Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil
Abstract
1. Introduction
- evaluation of chemical composition of EOs,
- description of some physical parameters,
- evaluation of microbiological activity,
- evaluation of inhibitory activity on cancer cell lines.
2. Materials and Methods
2.1. Location and Climate
2.2. Biological Material and Cultivation
2.3. Essential Oil Extraction Method
2.4. GC-MS Qualitative Volatile Profile of Essential Oils
2.5. Physical Parameters of EOs
2.5.1. FT-IR Assay
2.5.2. Refractive Index
2.6. Inhibitory Activity of EO against Bacterial Strains
2.6.1. Determination of the Minimum Inhibitory Concentration (MIC)
2.6.2. Determination of the Minimum Bactericidal Concentration (MBC)
2.7. Cytotoxicity Screening of EO in Cancer Cell Lines
2.8. Statistical Analysis for Microbiological Activity
3. Results
3.1. GC-MS Results
3.2. Physical Characteristics of EOs
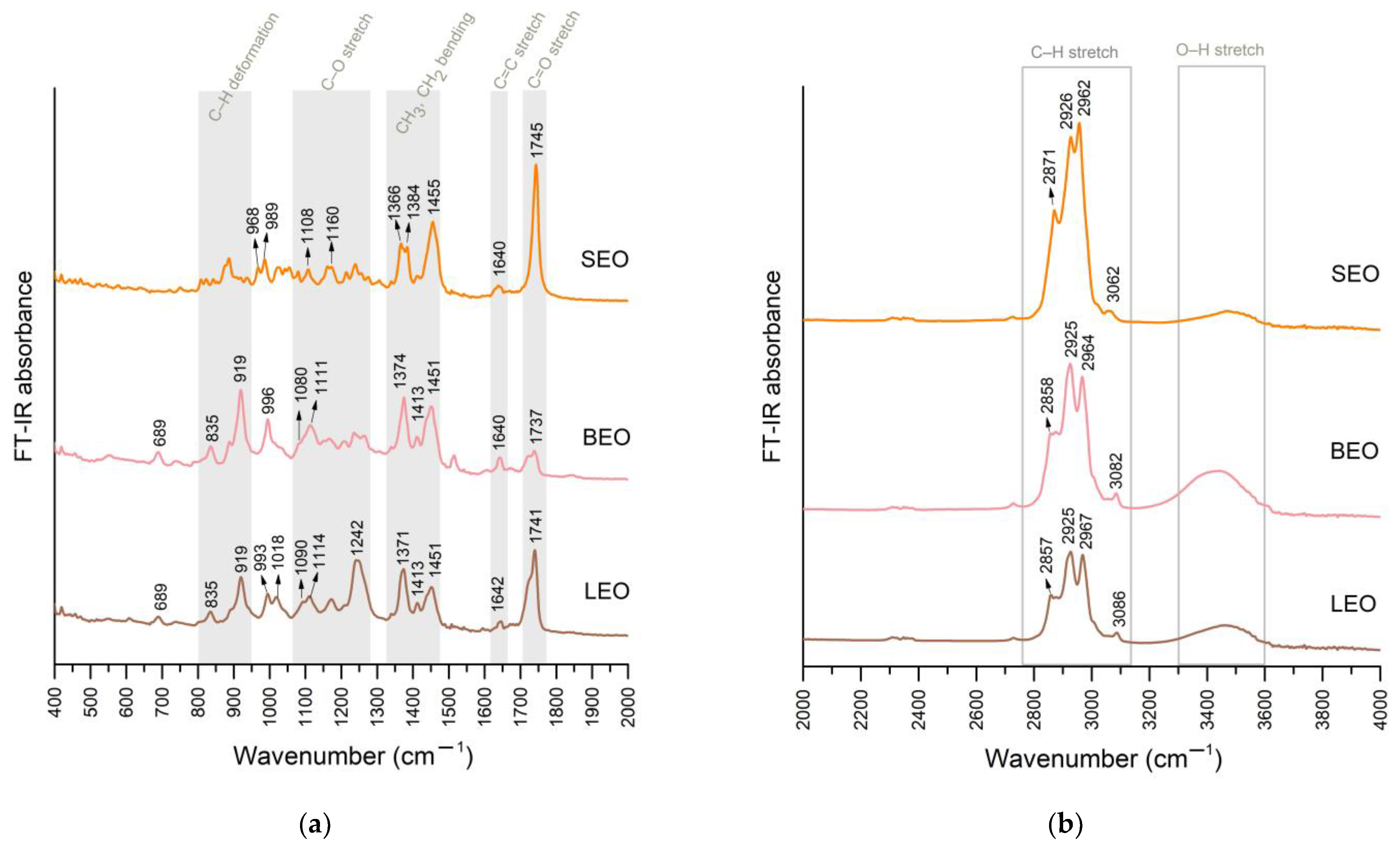
3.2.1. FT-IR Results
3.2.2. Refractive Index
3.3. Inhibitory Activity of EO against Bacterial Strains
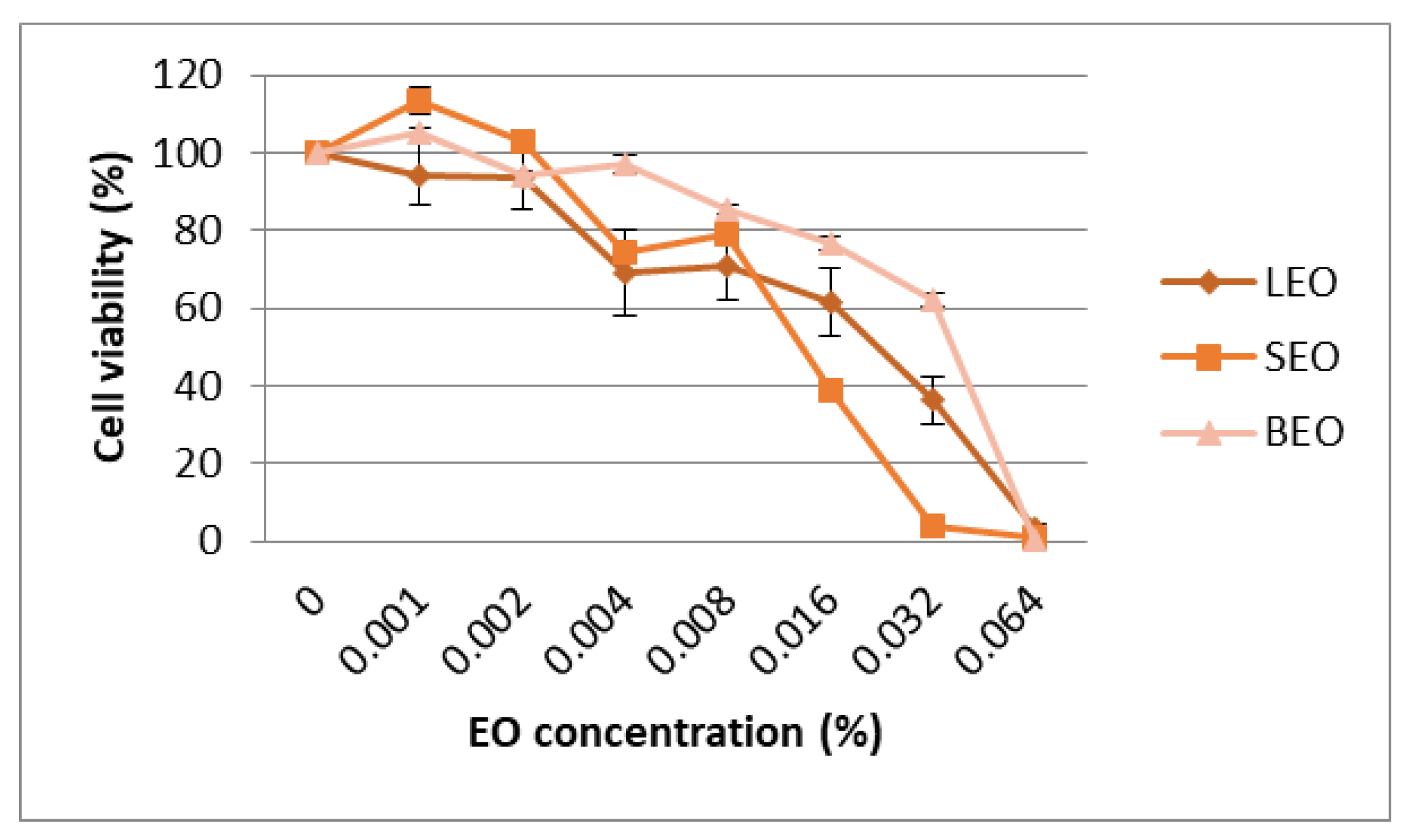
3.4. Cytotoxicity of EO in Cancer Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation; IntechOpen: Houston, TX, USA, 2018; ISBN 978-1-78923-780-1. [Google Scholar]
- Răileanu, M.; Todan, L.; Voicescu, M.; Ciuculescu, C.; Maganu, M. A Way for Improving the Stability of the Essential Oils in an Environmental Friendly Formulation. Mater. Sci. Eng. C 2013, 33, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric Comparison and Classification of Some Essential Oils Extracted from Plants Belonging to Apiaceae and Lamiaceae Families Based on Their Chemical Composition and Biological Activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, e9268468. [Google Scholar] [CrossRef]
- Sendra, E. Essential Oils in Foods: From Ancient Times to the 21st Century. Foods 2016, 5, 43. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ding, P. Production of Essential Oil in Plants: Ontogeny, Secretory Structures and Seasonal Variations. Pertanika J. Sch. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential Accumulation of Volatile Terpene and Terpene Synthase MRNAs during Lavender (Lavandula Angustifolia and L. x Intermedia) Inflorescence Development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef]
