2020, 20, 193. [Google Scholar] [CrossRef]
- Benítez Del Castillo, J.M.; Pinazo-Duran, M.D.; Sanz-González, S.M.; Muñoz-Hernández, A.M.; Garcia-Medina, J.J.; Zanón-Moreno, V. Tear 1H Nuclear Magnetic Resonance-Based Metabolomics Application to the Molecular Diagnosis of Aqueous Tear Deficiency and Meibomian Gland Dysfunction. Ophthalmic Res. 2021, 64, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear magnetic resonance and surface-assisted laser desorption/ionization mass spectrometry-based serum metabolomics of kidney cancer. Anal. BioAnal. Chem. 2020, 412, 5827–5841. [Google Scholar] [CrossRef]
- Liang, K.H.; Cheng, M.L.; Lo, C.J.; Lin, Y.H.; Lai, M.W.; Lin, W.R.; Yeh, C.T. Plasma phenylalanine and glutamine concentrations correlate with subsequent hepatocellular carcinoma occurrence in liver cirrhosis patients: An exploratory study. Sci. Rep. 2020, 10, 10926. [Google Scholar] [CrossRef]
- Lalwani, A.M.; Yilmaz, A.; Bisgin, H.; Ugur, Z.; Akyol, S.; Graham, S.F. The Biochemical Profile of Post-Mortem Brain from People Who Suffered from Epilepsy Reveals Novel Insights into the Etiopathogenesis of the Disease. Metabolites 2020, 10, 261. [Google Scholar] [CrossRef]
- Maltesen, R.G.; Wimmer, R.; Rasmussen, B.S. A longitudinal serum NMR-based metabolomics dataset of ischemia-reperfusion injury in adult cardiac surgery. Sci. Data 2020, 7, 198. [Google Scholar] [CrossRef]
- Urman, J.M.; Herranz, J.M.; Uriarte, I.; Rullán, M.; Oyón, D.; González, B.; Fernandez-Urién, I.; Carrascosa, J.; Bolado, F.; Zabalza, L.; et al. Pilot Multi-Omic Analysis of Human Bile from Benign and Malignant Biliary Strictures: A Machine-Learning Approach. Cancers 2020, 12, 1644. [Google Scholar] [CrossRef]
- Zennaro, L.; Nicolè, L.; Vanzani, P.; Cappello, F.; Fassina, A. 1H-NMR spectroscopy metabonomics of reactive, ovarian carcinoma and hepatocellular carcinoma ascites. Pleura Peritoneum 2020, 5, 20200113. [Google Scholar] [CrossRef]
- Acar, İ.E.; Lores-Motta, L.; Colijn, J.M.; Meester-Smoor, M.A.; Verzijden, T.; Cougnard-Gregoire, A.; Ajana, S.; Merle, B.M.J.; de Breuk, A.; Heesterbeek, T.J.; et al. Integrating Metabolomics, Genomics, and Disease Pathways in Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2020, 127, 1693–1709. [Google Scholar] [CrossRef]
- Xie, J.; Chen, C.; Hou, L.J.; Zhou, C.J.; Fang, L.; Chen, J.J. Dual Metabolomic Platforms Identified a Novel Urinary Metabolite Signature for Hepatitis B Virus-Infected Patients with Depression. Diabetes Metab. Syndr. Obes. 2020, 13, 1677–1683. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhuang, Y.; Zhang, P.; Chen, S.; Asakawa, T.; Gao, B. Serum Metabolomic Profiles Associated With Untreated Metabolic Syndrome Patients in the Chinese Population. Clin. Transl. Sci. 2020, 13, 1271–1278. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; Bastos, M.L.; Carvalho, M.; Guedes Pinho, P. New findings on urinary prostate cancer metabolome through combined GC-MS and 1H NMR analytical platforms. Metabolomics 2020, 16, 70. [Google Scholar] [CrossRef]
- Lee, C.W.; Yu, M.C.; Lin, G.; Chiu, J.C.; Chiang, M.H.; Sung, C.M.; Hsieh, Y.C.; Kuo, T.; Lin, C.Y.; Tsai, H.I. Serum metabolites may be useful markers to assess vascular invasion and identify normal alpha-fetoprotein in hepatocellular carcinoma undergoing liver resection: A pilot study. World J. Surg. Oncol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Palomino-Schätzlein, M.; Lamas-Domingo, R.; Ciudin, A.; Gutiérrez-Carcedo, P.; Marés, R.; Aparicio-Gómez, C.; Hernández, C.; Simó, R.; Herance, J.R. A Translational In Vivo and In Vitro Metabolomic Study Reveals Altered Metabolic Pathways in Red Blood Cells of Type 2 Diabetes. J. Clin. Med. 2020, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Metere, A.; Graves, C.E.; Chirico, M.; Caramujo, M.J.; Pisanu, M.E.; Iorio, E. Metabolomic Reprogramming Detected by 1H-NMR Spectroscopy in Human Thyroid Cancer Tissues. Biology 2020, 9, 112. [Google Scholar] [CrossRef]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Ostuni, A. Metabolic changes in follicular fluids of patients treated with recombinant versus urinary human chorionic gonadotropin for triggering ovulation in assisted reproductive technologies: A metabolomics pilot study. Arch. Gynecol. Obstet. 2020, 302, 741–751. [Google Scholar] [CrossRef]
- Capolongo, G.; Zacchia, M.; Beneduci, A.; Costantini, S.; Cinque, P.; Spasiano, A.; De Luca, G.; Di Pietro, M.E.; Ricchi, P.; Trepiccione, F.; et al. Urinary Metabolic Profile of Patients with Transfusion-Dependent β-Thalassemia Major Undergoing Deferasirox Therapy. Kidney Blood Press. Res. 2020, 45, 455–466. [Google Scholar] [CrossRef]
- Dudka, I.; Thysell, E.; Lundquist, K.; Antti, H.; Iglesias-Gato, D.; Flores-Morales, A.; Bergh, A.; Wikström, P.; Gröbner, G. Comprehensive metabolomics analysis of prostate cancer tissue in relation to tumor aggressiveness and TMPRSS2-ERG fusion status. BMC Cancer 2020, 20, 437. [Google Scholar] [CrossRef]
- Lunde, S.; Nguyen, H.T.; Petersen, K.K.; Arendt-Nielsen, L.; Krarup, H.B.; Søgaard-Andersen, E. Chronic Postoperative Pain After Hysterectomy for Endometrial Cancer: A Metabolic Profiling Study. Mol. Pain 2020, 16, 1744806920923885. [Google Scholar] [CrossRef]
- Malo, A.I.; Rull, A.; Girona, J.; Domingo, P.; Fuertes-Martín, R.; Amigó, N.; Rodríguez-Borjabad, C.; Martínez-Micaelo, N.; Leal, M.; Peraire, J.; et al. Glycoprotein Profile Assessed by 1H-NMR as a Global Inflammation Marker in Patients with HIV Infection. A Prospective Study. J. Clin. Med. 2020, 9, 1344. [Google Scholar] [CrossRef]
- Andreas, N.J.; Basu Roy, R.; Gomez-Romero, M.; Horneffer-van der Sluis, V.; Lewis, M.R.; Camuzeaux, S.S.M.; Jiménez, B.; Posma, J.M.; Tientcheu, L.; Egere, U.; et al. Performance of metabonomic serum analysis for diagnostics in paediatric tuberculosis. Sci. Rep. 2020, 10, 7302. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Nagana Gowda, G.A.; Djukovic, D.; Raftery, D. Distinguishing NASH Histological Severity Using a Multiplatform Metabolomics Approach. Metabolites 2020, 10, 168. [Google Scholar] [CrossRef]
- Nong, Q.; Zhang, C.; Liu, Q.; Xie, R.; Dong, M. Effect of daunorubicin on acute promyelocytic leukemia cells using nuclear magnetic resonance spectroscopy-based metabolomics. Environ. Toxicol. Pharmacol. 2020, 78, 103382. [Google Scholar] [CrossRef]
