Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond
Abstract
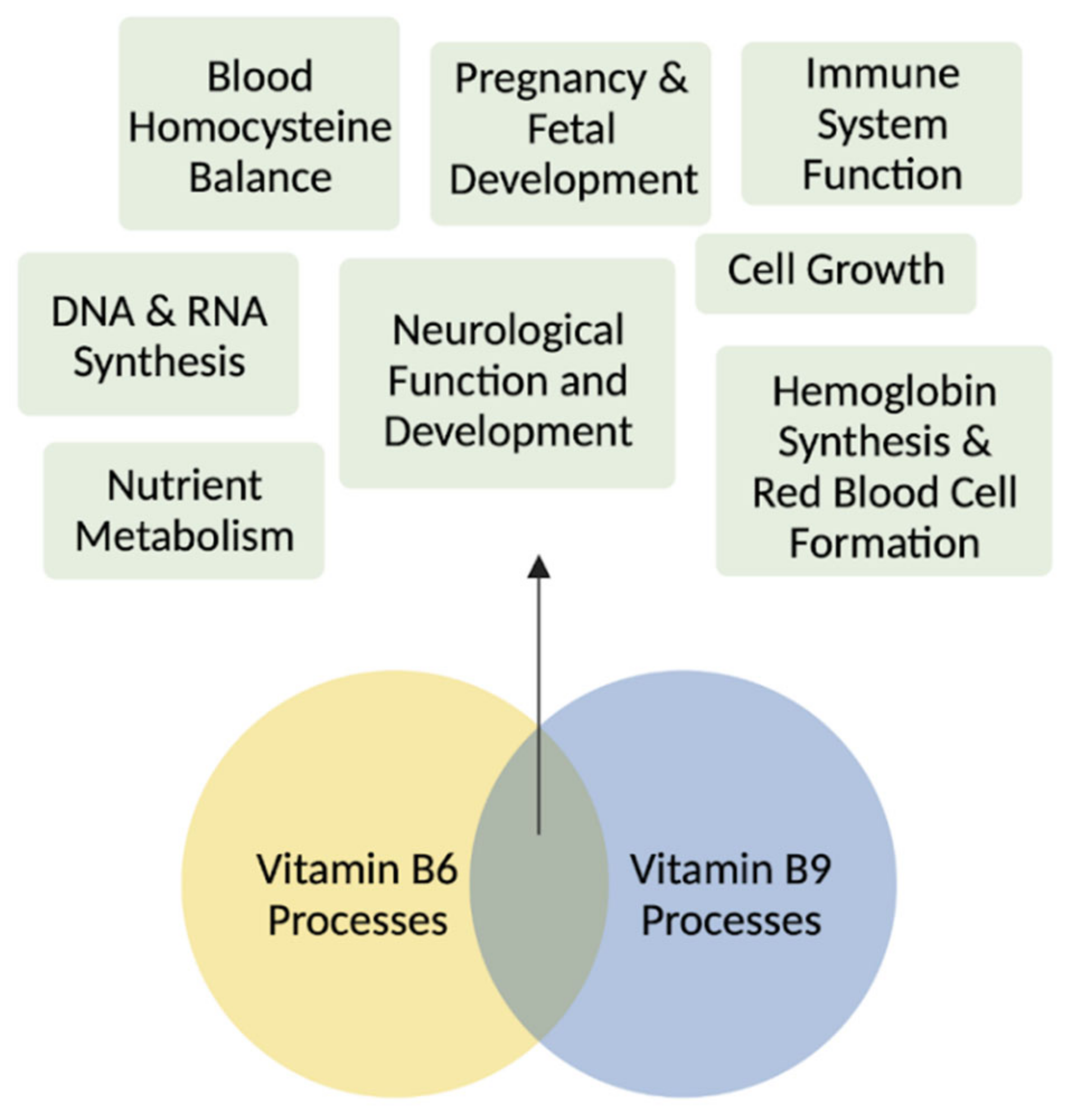
1. Introduction
2. Vitamin B9: Folate
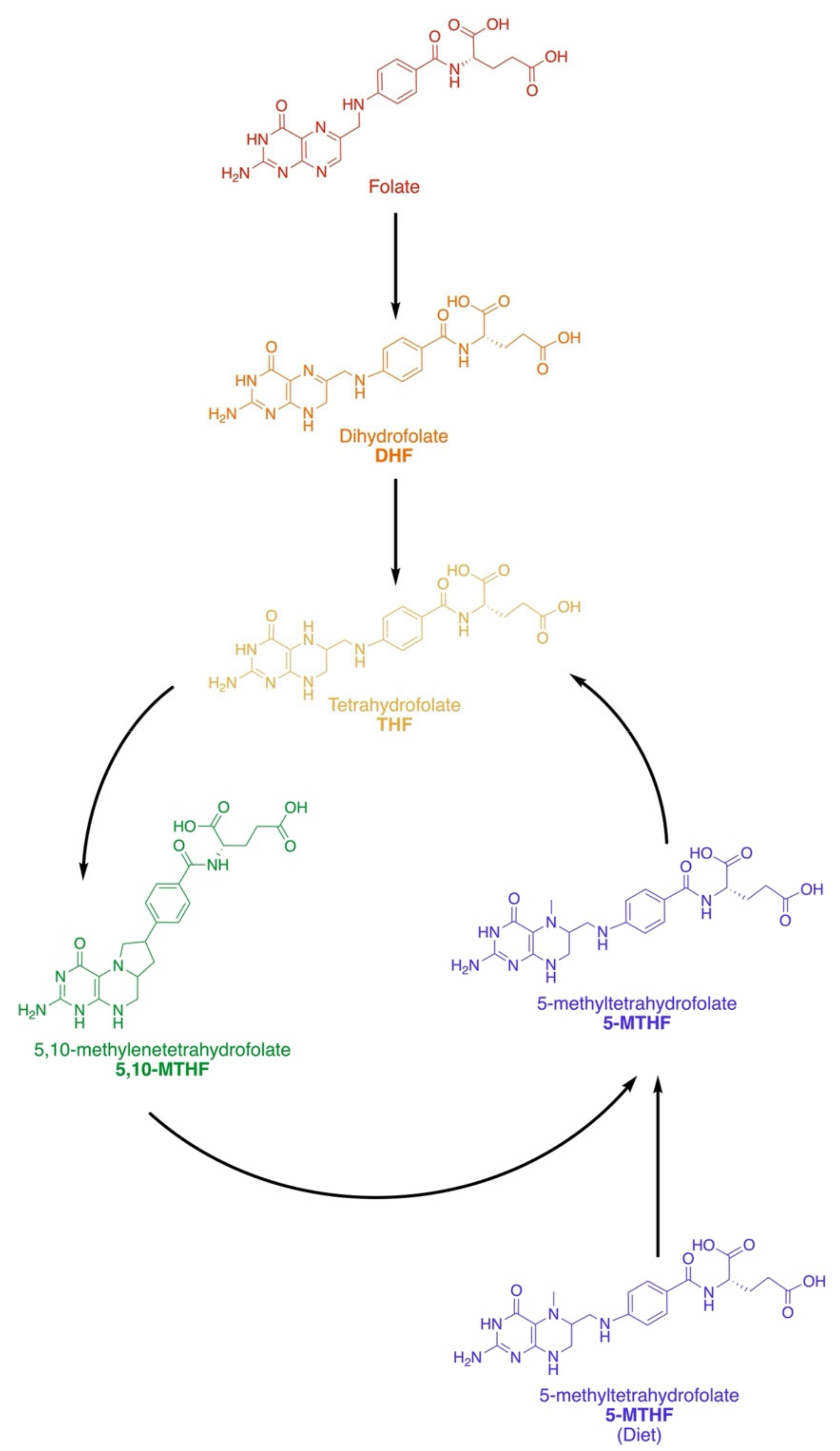
2.1. Dietary and Active Forms
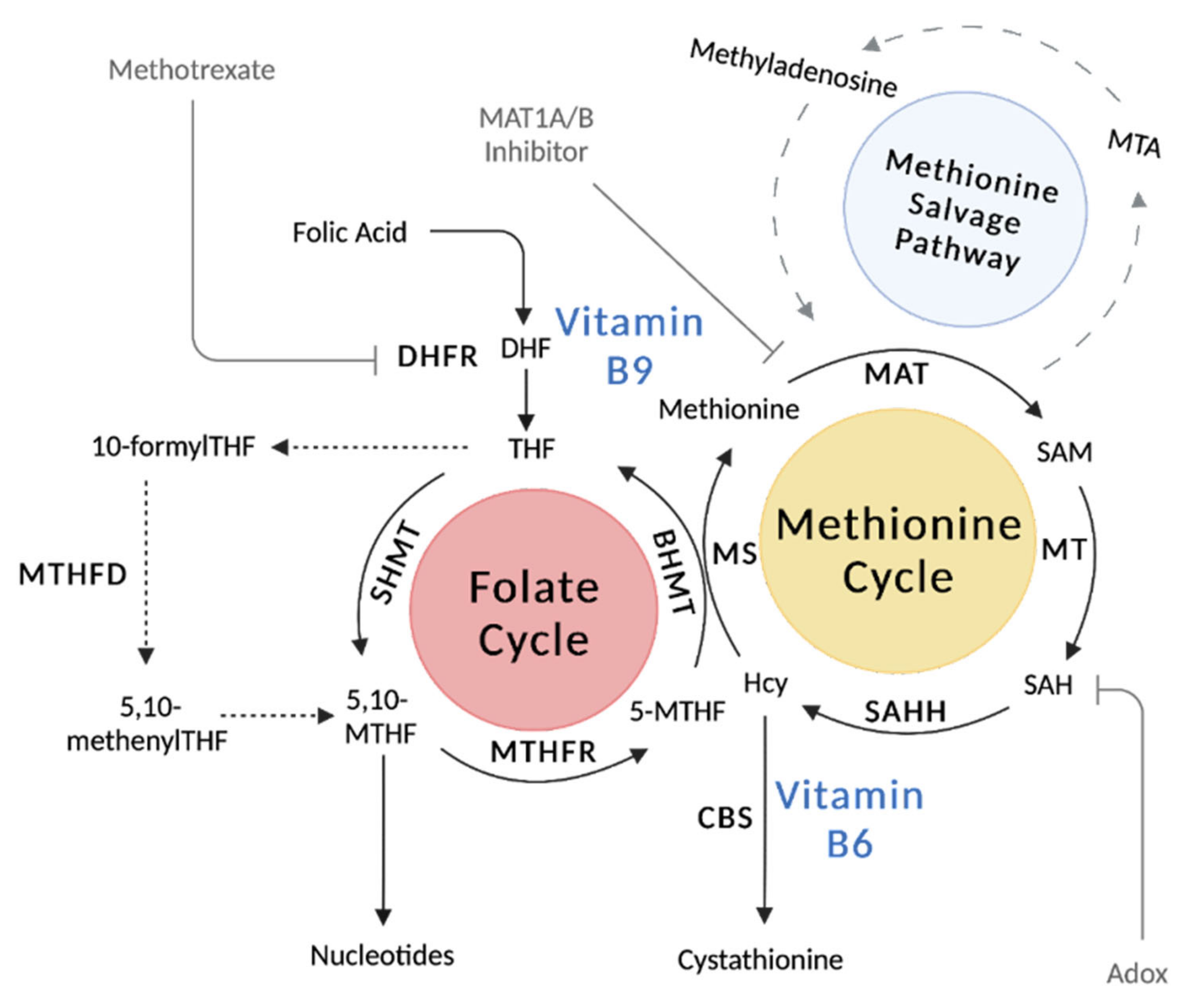
2.2. Metabolic Pathways and Key Enzymes of the Folate Cycle
2.3. Vitamin B9 Regulation
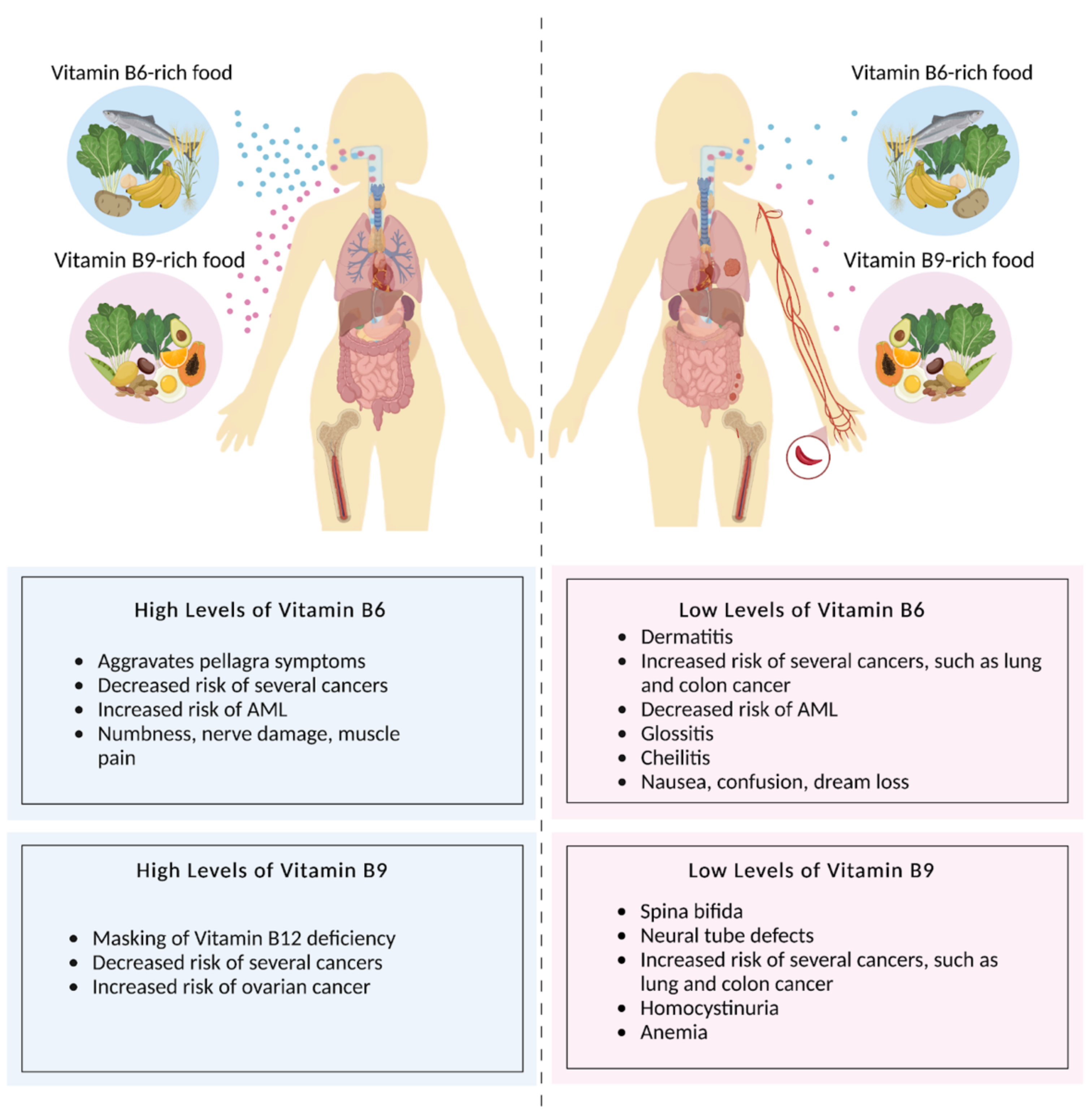
2.4. Folate Deficiency and Disorders
2.5. Folate in Cancer
2.5.1. Lung Cancer
2.5.2. Colon Cancer
2.5.3. Pancreatic Cancer
2.5.4. Ovarian Cancer
2.5.5. Esophageal, Liver, and Gastric Cancer
2.5.6. Prostate Cancer
2.5.7. Breast Cancer
3. Vitamin B6: Pyridoxine
3.1. Dietary and Active Forms
3.2. Metabolic Pathways and Key Enzymes in Pyrixodine Metabolism
3.3. Vitamin B6 Regulation
3.4. Pyridoxine Deficiencies and Disorders
3.5. Pyridoxine and Cancer
3.5.1. Colorectal Cancer