- Gudi, G.; Krähmer, A.; Krüger, H.; Schulz, H. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy on Intact Dried Leaves of Sage (Salvia Officinalis L.): Accelerated Chemotaxonomic Discrimination and Analysis of Essential Oil Composition. J. Agric. Food Chem. 2015, 63, 8743–8750. [Google Scholar] [CrossRef]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula Angustifolia Mill., Citrus Limon (L.) Osbeck and Melaleuca Alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of Essential Oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; Moreno-Pérez, G.F.; Martínez-Gordillo, M.; Aguirre-Hernández, E.; Valle-Dorado, M.G.; Díaz-Reval, M.I.; González-Trujano, M.E.; Pellicer, F. Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief. Molecules 2021, 26, 7632. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. Plant Systematics; Elsevier Academic Press: Amsterdam, The Netherlands, 2006; ISBN 0-12-644460-9. [Google Scholar]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Vârban, R.; Vidican, R.; Ona, A.D.; Vârban, D.; Stoie, A.; Gâdea, Ș.; Vâtcă, S.; Stoian, V.; Crișan, I.; Stoian, V. Modelling Plant Morphometric Parameters as Predictors for Successful Cultivation of Some Medicinal Agastache Species. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12638. [Google Scholar] [CrossRef]
- Muntean, L.S.; Tămaș, M.; Muntean, S.; Muntean, L.; Duda, M.M.; Vârban, D.I.; Florian, S. Tratat de Plante Medicinale Cultivate Şi Spontane; Risoprint: Cluj-Napoca, Romania, 2016; ISBN 978-973-53-1873-4. [Google Scholar]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory Structures and Essential Oil Composition of Selected Industrial Species of Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Serrato-Valenti, G.; Bisio, A.; Cornara, L.; Ciarallo, G. Structural and Histochemical Investigation of the Glandular Trichomes of Salvia Aurea L. Leaves, and Chemical Analysis of the Essential Oil. Ann. Bot. 1997, 79, 329–336. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Tundis, R.; Upson, T.M. A New Species of Lavandula Sect. Lavandula (Lamiaceae) and Review of Species Boundaries in Lavandula Angustifolia. Phytotaxa 2017, 292, 161–170. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia Officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- DeBaggio, T.; Tucker, A.O. The Encyclopedia of Herbs: A Comprehensive Reference to Herbs of Flavor and Fragrance; Timber Press: Portland, OR, USA, 2009; ISBN 978-1-60469-134-4. [Google Scholar]