- Ganguly, S.; Kumar, U.; Gupta, N.; Guleria, A.; Majumdar, S.; Phatak, S.; Chaurasia, S.; Kumar, S.; Aggarwal, A.; Kumar, D.; et al. Nuclear magnetic resonance-based targeted profiling of urinary acetate and citrate following cyclophosphamide therapy in patients with lupus nephritis. Lupus 2020, 29, 782–786. [Google Scholar] [CrossRef]
- McCann, M.R.; McHugh, C.E.; Kirby, M.; Jennaro, T.S.; Jones, A.E.; Stringer, K.A.; Puskarich, M.A. A Multivariate Metabolomics Method for Estimating Platelet Mitochondrial Oxygen Consumption Rates in Patients with Sepsis. Metabolites 2020, 10, 139. [Google Scholar] [CrossRef]
- Murgia, F.; Lorefice, L.; Poddighe, S.; Fenu, G.; Secci, M.A.; Marrosu, M.G.; Cocco, E.; Atzori, L. Multi-Platform Characterization of Cerebrospinal Fluid and Serum Metabolome of Patients Affected by Relapsing-Remitting and Primary Progressive Multiple Sclerosis. J. Clin. Med. 2020, 9, 863. [Google Scholar] [CrossRef]
- Tsai, C.K.; Lin, C.Y.; Kang, C.J.; Liao, C.T.; Wang, W.L.; Chiang, M.H.; Yen, T.C.; Lin, G. Nuclear Magnetic Resonance Metabolomics Biomarkers for Identifying High Risk Patients with Extranodal Extension in Oral Squamous Cell Carcinoma. J. Clin. Med. 2020, 9, 951. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Men, Y.; Wang, W.; Zhang, W. Heavy metals interfere with plasma metabolites, including lipids and amino acids, in patients with breast cancer. Oncol. Lett. 2020, 19, 2925–2933. [Google Scholar] [CrossRef]
- Insenser, M.; Moncayo, S.; Martínez-García, M.; Fernández-Durán, E.; Samino, S.; Álvarez-Blasco, F.; Luque-Ramírez, M.; Escobar-Morreale, H.F. 2D Diffusion-Ordered 1H-NMR Spectroscopy Lipidomic Profiling after Oral Single Macronutrient Loads: Influence of Obesity, Sex, and Female Androgen Excess. Mol. Nutr. Food Res. 2020, 64, e1900928. [Google Scholar] [CrossRef]
- Kalantari, S.; Chashmniam, S.; Nafar, M.; Zakeri, Z.; Parvin, M. Metabolomics approach reveals urine biomarkers and pathways associated with the pathogenesis of lupus nephritis. Iran. J. Basic Med. Sci. 2019, 22, 1288–1295. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.Y.; Chun, Y.S.; Chun, Y.J.; Shin, S.Y.; Choi, C.H.; Choi, H.K. Characteristics of fecal metabolic profiles in patients with irritable bowel syndrome with predominant diarrhea investigated using 1H-NMR coupled with multivariate statistical analysis. Neurogastroenterol. Motil. 2020, 32, e13830. [Google Scholar] [CrossRef]
- Vignoli, A.; Paciotti, S.; Tenori, L.; Eusebi, P.; Biscetti, L.; Chiasserini, D.; Scheltens, P.; Turano, P.; Teunissen, C.; Luchinat, C.; et al. Fingerprinting Alzheimer’s Disease by 1H Nuclear Magnetic Resonance Spectroscopy of Cerebrospinal Fluid. J. Proteome Res. 2020, 19, 1696–1705. [Google Scholar] [CrossRef]
- Merolle, L.; Marraccini, C.; Latorrata, A.; Quartieri, E.; Farioli, D.; Scarano, L.; Fasano, T.; Bergamini, S.; Bellei, E.; Monari, E.; et al. Heparin-induced lipoprotein precipitation apheresis in dyslipidemic patients: A multiparametric assessment. J. Clin. Apher. 2020, 35, 146–153. [Google Scholar] [CrossRef]
- Souto-Carneiro, M.; Tóth, L.; Behnisch, R.; Urbach, K.; Klika, K.D.; Carvalho, R.A.; Lorenz, H.M. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 499–506. [Google Scholar] [CrossRef]
- Jaurila, H.; Koivukangas, V.; Koskela, M.; Gäddnäs, F.; Myllymaa, S.; Kullaa, A.; Salo, T.; Ala-Kokko, T.I. 1H NMR Based Metabolomics in Human Sepsis and Healthy Serum. Metabolites 2020, 10, 70. [Google Scholar] [CrossRef]
- Liu, F.; Ruze, A.; Liu, W.; Xiong, K.; Yiming, A. Metabonomic profiling of blood plasma from erectile dysfunction patients using 1H nuclear magnetic resonance spectroscopy. Acta Biochim. Biophys. Sin. (Shanghai) 2020, 52, 332–335. [Google Scholar] [CrossRef]
- Gupta, A.; Bansal, N.; Mitash, N.; Kumar, D.; Kumar, M.; Sankhwar, S.N.; Mandhani, A.; Singh, U.P. NMR-derived targeted serum metabolic biomarkers appraisal of bladder cancer: A pre- and post-operative evaluation. J. Pharm. Biomed. Anal. 2020, 183, 113134. [Google Scholar] [CrossRef]
- Vroegindewey, M.M.; van den Berg, V.J.; Oemrawsingh, R.M.; Kardys, I.; Asselbergs, F.W.; van der Harst, P.; Kietselaer, B.; Lenderink, T.; Akkerhuis, K.M.; Boersma, E. High-frequency metabolite profiling and the incidence of recurrent cardiac events in patients with post-acute coronary syndrome. Biomarkers 2020, 25, 235–240. [Google Scholar] [CrossRef]
- Muhle-Goll, C.; Eisenmann, P.; Luy, B.; Kölker, S.; Tönshoff, B.; Fichtner, A.; Westhoff, J.H. Urinary NMR Profiling in Pediatric Acute Kidney Injury-A Pilot Study. Int. J. Mol. Sci. 2020, 21, 1187. [Google Scholar] [CrossRef]
- Murgia, F.; Corda, V.; Serrenti, M.; Usai, V.; Santoru, M.L.; Hurt, K.J.; Passaretti, M.; Monni, M.C.; Atzori, L.; Monni, G. Seminal Fluid Metabolomic Markers of Oligozoospermic Infertility in Humans. Metabolites 2020, 10, 64. [Google Scholar] [CrossRef]
- Cortese, N.; Capretti, G.; Barbagallo, M.; Rigamonti, A.; Takis, P.G.; Castino, G.F.; Vignali, D.; Maggi, G.; Gavazzi, F.; Ridolfi, C.; et al. Metabolome of Pancreatic Juice Delineates Distinct Clinical Profiles of Pancreatic Cancer and Reveals a Link between Glucose Metabolism and PD-1(+) Cells. Cancer Immunol. Res. 2020, 8, 493–505. [Google Scholar] [CrossRef]
- Jääskeläinen, O.; Hall, A.; Tiainen, M.; van Gils, M.; Lötjönen, J.; Kangas, A.J.; Helisalmi, S.; Pikkarainen, M.; Hallikainen, M.; Koivisto, A.; et al. Metabolic Profiles Help Discriminate Mild Cognitive Impairment from Dementia Stage in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 74, 277–286. [Google Scholar] [CrossRef]
- Men, Y.; Li, L.; Zhang, F.; Kong, X.; Zhang, W.; Hao, C.; Wang, G. Evaluation of heavy metals and metabolites in the urine of patients with breast cancer. Oncol. Lett. 2020, 19, 1331–1337. [Google Scholar] [CrossRef]
- West, K.A.; Kanu, C.; Maric, T.; McDonald, J.A.K.; Nicholson, J.K.; Li, J.V.; Johnson, M.R.; Holmes, E.; Savvidou, M.D. Longitudinal metabolic and gut bacterial profiling of pregnant women with previous bariatric surgery. Gut 2020, 69, 1452–1459. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Del Coco, L.; Marisi, G.; Conti, F.; Rovesti, G.; Ulivi, P.; Canale, M.; Frassineti, G.L.; Foschi, F.G.; Longo, S.; et al. 1H-NMR Based Serum Metabolomics Highlights Different Specific Biomarkers between Early and Advanced Hepatocellular Carcinoma Stages. Cancers 2020, 12, 241. [Google Scholar] [CrossRef]