3.5.2. Pancreatic Cancer
3.5.3. Lung Cancer
3.5.4. Breast Cancer
3.5.5. Prostate Cancer
3.5.6. Skin Cancer
3.5.7. Kidney Cancer
3.5.8. Acute Myeloid Leukemia
3.5.9. Brain Cancer
4. Key Advances and Implementation of Innovative Tools and Instrumentation
5. Exploiting One Carbon Metabolism for New Therapeutics
6. Future Perspective and Ending Notes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Funk, C. The Etiology of the Deficiency Diseases. Beri-Beri, Polyneuritis in Birds, Epidemic Deopsy, Scurvy, Experimental Scurvy in Animals, Infantile Scurvy, Ship Beri-Beri, Pellagra. Anal. Chim. Acta 1912, 76, 341–368. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Head Zauche, L.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
- Wills, L. Treatment of “Pernicious Anameia of Pregnancy” and “Tropical Anaemia”. Br. Med. J. 1931, 1, 1059–1064. [Google Scholar] [CrossRef]
- Wills, L. A Note on the Use of Marmite in Tropical Macrocytic Anæmia, Including Pernicious Anæmia of Pregnancy. Indian Med. Gaz. 1933, 68, 133–134. [Google Scholar]
- Momb, J.; Appling, D.R. Mitochondrial One-Carbon Metabolism and Neural Tube Defects. Birt. Defects Res. A. Clin. Mol. Teratol. 2014, 100, 576–583. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Dhur, A.; Galan, P.; Hercberg, S. Folate Status and the Immune System. Prog. Food Nutr. Sci. 1991, 15, 43–60. [Google Scholar]
- Yang, M.; Vousden, K.H. Serine and One-Carbon Metabolism in Cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Reed, M.C.; Budu, P.; Ulrich, C.M. A Mathematical Model of the Folate Cycle: New insights into folate homeostasis*. J. Biol. Chem. 2004, 279, 55008–55016. [Google Scholar] [CrossRef]