- Onofrei, V.; Teliban, G.-C.; Clinciu-Radu, R.-A.; Teliban, I.-V.; Galea, F.-M.; Robu, T. Ocimum Basilicum L.: Presence, influence and evolution. Sci. Papers Agron. Ser. 2015, 58, 161–166. [Google Scholar]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and Developmental Factors Affect Essential Oil Production and Quality of Lavandula Angustifolia during Flowering Period. Ind. Crop. Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Corsi, G. Glandular Hairs of Salvia Officinalis: New Data on Morphology, Localization and Histochemistry in Relation to Function. Ann. Bot. 1999, 84, 657. [Google Scholar] [CrossRef]
- Tirillini, B.; Ricci, A.; Pellegrino, R. Secretion Constituents of Leaf Glandular Trichome of Salvia Officinalis L. J. Essent. Oil Res. 1999, 11, 565–569. [Google Scholar] [CrossRef]
- Gang, D.R.; Simon, J.; Lewinsohn, E.; Pichersky, E. Peltate Glandular Trichomes of Ocimum Basilicum L. (Sweet Basil) Contain High Levels of Enzymes Involved in the Biosynthesis of Phenylpropenes. J. Herbs Spices Med. Plants 2002, 9, 189–195. [Google Scholar] [CrossRef]
- Iriti, M.; Colnaghi, G.; Chemat, F.; Smadja, J.; Faoro, F.; Visinoni, F.A. Histo-Cytochemistry and Scanning Electron Microscopy of Lavender Glandular Trichomes Following Conventional and Microwave-Assisted Hydrodistillation of Essential Oils: A Comparative Study. Flavour Fragr. J. 2006, 21, 704–712. [Google Scholar] [CrossRef]
- Maurya, S.; Chandra, M.; Yadav, R.K.; Narnoliya, L.K.; Sangwan, R.S.; Bansal, S.; Sandhu, P.; Singh, U.; Kumar, D.; Sangwan, N.S. Interspecies Comparative Features of Trichomes in Ocimum Reveal Insights for Biosynthesis of Specialized Essential Oil Metabolites. Protoplasma 2019, 256, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Hassiotis, C.N.; Lazari, D.; Vlachonasios, K. The Effects of Habitat Type and Diurnal Harvest on Essential Oil Yield and Composition of Lavandula Angustifolia Mill. Fresenius Environ. Bull. 2010, 19, 1491–1498. [Google Scholar]