- Dogan, B.; Karaer, A.; Tuncay, G.; Tecellioglu, N.; Mumcu, A. High-resolution 1H-NMR spectroscopy indicates variations in metabolomics profile of follicular fluid from women with advanced maternal age. J. Assist. Reprod. Genet. 2020, 37, 321–330. [Google Scholar] [CrossRef]
- Gooding, J.R.; Agrawal, S.; McRitchie, S.; Acuff, Z.; Merchant, M.L.; Klein, J.B.; Smoyer, W.E.; Sumner, S.J. Predicting and Defining Steroid Resistance in Pediatric Nephrotic Syndrome Using Plasma Metabolomics. Kidney Int. Rep. 2020, 5, 81–93. [Google Scholar] [CrossRef]
- Hsu, W.H.; Wang, S.J.; Chao, Y.M.; Chen, C.J.; Wang, Y.F.; Fuh, J.L.; Chen, S.P.; Lin, Y.L. Urine metabolomics signatures in reversible cerebral vasoconstriction syndrome. Cephalalgia 2020, 40, 735–747. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Giusti, B.; Valente, S.; Carrabba, N.; Balzi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; Marcucci, R.; et al. Differential Network Analysis Reveals Metabolic Determinants Associated with Mortality in Acute Myocardial Infarction Patients and Suggests Potential Mechanisms Underlying Different Clinical Scores Used To Predict Death. J. Proteome Res. 2020, 19, 949–961. [Google Scholar] [CrossRef]
- Kumar, U.; Jain, A.; Guleria, A.; Kumar, R.V.; Misra, D.P.; Goel, R.; Danda, D.; Misra, R.; Kumar, D. Circulatory Glutamine/Glucose ratio for evaluating disease activity in Takayasu arteritis: A NMR based serum metabolomics study. J. Pharm. Biomed. Anal. 2020, 180, 113080. [Google Scholar] [CrossRef]
- Banoei, M.M.; Iupe, I.; Bazaz, R.D.; Campos, M.; Vogel, H.J.; Winston, B.W.; Mirsaeidi, M. Metabolomic and metallomic profile differences between Veterans and Civilians with Pulmonary Sarcoidosis. Sci. Rep. 2019, 9, 19584. [Google Scholar] [CrossRef]
- Cardner, M.; Yalcinkaya, M.; Goetze, S.; Luca, E.; Balaz, M.; Hunjadi, M.; Hartung, J.; Shemet, A.; Kränkel, N.; Radosavljevic, S.; et al. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight 2020, 5, e131491. [Google Scholar] [CrossRef]
- Righi, V.; Cavallini, N.; Valentini, A.; Pinna, G.; Pavesi, G.; Rossi, M.C.; Puzzolante, A.; Mucci, A.; Cocchi, M. A metabolomic data fusion approach to support gliomas grading. NMR Biomed. 2020, 33, e4234. [Google Scholar] [CrossRef]
- Gilany, K.; Mohamadkhani, A.; Chashmniam, S.; Shahnazari, P.; Amini, M.; Arjmand, B.; Malekzadeh, R.; Nobakht Motlagh Ghoochani, B.F. Metabolomics analysis of the saliva in patients with chronic hepatitis B using nuclear magnetic resonance: A pilot study. Iran. J. Basic Med. Sci. 2019, 22, 1044–1049. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yang, Z.X.; Ma, L.M.; Wen, X.Q.; Ji, H.L.; Li, K. 1H-NMR spectroscopy identifies potential biomarkers in serum metabolomic signatures for early stage esophageal squamous cell carcinoma. PeerJ 2019, 7, e8151. [Google Scholar] [CrossRef]
- Erasmus, E.; Mason, S.; van Reenen, M.; Steffens, F.E.; Vorster, B.C.; Reinecke, C.J. A laboratory approach for characterizing chronic fatigue: What does metabolomics tell us? Metabolomics 2019, 15, 158. [Google Scholar] [CrossRef]
- Frick, M.A.; Barba, I.; Fenoy-Alejandre, M.; López-López, P.; Baquero-Artigao, F.; Rodríguez-Molino, P.; Noguera-Julian, A.; Nicolás-López, M.; de la Fuente-Juárez, A.; Codina-Grau, M.G.; et al. 1H-NMR Urinary Metabolic Profile, A Promising Tool for the Management of Infants with Human Cytomegalovirus-Infection. Metabolites 2019, 9, 288. [Google Scholar] [CrossRef]
- Zheng, H.; Dong, B.; Ning, J.; Shao, X.; Zhao, L.; Jiang, Q.; Ji, H.; Cai, A.; Xue, W.; Gao, H. NMR-based metabolomics analysis identifies discriminatory metabolic disturbances in tissue and biofluid samples for progressive prostate cancer. Clin. Chim. Acta 2020, 501, 241–251. [Google Scholar] [CrossRef]
- Silva, C.L.; Olival, A.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Untargeted Urinary 1H NMR-Based Metabolomic Pattern as a Potential Platform in Breast Cancer Detection. Metabolites 2019, 9, 269. [Google Scholar] [CrossRef]
- Kevat, A.C.; Carzino, R.; Vidmar, S.; Ranganathan, S. Glycoprotein A as a biomarker of pulmonary infection and inflammation in children with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Lee, S.H.; Chen, Y.C.; Wu, C.K.; Chuang, J.Y.; Lo, S.C.; Yeh, H.M.; Yeh, S.S.; Hsu, C.A.; Lin, B.B.; et al. Metabolomic Analysis of Platelets of Patients With Aspirin Non-Response. Front. Pharmacol. 2019, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.; Probert, F.; Jurynczyk, M.; Sealey, M.; Cavey, A.; Claridge, T.D.W.; Woodhall, M.; Waters, P.; Leite, M.I.; Anthony, D.C.; et al. Classifying the antibody-negative NMO syndromes: Clinical, imaging, and metabolomic modeling. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e626. [Google Scholar] [CrossRef]
- Ahmed, N.; Kidane, B.; Wang, L.; Qing, G.; Tan, L.; Buduhan, G.; Srinathan, S.; Aliani, M. Non-invasive exploration of metabolic profile of lung cancer with Magnetic Resonance Spectroscopy and Mass Spectrometry. Contemp. Clin. Trials Commun. 2019, 16, 100445. [Google Scholar] [CrossRef]
- D’Amato, M.; Paris, D.; Molino, A.; Cuomo, P.; Fulgione, A.; Sorrentino, N.; Palomba, L.; Maniscalco, M.; Motta, A. The Immune-Modulator Pidotimod Affects the Metabolic Profile of Exhaled Breath Condensate in Bronchiectatic Patients: A Metabolomics Pilot Study. Front. Pharmacol. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Vignoli, A.; Santini, G.; Tenori, L.; Macis, G.; Mores, N.; Macagno, F.; Pagano, F.; Higenbottam, T.; Luchinat, C.; Montuschi, P. NMR-Based Metabolomics for the Assessment of Inhaled Pharmacotherapy in Chronic Obstructive Pulmonary Disease Patients. J. Proteome Res. 2020, 19, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mehrparvar, B.; Chashmniam, S.; Nobakht, F.; Amini, M.; Javidi, A.; Minai-Tehrani, A.; Arjmand, B.; Gilany, K. Metabolic profiling of seminal plasma from teratozoospermia patients. J. Pharm. Biomed. Anal. 2020, 178, 112903. [Google Scholar] [CrossRef]
- An, J.N.; Hyeon, J.S.; Jung, Y.; Choi, Y.W.; Kim, J.H.; Yang, S.H.; Oh, S.; Kwon, S.; Lee, S.H.; Cho, J.H.; et al. Urinary myo-inositol is associated with the clinical outcome in focal segmental glomerulosclerosis. Sci. Rep. 2019, 9, 14707. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Wu, J.; Kang, X.; Xie, Q.; Sheng, J.; Xu, W.; Liu, D.; Zheng, W. Plasma metabolite profiling reveals potential biomarkers of giant cell tumor of bone by using NMR-based metabolic profiles: A cross-sectional study. Medicine (Baltimore) 2019, 98, e17445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.Y.; Xie, C.; Zhang, M.L.; Wang, Y.J.; Liu, G.H. Metabolomics as a potential method for predicting thyroid malignancy in children and adolescents. Pediatr. Surg. Int. 2020, 36, 145–153. [Google Scholar] [CrossRef]