- Blakley, R.L. Nomenclature and Symbols for Folic Acid and Related Compounds. Eur. J. Biochem. 1987, 168, 251–253. [Google Scholar] [CrossRef]
- Ducker, G.S.; Chen, L.; Morscher, R.J.; Ghergurovich, J.M.; Esposito, M.; Teng, X.; Kang, Y.; Rabinowitz, J.D. Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016, 23, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Hol, F.A.; van der Put, N.M.; Geurds, M.P.; Heil, S.G.; Trijbels, F.J.; Hamel, B.C.; Mariman, E.C.; Blom, H.J. Molecular Genetic Analysis of the Gene Encoding the Trifunctional Enzyme MTHFD (Methylenetetrahydrofolate-Dehydrogenase, Methenyltetrahydrofolate-Cyclohydrolase, Formyltetrahydrofolate Synthetase) in Patients with Neural Tube Defects. Clin. Genet. 1998, 53, 119–125. [Google Scholar] [CrossRef]
- Sun, Y.; Locasale, J.W. Rethinking the Bioavailability and Cellular Transport Properties of S-Adenosylmethionine. Cell Stress 2021, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, G.L. The Nature of the Active Methyl Donor Formed Enzymatically from L-Methionine and Adenosinetriiphosphate1,2. J. Am. Chem. Soc. 1952, 74, 2942–2943. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine Metabolism in Health and Cancer: A Nexus of Diet and Precision Medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Krall, A.S.; Mullen, P.J.; Surjono, F.; Momcilovic, M.; Schmid, E.W.; Halbrook, C.J.; Thambundit, A.; Mittelman, S.D.; Lyssiotis, C.A.; Shackelford, D.B.; et al. Asparagine Couples Mitochondrial Respiration to ATF4 Activity and Tumor Growth. Cell Metab. 2021, 33, 1013–1026.e6. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Chapter 7: Vitamin B6. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academics Press: Washington, DC, USA, 1998.