- Hazrati, S.; Beidaghi, P.; Beyraghdar Kashkooli, A.; Hosseini, S.J.; Nicola, S. Effect of Harvesting Time Variations on Essential Oil Yield and Composition of Sage (Salvia Officinalis). Horticulturae 2022, 8, 149. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Bogucka-Kocka, A.; Kowalski, R.; Borowski, B. Changes in the Chemical Composition of the Essential Oil of Sweet Basil (Ocimum Basilicum L.) Depending on the Plant Growth Stage. Chemija 2012, 23, 216–222. [Google Scholar]
- Hortus Agrobotanicus Napocensis—Index Seminum; Academic Press: Cluj-Napoca, Romania, 2021; ISSN 1223-6055.
- Vârban, R.; Ona, A.; Stoie, A.; Vârban, D.; Crișan, I. Phenological Assessment for Agronomic Suitability of Some Agastache Species Based on Standardized BBCH Scale. Agronomy 2021, 11, 2280. [Google Scholar] [CrossRef]
- ICPA, B. Coduri de Bune Practici. Available online: https://www.icpa.ro/coduri.shtml (accessed on 5 July 2022).
- Michelina, C.; Naviglio, D.; Gallo, M.; Severina, P. FT-IR and GC-MS Analyses of an Antioxidant Leaf Essential Oil from Sage Plants Cultivated as an Alternative to Tobacco Production. J. Essent. Oil Res. 2019, 31, 138–144. [Google Scholar] [CrossRef]
- McFARLAND, J. Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. J. Am. Med. Assoc. 1907, XLIX, 1176–1178. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Porto, C.D.; Decorti, D. Analysis of the Volatile Compounds of Flowers and Essential Oils from Lavandula Angustifolia Cultivated in Northeastern Italy by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry. Planta Med. 2008, 74, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Stešević, D.; Ristić, M.; Nikolić, V.; Nedović, M.; Caković, D.; Šatović, Z. Chemotype Diversity of Indigenous Dalmatian Sage (Salvia Officinalis L.) Populations in Montenegro. Chem. Biodivers. 2014, 11, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Karapandzova, M.; Dimitrovska, A.; Kulevanova, S. Headspace Screening: A Novel Approach for Fast Quality Assessment of the Essential Oil from Culinary Sage. Food Chem. 2016, 202, 133–140. [Google Scholar] [CrossRef]
- 14:00–17:00 ISO 9909:1997. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/77/17791.html (accessed on 30 August 2022).
- German Drug Codex (DAC)—Glossary—Kooperation Phytopharmaka. Available online: https://arzneipflanzenlexikon.info/en/german-drug-codex-dac.php (accessed on 30 August 2022).