- Tasic, L.; Larcerda, A.L.T.; Pontes, J.G.M.; da Costa, T.; Nani, J.V.; Martins, L.G.; Santos, L.A.; Nunes, M.F.Q.; Adelino, M.P.M.; Pedrini, M.; et al. Peripheral biomarkers allow differential diagnosis between schizophrenia and bipolar disorder. J. Psychiatr. Res. 2019, 119, 67–75. [Google Scholar] [CrossRef]
- Diao, W.; Labaki, W.W.; Han, M.K.; Yeomans, L.; Sun, Y.; Smiley, Z.; Kim, J.H.; McHugh, C.; Xiang, P.; Shen, N.; et al. Disruption of histidine and energy homeostasis in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2015–2025. [Google Scholar] [CrossRef]
- Jääskeläinen, O.; Solje, E.; Hall, A.; Katisko, K.; Korhonen, V.; Tiainen, M.; Kangas, A.J.; Helisalmi, S.; Pikkarainen, M.; Koivisto, A.; et al. Low Serum High-Density Lipoprotein Cholesterol Levels Associate with the C9orf72 Repeat Expansion in Frontotemporal Lobar Degeneration Patients. J. Alzheimers Dis. 2019, 72, 127–137. [Google Scholar] [CrossRef]
- Seow, W.J.; Shu, X.O.; Nicholson, J.K.; Holmes, E.; Walker, D.I.; Hu, W.; Cai, Q.; Gao, Y.T.; Xiang, Y.B.; Moore, S.C.; et al. Association of Untargeted Urinary Metabolomics and Lung Cancer Risk Among Never-Smoking Women in China. JAMA Netw. Open 2019, 2, e1911970. [Google Scholar] [CrossRef] [PubMed]
- Bund, C.; Lhermitte, B.; Cicek, A.E.; Ruhland, E.; Proust, F.; Namer, I.J. What Does Reduced FDG Uptake Mean in High-Grade Gliomas? Clin. Nucl. Med. 2019, 44, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.G.; Gonçalves, L.G.; Cunha, N.; Bugalho, M.J. Metabolomic Urine Profile: Searching for New Biomarkers of SDHx-Associated Pheochromocytomas and Paragangliomas. J. Clin. Endocrinol. Metab. 2019, 104, 5467–5477. [Google Scholar] [CrossRef]
- Ose, J.; Gigic, B.; Lin, T.; Liesenfeld, D.B.; Böhm, J.; Nattenmüller, J.; Scherer, D.; Zielske, L.; Schrotz-King, P.; Habermann, N.; et al. Multiplatform Urinary Metabolomics Profiling to Discriminate Cachectic from Non-Cachectic Colorectal Cancer Patients: Pilot Results from the ColoCare Study. Metabolites 2019, 9, 178. [Google Scholar] [CrossRef]
- Taherkhani, A.; Nafar, M.; Arefi-Oskouie, A.; Broumandnia, N.; Parvin, M.; Mahmoudieh, L.; Kalantari, S. Metabolomic Analysis of Membranous Glomerulonephritis: Identification of a Diagnostic Panel and Pathogenic Pathways. Arch. Med. Res. 2019, 50, 159–169. [Google Scholar] [CrossRef]
- Akhbari, P.; Jaggard, M.K.; Boulangé, C.L.; Vaghela, U.; Graça, G.; Bhattacharya, R.; Lindon, J.C.; Williams, H.R.T.; Gupte, C.M. Differences in the composition of hip and knee synovial fluid in osteoarthritis: A nuclear magnetic resonance (NMR) spectroscopy study of metabolic profiles. Osteoarthr. Cartil. 2019, 27, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Debik, J.; Euceda, L.R.; Lundgren, S.; Gythfeldt, H.V.L.; Garred, Ø.; Borgen, E.; Engebraaten, O.; Bathen, T.F.; Giskeødegård, G.F. Assessing Treatment Response and Prognosis by Serum and Tissue Metabolomics in Breast Cancer Patients. J. Proteome Res. 2019, 18, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Bawadikji, A.A.; Teh, C.H.; Sheikh Abdul Kader, M.A.B.; Abdul Wahab, M.J.B.; Syed Sulaiman, S.A.; Ibrahim, B. Plasma Metabolites as Predictors of Warfarin Outcome in Atrial Fibrillation. Am. J. Cardiovasc. Drugs 2020, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Righi, V.; Tarentini, E.; Mucci, A.; Reggiani, C.; Rossi, M.C.; Ferrari, F.; Casari, A.; Magnoni, C. Field cancerization therapy with ingenol mebutate contributes to restoring skin-metabolism to normal-state in patients with actinic keratosis: A metabolomic analysis. Sci. Rep. 2019, 9, 11515. [Google Scholar] [CrossRef]
- Wildberg, C.; Masuch, A.; Budde, K.; Kastenmüller, G.; Artati, A.; Rathmann, W.; Adamski, J.; Kocher, T.; Völzke, H.; Nauck, M.; et al. Plasma Metabolomics to Identify and Stratify Patients With Impaired Glucose Tolerance. J. Clin. Endocrinol. Metab. 2019, 104, 6357–6370. [Google Scholar] [CrossRef] [PubMed]
- Huart, J.; Leenders, J.; Taminiau, B.; Descy, J.; Saint-Remy, A.; Daube, G.; Krzesinski, J.M.; Melin, P.; de Tullio, P.; Jouret, F. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension 2019, 74, 1005–1013. [Google Scholar] [CrossRef]
- Falegan, O.S.; Arnold Egloff, S.A.; Zijlstra, A.; Hyndman, M.E.; Vogel, H.J. Urinary Metabolomics Validates Metabolic Differentiation Between Renal Cell Carcinoma Stages and Reveals a Unique Metabolic Profile for Oncocytomas. Metabolites 2019, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Lin, Y.; Ouyang, T.; Tang, W.; Huang, Y.; Ye, W.; Zhao, J.Y.; Wang, Z.N.; Ma, C.C. Nuclear magnetic resonance-based metabolomics and metabolic pathway networks from patient-matched esophageal carcinoma, adjacent noncancerous tissues and urine. World J. Gastroenterol. 2019, 25, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- López-Garrido, L.; Bañuelos-Hernández, A.E.; Pérez-Hernández, E.; Tecualt-Gómez, R.; Quiroz-Williams, J.; Ariza-Castolo, A.; Becerra-Martínez, E.; Pérez-Hernández, N. Metabolic profiling of serum in patients with cartilage tumours using 1H-NMR spectroscopy: A pilot study. Magn. Reson. Chem. 2020, 58, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tomàs, E.; Murcia, M.; Arenas, M.; Arguís, M.; Gil, M.; Amigó, N.; Correig, X.; Torres, L.; Sabater, S.; Baiges-Gayà, G.; et al. Serum Paraoxonase-1-Related Variables and Lipoprotein Profile in Patients with Lung or Head and Neck Cancer: Effect of Radiotherapy. Antioxidants 2019, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Gawron, K.; Wojtowicz, W.; Łazarz-Bartyzel, K.; Łamasz, A.; Qasem, B.; Mydel, P.; Chomyszyn-Gajewska, M.; Potempa, J.; Mlynarz, P. Metabolomic Status of The Oral Cavity in Chronic Periodontitis. In Vivo 2019, 33, 1165–1174. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Milani, C.; Gutiérrez-Díaz, I.; Sánchez, B.; Mangifesta, M.; Segura, J.; Cambero, I.; Campelo, A.B.; García-Bernardo, C.M.; et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome 2019, 7, 100. [Google Scholar] [CrossRef]
- Loras, A.; Martínez-Bisbal, M.C.; Quintás, G.; Gil, S.; Martínez-Máñez, R.; Ruiz-Cerdá, J.L. Urinary Metabolic Signatures Detect Recurrences in Non-Muscle Invasive Bladder Cancer. Cancers 2019, 11, 914. [Google Scholar] [CrossRef]