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary Methionine Influences Therapy in Mouse Cancer Models and Alters Human Metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Hinze, L.; Labrosse, R.; Degar, J.; Han, T.; Schatoff, E.M.; Schreek, S.; Karim, S.; McGuckin, C.; Sacher, J.R.; Wagner, F.; et al. Exploiting the Therapeutic Interaction of WNT Pathway Activation and Asparaginase for Colorectal Cancer Therapy. Cancer Discov. 2020, 10, 1690–1705. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the Therapeutic Response of Tumours to Dietary Serine and Glycine Starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W.; Grassian, A.R.; Melman, T.; Lyssiotis, C.A.; Mattaini, K.R.; Bass, A.J.; Heffron, G.; Metallo, C.M.; Muranen, T.; Sharfi, H.; et al. Phosphoglycerate Dehydrogenase Diverts Glycolytic Flux and Contributes to Oncogenesis. Nat. Genet. 2011, 43, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional Genomics Reveal That the Serine Synthesis Pathway Is Essential in Breast Cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Wang, Z.; Yip, L.Y.; Lee, J.H.J.; Wu, Z.; Chew, H.Y.; Chong, P.K.W.; Teo, C.C.; Ang, H.Y.-K.; Peh, K.L.E.; Yuan, J.; et al. Methionine Is a Metabolic Dependency of Tumor-Initiating Cells. Nat. Med. 2019, 25, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Di Palma, S.; Preisinger, C.; Peng, M.; Polat, A.N.; Heck, A.J.R.; Mohammed, S. Toward a Comprehensive Characterization of a Human Cancer Cell Phosphoproteome. J. Proteome Res. 2013, 12, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Knight, Z.A.; Shokat, K.M. Features of Selective Kinase Inhibitors. Chem. Biol. 2005, 12, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Olopade, O.I.; Savarese, T.M. Expression of Methylthioadenosine Phosphorylase CDNA in P16-, MTAP- Malignant Cells: Restoration of Methylthioadenosine Phosphorylase-Dependent Salvage Pathways and Alterations of Sensitivity to Inhibitors of Purine de Novo Synthesis. Mol. Pharmacol. 1997, 52, 903–911. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Wilson, F.H.; Ruth, J.R.; Paulk, J.; Tsherniak, A.; Marlow, S.E.; Vazquez, F.; Weir, B.A.; Fitzgerald, M.E.; Tanaka, M.; et al. MTAP Deletion Confers Enhanced Dependency on the PRMT5 Arginine Methyltransferase in Cancer Cells. Science 2016, 351, 1214–1218. [Google Scholar] [CrossRef]
- Clayton, P.T. B6-Responsive Disorders: A Model of Vitamin Dependency. J. Inherit. Metab. Dis. 2006, 29, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Marjon, K.; Cameron, M.J.; Quang, P.; Clasquin, M.F.; Mandley, E.; Kunii, K.; McVay, M.; Choe, S.; Kernytsky, A.; Gross, S.; et al. MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell Rep. 2016, 15, 574–587. [Google Scholar] [CrossRef]
- Mavrakis, K.J.; McDonald, E.R.; Schlabach, M.R.; Billy, E.; Hoffman, G.R.; deWeck, A.; Ruddy, D.A.; Venkatesan, K.; Yu, J.; McAllister, G.; et al. Disordered Methionine Metabolism in MTAP/CDKN2A-Deleted Cancers Leads to Dependence on PRMT5. Science 2016, 351, 1208–1213. [Google Scholar] [CrossRef]
- Mullen, P.; Christofk, H.R. The Metabolic Relationship Between Viral Infection and Cancer. Annu. Rev. Cancer Biol. 2022. [Google Scholar] [CrossRef]
- Gu, T.-L.; Deng, X.; Huang, F.; Tucker, M.; Crosby, K.; Rimkunas, V.; Wang, Y.; Deng, G.; Zhu, L.; Tan, Z.; et al. Survey of Tyrosine Kinase Signaling Reveals ROS Kinase Fusions in Human Cholangiocarcinoma. PLoS ONE 2011, 6, e15640. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of Cellular Metabolism by Protein Lysine Acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Larsen, S.C.; Sylvestersen, K.B.; Mund, A.; Lyon, D.; Mullari, M.; Madsen, M.V.; Daniel, J.A.; Jensen, L.J.; Nielsen, M.L. Proteome-Wide Analysis of Arginine Monomethylation Reveals Widespread Occurrence in Human Cells. Sci. Signal. 2016, 9, rs9. [Google Scholar] [CrossRef]