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Amanpour, A.; Kelebek, H.; Selli, S. The Most Aroma-Active Compounds in Shade-Dried Aerial Parts of Basil Obtained from Iran and Turkey. Ind. Crop. Prod. 2018, 124, 692–698. [Google Scholar] [CrossRef]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R.; Motelica-Heino, M.S.; Cimpeanu, C. Properties of Basil and Lavender Essential Oils Adsorbed on the Surface of Hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef]
- Lafhal, S.; Vanloot, P.; Bombarda, I.; Kister, J.; Dupuy, N. Identification of Metabolomic Markers of Lavender and Lavandin Essential Oils Using Mid-Infrared Spectroscopy. Vib. Spectrosc. 2016, 85, 79–90. [Google Scholar] [CrossRef]
- Ciko, L.; Andoni, A.; Ylli, F.; Plaku, E.; Taraj, K.; Çomo, A. Extraction of Essential Oil from Albanian Salvia Officinalis L. and Its Characterization by FTIR Spectroscopy. Asian J. Chem. 2016, 28, 1401–1402. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Gaio, I.; Saggiorato, A.G.; Treichel, H.; Cichoski, A.J.; Astolfi, V.; Cardoso, R.I.; Toniazzo, G.; Valduga, E.; Paroul, N.; Cansian, R.L. Antibacterial Activity of Basil Essential Oil (Ocimum Basilicum L.) in Italian-Type Sausage. J. Für Verbraucherschutz Leb. 2015, 10, 323–329. [Google Scholar] [CrossRef]
- Bounaas, K.; Bouzidi, N.; Daghbouche, Y.; Garrigues, S.; de la Guardia, M.; El Hattab, M. Essential Oil Counterfeit Identification through Middle Infrared Spectroscopy. Microchem. J. 2018, 139, 347–356. [Google Scholar] [CrossRef]
- Máthé, Á.; Franz, C. Good Agricultural Practice and the Quality of Phytomedicines. J. Herbs Spices Med. Plants 1999, 6, 101–113. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006—Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH); European Chemicals Agency: Helsinki, Finland, 2014.
- About Us—ECHA. Available online: https://echa.europa.eu/about-us (accessed on 12 June 2022).
- Essential Oils—ECHA. Available online: https://echa.europa.eu/support/substance-identification/sector-specific-support-for-substance-identification/essential-oils (accessed on 12 June 2022).
- Lis-Balchin, M.; Hart, S.; Deans, S.G.; Eaglesham, E. Comparison of the Pharmacological and Antimicrobial Action of Commercial Plant Essential Oils. J. Herbs Spices Med. Plants 1996, 4, 69–86. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between Bioactivity and Chemical Composition of Commercial Essential Oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Samfira, I.; Rodino, S.; Petrache, P.; Cristina, R.; Butu, M.; BUTNARIU, M. Characterization and Identity Confirmation of Essential Oils by Mid Infrared Absorption Spectrophotometry. Dig. J. Nanomater. Biostructures 2015, 10, 557–566. [Google Scholar]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella Putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-Listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander Essential Oil and Linalool—Interactions with Antibiotics against Gram-Positive and Gram-Negative Bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The Antimicrobial Activity of Lavender Essential Oil (Lavandula Angustifolia) and Its Influence on the Production Performance of Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.-I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G.; et al. Chemical Composition and Assessment of Antimicrobial Activity of Lavender Essential Oil and Some By-Products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.-J. Antibacterial Activity of Essential Oil from Lavender (Lavandula Angustifolia) against Pet Turtle-Borne Pathogenic Bacteria. Lab. Anim. Res. 2017, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical Composition and Some Biological Activities of the Essential Oils from Basil Ocimum Different Cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical Composition and Antibacterial Activity of Essential Oil Isolated from Omani Basil (Ocimum Basilicum Linn.). Asian Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Głowacka, A.; Poznańska-Kurowska, K.; Kaszuba, A.; Urbaniak, A.; Kowalczyk, E. The Effect of Clary Sage Oil on Staphylococci Responsible for Wound Infections. Adv. Dermatol. Allergol. Dermatol. Alergol. 