- Lin, H.T.; Cheng, M.L.; Lo, C.J.; Lin, G.; Lin, S.F.; Yeh, J.T.; Ho, H.Y.; Lin, J.R.; Liu, F.C. 1H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef]
- Dalili, N.; Chashmniam, S.; Khoormizi, S.M.H.; Salehi, L.; Jamalian, S.A.; Nafar, M.; Kalantari, S. Urine and serum NMR-based metabolomics in pre-procedural prediction of contrast-induced nephropathy. Intern. Emerg. Med. 2020, 15, 95–103. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Z.; Zhang, L.; Wei, Z.; Cheng, K.K.; Liu, Y.; Shen, G.; Fan, H.; Dong, J. Deciphering the metabolic perturbation in hepatic alveolar echinococcosis: A 1H NMR-based metabolomics study. Parasit. Vectors 2019, 12, 300. [Google Scholar] [CrossRef]
- Mongan, A.M.; Lynam-Lennon, N.; Doyle, S.L.; Casey, R.; Carr, E.; Cannon, A.; Conroy, M.J.; Pidgeon, G.P.; Brennan, L.; Lysaght, J.; et al. Visceral Adipose Tissue Modulates Radiosensitivity in Oesophageal Adenocarcinoma. Int. J. Med. Sci. 2019, 16, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wijeyesekera, A.; Wagner, J.; De Goffau, M.; Thurston, S.; Rodrigues Sabino, A.; Zaher, S.; White, D.; Ridout, J.; Peters, M.J.; Ramnarayan, P.; et al. Multi-Compartment Profiling of Bacterial and Host Metabolites Identifies Intestinal Dysbiosis and Its Functional Consequences in the Critically Ill Child. Crit. Care Med. 2019, 47, e727–e734. [Google Scholar] [CrossRef]
- Ghosh, N.; Choudhury, P.; Subramani, E.; Saha, D.; Sengupta, S.; Joshi, M.; Banerjee, R.; Roychowdhury, S.; Bhattacharyya, P.; Chaudhury, K. Metabolomic signatures of asthma-COPD overlap (ACO) are different from asthma and COPD. Metabolomics 2019, 15, 87. [Google Scholar] [CrossRef]
- Fest, J.; Vijfhuizen, L.S.; Goeman, J.J.; Veth, O.; Joensuu, A.; Perola, M.; Männistö, S.; Ness-Jensen, E.; Hveem, K.; Haller, T.; et al. Search for Early Pancreatic Cancer Blood Biomarkers in Five European Prospective Population Biobanks Using Metabolomics. Endocrinology 2019, 160, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.F.; Holle, S.L.K.; Andersen, M.H.; Pedersen, A.; Bundgaard, H.; Iversen, K.K.; Malmendal, A. In-hospital metabolite changes in infective endocarditis-a longitudinal 1H NMR-based study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1553–1560. [Google Scholar] [CrossRef]
- Del Coco, L.; Vergara, D.; De Matteis, S.; Mensà, E.; Sabbatinelli, J.; Prattichizzo, F.; Bonfigli, A.R.; Storci, G.; Bravaccini, S.; Pirini, F.; et al. NMR-Based Metabolomic Approach Tracks Potential Serum Biomarkers of Disease Progression in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 720. [Google Scholar] [CrossRef]
- Loras, A.; Suárez-Cabrera, C.; Martínez-Bisbal, M.C.; Quintás, G.; Paramio, J.M.; Martínez-Máñez, R.; Gil, S.; Ruiz-Cerdá, J.L. Integrative Metabolomic and Transcriptomic Analysis for the Study of Bladder Cancer. Cancers 2019, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Triba, M.N.; Amathieu, R.; Lin, X.; Bouchemal, N.; Hantz, E.; Le Moyec, L.; Savarin, P. Nuclear magnetic resonance-based serum metabolomic analysis reveals different disease evolution profiles between septic shock survivors and non-survivors. Crit. Care 2019, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Chashmniam, S.; Kalantari, S.; Nafar, M.; Boroumandnia, N. The metabolomics signature associated with responsiveness to steroid therapy in focal segmental glomerulosclerosis: A pilot study. Rev. Investig. Clin. 2019, 71, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Stryeck, S.; Gastrager, M.; Degoricija, V.; Trbušić, M.; Potočnjak, I.; Radulović, B.; Pregartner, G.; Berghold, A.; Madl, T.; Frank, S. Serum Concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate are Associated with Increased 3-Month Mortality in Acute Heart Failure Patients. Sci. Rep. 2019, 9, 6743. [Google Scholar] [CrossRef] [PubMed]
- Bund, C.; Guergova-Kuras, M.; Cicek, A.E.; Moussallieh, F.M.; Dali-Youcef, N.; Piotto, M.; Schneider, P.; Heller, R.; Entz-Werle, N.; Lhermitte, B.; et al. An integrated genomic and metabolomic approach for defining survival time in adult oligodendrogliomas patients. Metabolomics 2019, 15, 69. [Google Scholar] [CrossRef]
- Rosado-Sánchez, I.; Rodríguez-Gallego, E.; Peraire, J.; Viladés, C.; Herrero, P.; Fanjul, F.; Gutiérrez, F.; Bernal, E.; Pelazas, R.; Leal, M.; et al. Glutaminolysis and lipoproteins are key factors in late immune recovery in successfully treated HIV-infected patients. Clin. Sci. 2019, 133, 997–1010. [Google Scholar] [CrossRef]
- Alborghetti, M.R.; Correa, M.E.P.; Whangbo, J.; Shi, X.; Aricetti, J.A.; da Silva, A.A.; Miranda, E.C.M.; Sforca, M.L.; Caldana, C.; Gerszten, R.E.; et al. Clinical Metabolomics Identifies Blood Serum Branched Chain Amino Acids as Potential Predictive Biomarkers for Chronic Graft vs. Host Disease. Front. Oncol. 2019, 9, 141. [Google Scholar] [CrossRef]
- Onderwater, G.L.J.; Ligthart, L.; Bot, M.; Demirkan, A.; Fu, J.; van der Kallen, C.J.H.; Vijfhuizen, L.S.; Pool, R.; Liu, J.; Vanmolkot, F.H.M.; et al. Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology 2019, 92, e1899–e1911. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, L.S.; Sun, J.L.; Guan, K.; Wei, J.F. 1H NMR-based metabolomic study of metabolic profiling for pollinosis. World Allergy Organ. J. 2019, 12, 100005. [Google Scholar] [CrossRef]
- Faitot, F.; Ruhland, E.; Oncioiu, C.; Besch, C.; Addeo, P.; Cicek, A.E.; Bachellier, P.; Namer, I.J. Metabolomic profiling highlights the metabolic bases of acute-on-chronic and post-hepatectomy liver failure. HPB (Oxford) 2019, 21, 1354–1361. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Ma, L.; Wen, X.; Ji, H.; Li, K. Exploring potential biomarkers of early stage esophageal squamous cell carcinoma in pre- and post-operative serum metabolomic fingerprint spectrum using 1H-NMR method. Am. J. Transl. Res. 2019, 11, 819–831. [Google Scholar] [PubMed]
- Kim, E.R.; Kwon, H.N.; Nam, H.; Kim, J.J.; Park, S.; Kim, Y.H. Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Sci. Rep. 2019, 9, 4786. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhu, H.; Zhang, C.; Shen, G.; Feng, J. Metabolomic analysis reveals metabolic characteristics of children with short stature caused by growth hormone deficiency. Clin. Sci. 2019, 133, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Meoni, G.; Lorini, S.; Monti, M.; Madia, F.; Corti, G.; Luchinat, C.; Zignego, A.L.; Tenori, L.; Gragnani, L. The metabolic fingerprints of HCV and HBV infections studied by Nuclear Magnetic Resonance Spectroscopy. Sci. Rep. 2019, 9, 4128. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Yanshole, L.V.; Snytnikova, O.A.; Tsentalovich, Y.P. Quantitative metabolomic analysis of changes in the lens and aqueous humor under development of age-related nuclear cataract. Metabolomics 2019, 15, 29. [Google Scholar] [CrossRef]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Cuviello, F.; Ostuni, A. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: A pilot study. Metabolomics 2019, 15, 19. [Google Scholar] [CrossRef]