- Kim, W.; Bennett, E.J.; Huttlin, E.L.; Guo, A.; Li, J.; Possemato, A.; Sowa, M.E.; Rad, R.; Rush, J.; Comb, M.J.; et al. Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome. Mol. Cell 2011, 44, 325–340. [Google Scholar] [CrossRef]
- Skierka, K.; Wilamowski, P.; Wielechowska, M.; Cysewski, D.; Senkara, E.; Wińska, P.; Bretner, M.; Cieśla, J. Human Dihydrofolate Reductase Is a Substrate of Protein Kinase CK2α. Biochem. Biophys. Res. Commun. 2019, 513, 368–373. [Google Scholar] [CrossRef]
- Mertins, P.; Qiao, J.W.; Patel, J.; Udeshi, N.D.; Clauser, K.R.; Mani, D.R.; Burgess, M.W.; Gillette, M.A.; Jaffe, J.D.; Carr, S.A. Integrated Proteomic Analysis of Post-Translational Modifications by Serial Enrichment. Nat. Methods 2013, 10, 634–637. [Google Scholar] [CrossRef]
- Sylvestersen, K.B.; Horn, H.; Jungmichel, S.; Jensen, L.J.; Nielsen, M.L. Proteomic Analysis of Arginine Methylation Sites in Human Cells Reveals Dynamic Regulation During Transcriptional Arrest. Mol. Cell. Proteomics MCP 2014, 13, 2072–2088. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Fan, W.; Li, Y.; Wang, J.; Li, T.W.H.; Barbier-Torres, L.; Mato, J.M.; Liu, T.; Seki, E.; et al. Deregulated 14-3-3ζ and Methionine Adenosyltransferase A1 Interplay Promotes Liver Cancer Tumorigenesis in Mice and Humans. Oncogene 2021, 40, 5866–5879. [Google Scholar] [CrossRef]
- Banerjee, R.V.; Matthews, R.G. Cobalamin-Dependent Methionine Synthase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1990, 4, 1450–1459. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, Y.-X.; Wei, C.; Wang, Z.-X.; Lin, J.-F.; Liao, K.; Luo, X.-J.; Yu, K.; Han, Y.; Li, J.-J.; et al. Arginine Methylation of MTHFD1 by PRMT5 Enhances Anoikis Resistance and Cancer Metastasis. Oncogene 2022, 41, 3912–3924. [Google Scholar] [CrossRef]
- Bhatia, M.; Thakur, J.; Suyal, S.; Oniel, R.; Chakraborty, R.; Pradhan, S.; Sharma, M.; Sengupta, S.; Laxman, S.; Masakapalli, S.K.; et al. Allosteric Inhibition of MTHFR Prevents Futile SAM Cycling and Maintains Nucleotide Pools in One-Carbon Metabolism. J. Biol. Chem. 2020, 295, 16037–16057. [Google Scholar] [CrossRef]
- Froese, D.S.; Kopec, J.; Rembeza, E.; Bezerra, G.A.; Oberholzer, A.E.; Suormala, T.; Lutz, S.; Chalk, R.; Borkowska, O.; Baumgartner, M.R.; et al. Structural Basis for the Regulation of Human 5,10-Methylenetetrahydrofolate Reductase by Phosphorylation and S-Adenosylmethionine Inhibition. Nat. Commun. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Lu, L. Vitamin B6 Deficiency, Genome Instability and Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5333–5338. [Google Scholar] [CrossRef]
- Oppermann, F.S.; Gnad, F.; Olsen, J.V.; Hornberger, R.; Greff, Z.; Kéri, G.; Mann, M.; Daub, H. Large-Scale Proteomics Analysis of the Human Kinome. Mol. Cell. Proteomics MCP 2009, 8, 1751–1764. [Google Scholar] [CrossRef]
- Bian, Y.; Song, C.; Cheng, K.; Dong, M.; Wang, F.; Huang, J.; Sun, D.; Wang, L.; Ye, M.; Zou, H. An Enzyme Assisted RP-RPLC Approach for in-Depth Analysis of Human Liver Phosphoproteome. J. Proteomics 2014, 96, 253–262. [Google Scholar] [CrossRef]
- Rush, J.; Moritz, A.; Lee, K.A.; Guo, A.; Goss, V.L.; Spek, E.J.; Zhang, H.; Zha, X.-M.; Polakiewicz, R.D.; Comb, M.J. Immunoaffinity Profiling of Tyrosine Phosphorylation in Cancer Cells. Nat. Biotechnol. 2005, 23, 94–101. [Google Scholar] [CrossRef]
- Jencks, D.A.; Mathews, R.G. Allosteric Inhibition of Methylenetetrahydrofolate Reductase by Adenosylmethionine. Effects of Adenosylmethionine and NADPH on the Equilibrium between Active and Inactive Forms of the Enzyme and on the Kinetics of Approach to Equilibrium. J. Biol. Chem. 1987, 262, 2485–2493. [Google Scholar] [CrossRef]