2015, 32, 21–26. [Google Scholar] [CrossRef]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical Composition and Antimicrobial Activity of Essential Oils Obtained from Leaves and Flowers of Salvia Hydrangea DC. Ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of Lavender Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced Biological Activity of a Novel Preparation of Lavandula Angustifolia Essential Oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef]
- Nikšić, H.; Kovač-Bešović, E.; Makarević, E.; Durić, K.; Kusturica, J.; Muratovic, S. Antiproliferative, Antimicrobial, and Antioxidant Activity of Lavandula Angustifolia Mill. Essential Oil. J. Health Sci. 2017, 7, 35–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In Vitro and In Vivo Efficacy Studies of Lavender Angustifolia Essential Oil and Its Active Constituents on the Proliferation of Human Prostate Cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Abutaha, N.; Al-Ghamdi, F.; Arbi Nehdi, I.; Nasr, F.A.; Mansour, L.; AL-Zharani, M.; Harrath, A.H. Analysis of the Chemical Composition and in Vitro Cytotoxic Activities of the Essential Oil of the Aerial Parts of Lavandula Atriplicifolia Benth. J. King Saud Univ. Sci. 2020, 32, 1476–1481. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula Angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-Inflammatory Effect of Lavender (Lavandula Angustifolia Mill.) Essential Oil Prepared during Different Plant Phenophases on THP-1 Macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential Oil of Common Sage (Salvia Officinalis L.) from Jordan: Assessment of Safety in Mammalian Cells and Its Antifungal and Anti-Inflammatory Potential. BioMed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- Tosun, A.; Khan, S.; Kim, Y.S.; Calín-Sánchez, A.; Hysenaj, X.; Carbonell-Barrachina, A. Essential Oil Composition and Anti-Inflammatory Activity of Salvia Officinalis L (Lamiaceae) in Murin Macrophages. Trop. J. Pharm. Res. 2014, 13, 937–942. [Google Scholar] [CrossRef]
- Lima, C.F.; Carvalho, F.; Fernandes, E.; Bastos, M.L.; Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Evaluation of Toxic/Protective Effects of the Essential Oil of Salvia Officinalis on Freshly Isolated Rat Hepatocytes. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2004, 18, 457–465. [Google Scholar] [CrossRef]
- Hadri, A.; Rio, M.; Sanz, J.; Coloma, A.; Idaomar, M.; Ozonas, B.; Benedi, J.; Reus, M. Cytotoxic Activity of Alpha-Humulene and Trans-Caryophyllene from Salvia Officinalis in Animal and Human Tumor Cells. An. Real Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical Composition and Anticancer Activity of Essential Oils of Mediterranean Sage (Salvia Officinalis L.) Grown in Different Environmental Conditions. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zare, H. Effects of Salvia Officinalis Extract on the Breast Cancer Cell Line. SciMedicine J. 2019, 1, 25–29. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Sajid, M.S.M.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y.; et al. Sage, Salvia Officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef] [PubMed]
- Itani, W.S.; El-Banna, S.H.; Hassan, S.B.; Larsson, R.L.; Bazarbachi, A.; Gali-Muhtasib, H.U. Anti Colon Cancer Components from Lebanese Sage (Salvia Libanotica) Essential Oil: Mechanistic Basis. Cancer Biol. Ther. 2008, 7, 1765–1773. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kalemba, D.; Różalski, M.; Różalska, B.; Więckowska-Szakiel, M.; Krajewska, U.; Wysokińska, H. Chemical Composition and Biological Activities of Essential Oil from Salvia Sclarea Plants Regenerated in Vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef]
- Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum Spp.): Current Insights and Future Prospects. Cancers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Mahmoud, G.I. Biological Effects, Antioxidant and Anticancer Activities of Marigold and Basil Essential Oils. J. Med. Plants Res. 2013, 7, 561–572. [Google Scholar] [CrossRef]
- Aburjai, T.A.; Mansi, K.; Azzam, H.; Alqudah, D.A.; Alshaer, W.; Abuirjei, M. Chemical Compositions and Anticancer Potential Of Essential Oil from Greenhouse-Cultivated Ocimum Basilicum Leaves. Indian J. Pharm. Sci. 2020, 82, 179–184. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical Composition of the Essential Oil from Basil (Ocimum Basilicum Linn.) and Its in Vitro Cytotoxicity against HeLa and HEp-2 Human Cancer Cell Lines and NIH 3T3 Mouse Embryonic Fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- Taie, H.; Salama, Z.; Samir, R. Potential Activity of Basil Plants as a Source of Antioxidants and Anticancer Agents as Affected by Organic and Bio-Organic Fertilization. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 119–127. [Google Scholar] [CrossRef]



| Botanic Name | Lavandula angustifolia Mill. | Salvia officinalis L. | Ocimum basilicum L. |
|---|---|---|---|
| Common name | lavender | sage | basil |
| Origin/native range of the species | mountainous regions of the Mediterranean [19] | Middle East and Mediterranean [20] | tropical regions of the Old World [21,22] |
| Structures containing/ accumulating EO | capitate and peltate trichomes [23] | capitate, peltate trichomes [24,25] and ambrate resinous droplets [25] | capitate and peltate trichomes [26] |
| Location of highest abundance in EO storage structures | flower calyx [8,23,27] | both sides of the leaf [17,25] | abaxial leaf surface [28] |
| Harvested plant part and optimal time for EO extraction | inflorescence/upper plant part at full flowering stage [23,27] after midday [29] | leaves and shoots at full flowering, in the evening [30] | herbs at bud flowering stage [31] |
| Parameter | Result | Interpretation 1 |
|---|---|---|
| Soil reaction (pH) | 7.50 | slightly alkaline |
| Humus % | 3.30 | middle range |
| Total nitrogen (Nt %) | 0.155 | middle range |
| Phosphorus (P ppm) | 22.0 | middle range for field crops |
| Potassium (K ppm) | 185 | good for field crops |
| Category | Compound | Concentration (% from Total Peak Area) | ||
|---|---|---|---|---|
| LEO | BEO | SEO | ||
| terpenes/ terpenoids | (Z)-beta-Farnesene | 6.45 | - | - |
| 1-Terpinen-4-ol | 1.89 | - | - | |
| 2-Cyclohexen-1-one, 4-(1-methylethyl)- | 0.35 | - | - | |
| 3-Carene | 0.19 | - | - | |
| 3-Thujol | - | 0.10 | 0.28 | |
| 4(10)-Thujene | 0.12 | - | - | |
| alpha-Bergamotene | 0.08 | - | - | |
| alpha-Caryophyllene | 0.14 | 2.47 | 3.33 | |
| alpha-Phellandrene | 0.06 | - | - | |
| alpha-Pinene | 0.17 | 2.88 | 7.75 | |
| alpha-Terpineol | 0.70 | 0.40 | - | |
| alpha-Terpinolen | - | 0.28 | - | |
| alpha-Thujene | 0.09 | 0.11 | 0.32 | |
| Anisole, p-allyl- | - | 0.86 | - | |
| beta-cis-Ocimene | - | 0.80 | 0.06 | |
| beta-Cubebene | 1.27 | - | - | |
| beta-Linalool | 30.91 | - | - | |
| beta-Myrcene | 0.95 | 1.18 | 1.22 | |
| beta-Phellandrene | 1.81 | 0.42 | 0.45 | |
| beta-Pinene | - | 2.48 | 2.48 | |
| beta-trans-Ocimene | - | 0.20 | 0.16 | |
| Borneol | 0.44 | 0.68 | 1.63 | |
| Bornyl acetate | - | 1.74 | 0.66 | |
| Camphene | 0.19 | 2.47 | 7.1 | |
| Camphor | 0.16 | 5.39 | 16.17 | |
| Caryophyllene | 5.66 | 1.50 | 1.75 | |
| Caryophyllene oxide | - | 0.16 | - | |
| cis-beta-Ocimene | 2.91 | - | - | |
| cis-Thujone | - | - | 34.28 | |
| Copaene | - | 0.38 | - | |
| delta-Cadinene | - | 0.07 | - | |
| D-Limonene | 0.92 | 1.20 | 2.29 | |
| Eucalyptol | 0.88 | 5.22 | 4.94 | |
| Eugenol | - | 2.84 | - | |