- Amin, A.M.; Mostafa, H.; Arif, N.H.; Abdul Kader, M.A.S.; Kah Hay, Y. Metabolomics profiling and pathway analysis of human plasma and urine reveal further insights into the multifactorial nature of coronary artery disease. Clin. Chim. Acta 2019, 493, 112–122. [Google Scholar] [CrossRef]
- Lorefice, L.; Murgia, F.; Fenu, G.; Frau, J.; Coghe, G.; Murru, M.R.; Tranquilli, S.; Visconti, A.; Marrosu, M.G.; Atzori, L.; et al. Assessing the Metabolomic Profile of Multiple Sclerosis Patients Treated with Interferon Beta 1a by 1H-NMR Spectroscopy. Neurotherapeutics 2019, 16, 797–807. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Altenbuchinger, M.; Schultheiss, U.T.; Samol, C.; Kotsis, F.; Poguntke, I.; Sekula, P.; Krumsiek, J.; Köttgen, A.; Spang, R.; et al. A Novel Metabolic Signature To Predict the Requirement of Dialysis or Renal Transplantation in Patients with Chronic Kidney Disease. J. Proteome Res. 2019, 18, 1796–1805. [Google Scholar] [CrossRef]
- Ahmed, S.; Dubey, D.; Chowdhury, A.; Chaurasia, S.; Guleria, A.; Kumar, S.; Singh, R.; Kumar, D.; Misra, R. Nuclear magnetic resonance-based metabolomics reveals similar metabolomics profiles in undifferentiated peripheral spondyloarthritis and reactive arthritis. Int. J. Rheum. Dis. 2019, 22, 725–733. [Google Scholar] [CrossRef]
- Khalid, A.; Siddiqui, A.J.; Ansari, S.H.; Musharraf, S.G. Reflection of treatment proficiency of hydroxyurea treated β-thalassemia serum samples through nuclear magnetic resonance based metabonomics. Sci. Rep. 2019, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Xie, J.; Zeng, L.; Zhou, C.J.; Zheng, P.; Xie, P. Urinary metabolite signature in bipolar disorder patients during depressive episode. Aging (Albany NY) 2019, 11, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ma, C.; Bezabeh, T.; Wang, Z.; Liang, J.; Huang, Y.; Zhao, J.; Liu, X.; Ye, W.; Tang, W.; et al. 1H NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int. J. Cancer 2019, 145, 1679–1689. [Google Scholar] [CrossRef]
- Laíns, I.; Duarte, D.; Barros, A.S.; Martins, A.S.; Carneiro, T.J.; Gil, J.Q.; Miller, J.B.; Marques, M.; Mesquita, T.S.; Barreto, P.; et al. Urine Nuclear Magnetic Resonance (NMR) Metabolomics in Age-Related Macular Degeneration. J. Proteome Res. 2019, 18, 1278–1288. [Google Scholar] [CrossRef]
- Rawat, A.; Misra, G.; Saxena, M.; Tripathi, S.; Dubey, D.; Saxena, S.; Aggarwal, A.; Gupta, V.; Khan, M.Y.; Prakash, A. 1H NMR based serum metabolic profiling reveals differentiating biomarkers in patients with diabetes and diabetes-related complication. Diabetes Metab. Syndr. 2019, 13, 290–298. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Giusti, B.; Takis, P.G.; Valente, S.; Carrabba, N.; Balzi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; et al. NMR-based metabolomics identifies patients at high risk of death within two years after acute myocardial infarction in the AMI-Florence II cohort. BMC Med. 2019, 17, 3. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Lang, Y.; Qu, Y.; Chen, J.; Cui, L. 1H nuclear magnetic resonance-based metabolic profiling of cerebrospinal fluid to identify metabolic features and markers for tuberculosis meningitis. Infect. Genet. Evol. 2019, 68, 253–264. [Google Scholar] [CrossRef]
- Vignoli, A.; Orlandini, B.; Tenori, L.; Biagini, M.R.; Milani, S.; Renzi, D.; Luchinat, C.; Calabrò, A.S. Metabolic Signature of Primary Biliary Cholangitis and Its Comparison with Celiac Disease. J. Proteome Res. 2019, 18, 1228–1236. [Google Scholar] [CrossRef]
- Harbaum, L.; Ghataorhe, P.; Wharton, J.; Jiménez, B.; Howard, L.S.G.; Gibbs, J.S.R.; Nicholson, J.K.; Rhodes, C.J.; Wilkins, M.R. Reduced plasma levels of small HDL particles transporting fibrinolytic proteins in pulmonary arterial hypertension. Thorax 2019, 74, 380–389. [Google Scholar] [CrossRef]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Mariani, G.M.; Cacciatore, S.; Tenori, L.; Aimetti, M. Effect of non-surgical periodontal therapy on salivary metabolic fingerprint of generalized chronic periodontitis using nuclear magnetic resonance spectroscopy. Arch. Oral. Biol. 2019, 97, 208–214. [Google Scholar] [CrossRef]
- Parra, S.; Lopez-Dupla, M.; Ibarretxe, D.; de Las Heras, M.; Amigó, N.; Català, A.; Benavent, M.; Garcés, E.; Navarro, A.; Castro, A. Patients With Systemic Lupus Erythematosus Show an Increased Arterial Stiffness That is Predicted by IgM Anti-β(2) -Glycoprotein I and Small Dense High-Density Lipoprotein Particles. Arthritis Care Res. (Hoboken) 2019, 71, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Sonek, J.; McKenna, D.; Cool, D.; Aydas, B.; Turkoglu, O.; Bjorndahl, T.; Mandal, R.; Wishart, D.; Friedman, P.; et al. Artificial intelligence and amniotic fluid multiomics: Prediction of perinatal outcome in asymptomatic women with short cervix. Ultrasound Obstet. Gynecol. 2019, 54, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pocate-Cheriet, K.; Santulli, P.; Kateb, F.; Bourdon, M.; Maignien, C.; Batteux, F.; Chouzenoux, S.; Patrat, C.; Wolf, J.P.; Bertho, G.; et al. The follicular fluid metabolome differs according to the endometriosis phenotype. Reprod. Biomed. Online 2020, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Jacyna, J.; Wawrzyniak, R.; Balayssac, S.; Gilard, V.; Malet-Martino, M.; Sawicka, A.; Kordalewska, M.; Nowicki, Ł.; Kurek, E.; Bulska, E.; et al. Urinary metabolomic signature of muscle-invasive bladder cancer: A multiplatform approach. Talanta 2019, 202, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ijare, O.B.; Baskin, D.S.; Pichumani, K. Ex Vivo 1H NMR study of pituitary adenomas to differentiate various immunohistochemical subtypes. Sci. Rep. 2019, 9, 3007. [Google Scholar] [CrossRef]
- Chen, J.; Ye, C.; Hu, X.; Huang, C.; Yang, Z.; Li, P.; Wu, A.; Xue, X.; Lin, D.; Yang, H. Serum metabolomics model and its metabolic characteristics in patients with different syndromes of dyslipidemia based on nuclear magnetic resonance. J. Pharm. Biomed. Anal. 2019, 167, 100–113. [Google Scholar] [CrossRef]