| gamma-Elemene | - | 0.76 | - | |
| gamma-Muurolene | 0.09 | - | - | |
| gamma-Terpinene | 0.13 | 0.18 | 0.19 | |
| Lavandulol | 0.85 | - | - | |
| Lavandulyl acetate | 4.71 | - | - | |
| Linalool | - | 26.27 | 2.18 | |
| Linalool acetate | 28.75 | 1.54 | 1.64 | |
| p-Cymene | - | 0.28 | 0.79 | |
| Terpinolene | 0.08 | - | - | |
| Thujone | - | 14.84 | - | |
| Thujone (stereoisomer) | - | 2.43 | - | |
| trans-beta-Ocimene | 3.46 | - | - | |
| trans-Thujone | - | - | 5.44 | |
| Tricyclo[2.2.1.0(2,6)]heptane, 1,7,7-trimethyl | - | 0.07 | 0.24 | |
| β-Elemene | - | 1.11 | - | |
| esters | Acetic acid, hexyl ester | 0.99 | - | - |
| Acetic acid, octyl ester | - | 0.20 | - | |
| Butanoic acid, hexyl ester | 0.31 | - | - | |
| Hexanoic acid, hexyl ester | 0.07 | - | - | |
| 4-Hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate | - | 0.21 | 0.23 | |
| 3-Octanol, acetate | 0.18 | - | - | |
| Octen-1-ol, acetate | 0.97 | - | - | |
| alcohols | 1-Octen-3-ol- | 0.17 | - | - |
| 3-Octanol | 0.43 | - | - | |
| 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)- | - | 0.23 | 0.39 | |
| 3-Cyclohexene-1-methanol, alpha, alpha4-trimethyl- | - | - | 0.25 | |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1.alpha,2.alpha,5.alpha)- | - | - | 0.06 | |
| ketones | 3-Octanone- | 1.99 | - | - |
| Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha,2.beta,5.alpha) | - | - | 0.10 | |
| other | (E,E)-1,3,5-Undecatriene | 0.04 | - | - |
| 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene-, (Z)- | - | 0.25 | - | |
| 1H-Cycloprop[e]azulene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha,7alpha,7a.beta, 7b.alpha)] | - | 1.12 | 1.58 | |
| 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene-, [1aR-(1a.alpha,4a.alpha,7alpha,7a.beta,7b.alpha)]- | - | 0.15 | - | |
| Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1alpha,4alpha,7alpha)]- | - | 0.88 | - | |
| Azulene, 1,2,3,5,6,7,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1alpha,7alpha,8a.beta)]- | - | 1.64 | - | |
| Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)- | - | 4.27 | - | |
| non-identified | non-identified | 0.47 | 10.76 | 2.04 |
| Samples | Escherichia coli ATCC 25922 | Salmonella enteritidis ATCC 13076 | Staphylococcus aureus ATCC 6538P | Listeria monocytogenes ATCC | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | |
| LEO | 3.795 ± 0.73 b | 5.14 ± 0.00 b | 5.14 ± 0.00 a | 5.14 ± 0.00 a | 3.795 ± 0.73 a | 5.14 ± 0.00 a | 22.68 ± 0.00 a | 22.68 ± 0.00 a |
| SEO | 16.74 ± 0.73 a | 22.68 ± 0.00 a | 5.14 ± 0.00 a | 5.14 ± 0.00 a | 3.795 ± 0.73 a | 5.14 ± 0.00 a | 10.80 ± 0.00 b | 10.80 ± 0.00 b |
| BEO | 1.17 ± 0.00 c | 1.17 ± 0.00 c | 2.45 ± 0.00 b | 2.45 ± 0.00 b | 2.45 ± 0.00 b | 2.45 ± 0.00 b | 10.80 ± 0.00 b | 10.80 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vârban, D.; Zăhan, M.; Pop, C.R.; Socaci, S.; Ștefan, R.; Crișan, I.; Bota, L.E.; Miclea, I.; Muscă, A.S.; Deac, A.M.; et al. Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites 2022, 12, 962. https://doi.org/10.3390/metabo12100962
Vârban D, Zăhan M, Pop CR, Socaci S, Ștefan R, Crișan I, Bota LE, Miclea I, Muscă AS, Deac AM, et al. Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites. 2022; 12(10):962. https://doi.org/10.3390/metabo12100962
Chicago/Turabian StyleVârban, Dan, Marius Zăhan, Carmen Rodica Pop, Sonia Socaci, Răzvan Ștefan, Ioana Crișan, Loredana Elena Bota, Ileana Miclea, Adriana Sebastiana Muscă, Alexandru Marius Deac, and et al. 2022. "Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil" Metabolites 12, no. 10: 962. https://doi.org/10.3390/metabo12100962
APA StyleVârban, D., Zăhan, M., Pop, C. R., Socaci, S., Ștefan, R., Crișan, I., Bota, L. E., Miclea, I., Muscă, A. S., Deac, A. M., & Vârban, R. (2022). Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites, 12(10), 962. https://doi.org/10.3390/metabo12100962