- Clendinen, C.S.; Gaul, D.A.; Monge, M.E.; Arnold, R.S.; Edison, A.S.; Petros, J.A.; Fernández, F.M. Preoperative Metabolic Signatures of Prostate Cancer Recurrence Following Radical Prostatectomy. J. Proteome Res. 2019, 18, 1316–1327. [Google Scholar] [CrossRef]
- Padayachee, T.; Khamiakova, T.; Louis, E.; Adriaensens, P.; Burzykowski, T. The impact of the method of extracting metabolic signal from 1H-NMR data on the classification of samples: A case study of binning and BATMAN in lung cancer. PLoS ONE 2019, 14, e0211854. [Google Scholar] [CrossRef]
- Noorbakhsh, H.; Yavarmanesh, M.; Mortazavi, S.A.; Adibi, P.; Moazzami, A.A. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. Eur. J. Nutr. 2019, 58, 3109–3119. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Whole Blood Metabolomics by 1H NMR Spectroscopy Provides a New Opportunity To Evaluate Coenzymes and Antioxidants. Anal. Chem. 2017, 89, 4620–4627. [Google Scholar] [CrossRef]
- Gomez-Archila, L.G.; Palomino-Schatzlein, M.; Zapata-Builes, W.; Galeano, E. Development of an optimized method for processing peripheral blood mononuclear cells for 1H-nuclear magnetic resonance-based metabolomic profiling. PLoS ONE 2021, 16, e0247668. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, V.V.; Barbas, C.; Dudzik, D. A review of blood sample handling and pre-processing for metabolomics studies. Electrophoresis 2017, 38, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Del Greco, F.M.; Sigurdsson, B.B.; Rainer, J.; Volani, C.; Hicks, A.A.; Pramstaller, P.P.; Smarason, S.V. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin. Chim. Acta 2018, 486, 320–328. [Google Scholar] [CrossRef]
- Bi, H.; Guo, Z.; Jia, X.; Liu, H.; Ma, L.; Xue, L. The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics 2020, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.A.; et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.; Hansen, S.H.; Jaroszewski, J.W.; Cornett, C. Human urine as test material in 1H NMR-based metabonomics: Recommendations for sample preparation and storage. Anal. Chem. 2007, 79, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Wurtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Jarvelin, M.R.; Kahonen, M.; Lehtimaki, T.; Viikari, J.; et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E.; Everett, J.R. Metabonomics: Metabolic processes studied by NMR spectroscopy of biofluids. Concepts Magn. Reson. 2000, 12, 289–320. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Wurtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef]
- Ludwig, C.; Viant, M.R. Two-dimensional J-resolved NMR spectroscopy: Review of a key methodology in the metabolomics toolbox. Phytochem. Anal. 2010, 21, 22–32. [Google Scholar] [CrossRef]
- de Graaf, R.A.; Behar, K.L. Quantitative 1H NMR spectroscopy of blood plasma metabolites. Anal. Chem. 2003, 75, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Mckay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts Magn. Reson. Part A 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Vehtari, A.; Makinen, V.P.; Soininen, P.; Ingman, P.; Makela, S.M.; Savolainen, M.J.; Hannuksela, M.L.; Kaski, K.; Ala-Korpela, M. A novel Bayesian approach to quantify clinical variables and to determine their spectroscopic counterparts in 1H NMR metabonomic data. BMC Bioinform. 2007, 8 (Suppl. S2), S8. [Google Scholar] [CrossRef] [PubMed]
- Crook, A.A.; Powers, R. Quantitative NMR-Based Biomedical Metabolomics: Current Status and Applications. Molecules 2020, 25, 5128. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.; Fetler, B.; Marchant, J.; Johnson, B.A. NMRFx Processor: A cross-platform NMR data processing program. J. Biomol. 2016, 65, 205–216. [Google Scholar] [CrossRef]
- Sousa, S.; Magalhaes, A.; Ferreira, M. Optimized bucketing for NMR spectra: Three case studies. Chemom. Intell. Lab. Syst. 2013, 122, 93–102. [Google Scholar] [CrossRef]
- Anderson, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; DelRaso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2011, 7, 179–190. [Google Scholar] [CrossRef]
- Davis, R.A.; Charlton, A.J.; Godward, J.; Jones, S.A.; Harrison, M.; Wilson, J.C. Adaptive binning: An improved binning method for metabolomics data using the undecimated wavelet transform. Chemom. Intell. Lab. Syst. 2007, 85, 144–154. [Google Scholar] [CrossRef]
- De Meyer, T.; Sinnaeve, D.; Van Gasse, B.; Tsiporkova, E.; Rietzschel, E.R.; De Buyzere, M.L.; Gillebert, T.C.; Bekaert, S.; Martins, J.C.; Van Criekinge, W. NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal. Chem. 2008, 80, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- Mercier, P.; Lewis, M.J.; Chang, D.; Baker, D.; Wishart, D.S. Towards automatic metabolomic profiling of high-resolution one-dimensional proton NMR spectra. J. Biomol. NMR 2011, 49, 307–323. [Google Scholar] [CrossRef]
- Tulpan, D.; Leger, S.; Belliveau, L.; Culf, A.; Cuperlovic-Culf, M. MetaboHunter: An automatic approach for identification of metabolites from 1(H)-NMR spectra of complex mixtures. BMC Bioinform. 2011, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Bjorndahl, T.C.; Tang, P.; Wishart, D.S. MetaboMiner--semi-automated identification of metabolites from 2D NMR spectra of complex biofluids. BMC Bioinform. 2008, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, S.; Liu, P.; Bjorndahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef]
- Hao, J.; Liebeke, M.; Astle, W.; De Iorio, M.; Bundy, J.G.; Ebbels, T.M. Bayesian deconvolution and quantification of metabolites in complex 1D NMR spectra using BATMAN. Nat. Protoc. 2014, 9, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Emwas, A.H.; Saccenti, E.; Gao, X.; McKay, R.T.; Dos Santos, V.; Roy, R.; Wishart, D.S. Recommended strategies for spectral processing and post-processing of 1D 1H-NMR data of biofluids with a particular focus on urine. Metabolomics 2018, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis--a marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; van Velzen, E.J.; Hoefsloot, H.C.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Altenbuchinger, M.; Gronwald, W. Statistical Analysis of NMR Metabolic Fingerprints: Established Methods and Recent Advances. Metabolites 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 2: Overview of Hypothesis Testing. Indian J. Dermatol. 2016, 61, 137–145. [Google Scholar] [CrossRef]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 3: Comparing Groups: Numerical Variables. Indian J. Dermatol. 2016, 61, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.S. How does multiple testing correction work? Nat. Biotechnol. 2009, 27, 1135–1137. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmuller, G.; He, Y.; Belcredi, P.; Moller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef] [PubMed]
- Denery, J.R.; Nunes, A.A.K.; Dickerson, T.J. Characterization of Differences between Blood Sample Matrices in Untargeted Metabolomics. Anal. Chem. 2011, 83, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Orozco, J.; Chen, S.Y.; Hertz-Picciotto, I.; Slupsky, C.M. A Comparison of Serum and Plasma Blood Collection Tubes for the Integration of Epidemiological and Metabolomics Data. Front. Mol. Biosci. 2021, 8, 682134. [Google Scholar] [CrossRef] [PubMed]
- Lesche, D.; Geyer, R.; Lienhard, D.; Nakas, C.T.; Diserens, G.; Vermathen, P.; Leichtle, A.B. Does centrifugation matter? Centrifugal force and spinning time alter the plasma metabolome. Metabolomics 2016, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Jobard, E.; Tredan, O.; Postoly, D.; Andre, F.; Martin, A.L.; Elena-Herrmann, B.; Boyault, S. A Systematic Evaluation of Blood Serum and Plasma Pre-Analytics for Metabolomics Cohort Studies. Int. J. Mol. Sci. 2016, 17, 2035. [Google Scholar] [CrossRef]
- Ammerlaan, W.; Trezzi, J.P.; Mathay, C.; Hiller, K.; Betsou, F. Method validation for preparing urine samples for downstream proteomic and metabolomic applications. Biopreserv. Biobank. 2014, 12, 351–357. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Khlichkina, A.A.; Sagdeev, R.Z.; Tsentalovich, Y.P. Evaluation of sample preparation protocols for quantitative NMR-based metabolomics. Metabolomics 2019, 15, 84. [Google Scholar] [CrossRef]
- McHugh, C.E.; Flott, T.L.; Schooff, C.R.; Smiley, Z.; Puskarich, M.A.; Myers, D.D.; Younger, J.G.; Jones, A.E.; Stringer, K.A. Rapid, Reproducible, Quantifiable NMR Metabolomics: Methanol and Methanol: Chloroform Precipitation for Removal of Macromolecules in Serum and Whole Blood. Metabolites 2018, 8, 93. [Google Scholar] [CrossRef]
- Sheedy, J.R.; Ebeling, P.R.; Gooley, P.R.; McConville, M.J. A sample preparation protocol for 1H nuclear magnetic resonance studies of water-soluble metabolites in blood and urine. Anal. Biochem. 2010, 398, 263–265. [Google Scholar] [CrossRef]
- Lane, A. Principles of NMR for Applications in Metabolomics; Humana Press: Totowa, NJ, USA, 2012; Volume 17, pp. 127–197. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A Guideline to Univariate Statistical Analysis for LC/MS-Based Untargeted Metabolomics-Derived Data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Statistical Modeling: The Two Cultures (with comments and a rejoinder by the author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried Blood Spot in Laboratory: Directions and Prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.U.W.; Prow, T.W. A review of microsampling techniques and their social impact. Biomed. Microdevices 2019, 21, 81. [Google Scholar] [CrossRef]
- Wishart, D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Wishart, D.S.; Cheng, L.L.; Copie, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B.; et al. NMR and Metabolomics-A Roadmap for the Future. Metabolites 2022, 12, 678. [Google Scholar] [CrossRef]
- Dey, A.; Charrier, B.; Martineau, E.; Deborde, C.; Gandriau, E.; Moing, A.; Jacob, D.; Eshchenko, D.; Schnell, M.; Melzi, R.; et al. Hyperpolarized NMR Metabolomics at Natural 13C Abundance. Anal. Chem. 2020, 92, 14867–14871. [Google Scholar] [CrossRef]
- Hackl, M.; Tauber, P.; Schweda, F.; Zacharias, H.U.; Altenbuchinger, M.; Oefner, P.J.; Gronwald, W. An R-Package for the Deconvolution and Integration of 1D NMR Data: MetaboDecon1D. Metabolites 2021, 11, 452. [Google Scholar] [CrossRef]
- Migdadi, L.; Lambert, J.; Telfah, A.; Hergenroder, R.; Wohler, C. Automated metabolic assignment: Semi-supervised learning in metabolic analysis employing two dimensional Nuclear Magnetic Resonance (NMR). Comput. Struct. Biotechnol J. 2021, 19, 5047–5058. [Google Scholar] [CrossRef]
- Markley, J.L.; Dashti, H.; Wedell, J.R.; Westler, W.M.; Eghbalnia, H.R. Tools for Enhanced NMR-Based Metabolomics Analysis. Methods Mol. Biol. 2019, 2037, 413–427. [Google Scholar] [CrossRef] [PubMed]
| Reference | Collection Tube | Sample Prep | Sample: Buffer Ratio | Buffer | % D2O | Chemical Shift Reference (mM) | NaN3 (mM) | pH | NMR Experiments | Temp (K) |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | ||||||||||
| Beckonert [184] | Li-heparin | 1:2 | 103 mM NaCl | 6.66 | noesy, cpmg, (jres, diff) | 310 | ||||
| Bernini [185] | EDTA or citrate | 1:1 | 35 mM Na2HPO4 | 10 | TSP (27.5) | 19 | 7.4 | noesy, cpmg | 310 | |
| Dona [3] | Li-heparin or EDTA | 1:1 | 37.5 mM NaH2PO4 | 10 | TSP (2.73) | 3.08 | 7.4 | noesy, cpmg, jres | 310 | |
| Soininen [190] | 1:1 | 37.5 mM Na2HPO4 | 10 | TSP-d4 (2.32) | 3.08 | 7.4 | noesy, cpmg | 310 | ||
| Chenomx | Ultra- filtration 3 kDa | 9:1 | D2O | 10 | DSS-d6 (0.5) | 1.54 | noesy | 298 | ||
| Urine | ||||||||||
| Beckonert [184] | 0.05% wt/vol NaN3 | 2:1 | 82.3 mM Na2HPO4 | 6.66 | TSP (0.33) | 1 | 7.4 | noesy | 300 | |
| Bernini [185] | 3 mM NaN3 | 9:1 | 150 mM K2HPO4 | 10 | TSP (1.0) | 7.4 | noesy, jres | 300 | ||
| Dona [3] | 0.05% wt/vol NaN3 | 9:1 | 150 mM KH2PO4 | 1 | TSP (0.68) | 0.2 | 7.4 | noesy, jres | 300 | |
| Chenomx | 9:1 | D2O | 10 | DSS-d6 (0.5) | 1.54 | noesy | 298 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Thomas, N.; Gooley, P.R.; Armstrong, C.W. Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research. Metabolites 2022, 12, 963. https://doi.org/10.3390/metabo12100963
Huang K, Thomas N, Gooley PR, Armstrong CW. Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research. Metabolites. 2022; 12(10):963. https://doi.org/10.3390/metabo12100963
Chicago/Turabian StyleHuang, Katherine, Natalie Thomas, Paul R. Gooley, and Christopher W. Armstrong. 2022. "Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research" Metabolites 12, no. 10: 963. https://doi.org/10.3390/metabo12100963
APA StyleHuang, K., Thomas, N., Gooley, P. R., & Armstrong, C. W. (2022). Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research. Metabolites, 12(10), 963. https://doi.org/10.3390/metabo12100963