- Zheng, Y.; Ramsamooj, S.; Li, Q.; Johnson, J.L.; Yaron, T.M.; Sharra, K.; Cantley, L.C. Regulation of Folate and Methionine Metabolism by Multisite Phosphorylation of Human Methylenetetrahydrofolate Reductase. Sci. Rep. 2019, 9, 4190. [Google Scholar] [CrossRef]
- Pinthong, C.; Maenpuen, S.; Amornwatcharapong, W.; Yuthavong, Y.; Leartsakulpanich, U.; Chaiyen, P. Distinct Biochemical Properties of Human Serine Hydroxymethyltransferase Compared with the Plasmodium Enzyme: Implications for Selective Inhibition. FEBS J. 2014, 281, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Physiological Concentrations of Purines and Pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Loussouarn, G.; Tucker, S.J.; Zhao, C.; Nichols, C.G.; Ashcroft, F.M. A Novel Method for Measurement of Submembrane ATP Concentration. J. Biol. Chem. 2000, 275, 30046–30049. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, S.M.; Mikhael, P.G.; Ramesh, V.; Dai, Z.; Locasale, J.W. Nutrient Availability Shapes Methionine Metabolism in P16/MTAP-Deleted Cells. Sci. Adv. 2019, 5, eaav7769. [Google Scholar] [CrossRef]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine Metabolism Regulates Maintenance and Differentiation of Human Pluripotent Stem Cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic Control of Methylation and Acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised Acyl-CoA Metabolism and Roles in Chromatin Regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Ghergurovich, J.M.; Mainolfi, N.; Suri, V.; Jeong, S.K.; Hsin-Jung Li, S.; Friedman, A.; Manfredi, M.G.; Gitai, Z.; Kim, H.; et al. Human SHMT Inhibitors Reveal Defective Glycine Import as a Targetable Metabolic Vulnerability of Diffuse Large B-Cell Lymphoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11404–11409. [Google Scholar] [CrossRef]
- Albrecht, L.V.; Bui, M.H.; De Robertis, E.M. Canonical Wnt Is Inhibited by Targeting One-Carbon Metabolism through Methotrexate or Methionine Deprivation. Proc. Natl. Acad. Sci. USA 2019, 116, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Saygili, U.; Tuna, B.; Gol, M.; Gürel, D.; Acar, B.; Koyuncuoğlu, M. P53 and Mdm2 as Prognostic Indicators in Patients with Epithelial Ovarian Cancer: A Multivariate Analysis. Gynecol. Oncol. 2005, 97, 46–52. [Google Scholar] [CrossRef]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Dietary Folate Intake and Incidence of Ovarian Cancer: The Swedish Mammography Cohort. JNCI J. Natl. Cancer Inst. 2004, 96, 396–402. [Google Scholar] [CrossRef][Green Version]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Folate Intake, MTHFR Polymorphisms, and Risk of Esophageal, Gastric, and Pancreatic Cancer: A Meta-Analysis. Gastroenterology 2006, 131, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Andrici, J.; Cox, M.R.; Eslick, G.D. Folate Intake and the Risk of Upper Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2014, 29, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; An, Q.Z.; Wang, Q.Z.; Liu, C.X. Folate Intake and Pancreatic Cancer Risk: An Overall and Dose–Response Meta-Analysis. Public Health 2013, 127, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher Dietary Folate Intake Reduces the Breast Cancer Risk: A Systematic Review and Meta-Analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef]
- Contestabile, R.; di Salvo, M.L.; Bunik, V.; Tramonti, A.; Vernì, F. The Multifaceted Role of Vitamin B 6 in Cancer: Drosophila as a Model System to Investigate DNA Damage. Open Biol. 2020, 10, 200034. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.-C. Folic Acid and Diseases–Supplement It or Not? Rev. Assoc. Medica Bras. 2016, 62, 90–100. [Google Scholar] [CrossRef]
- Liu, M.; Cui, L.-H.; Ma, A.-G.; Li, N.; Piao, J.-M. Lack of Effects of Dietary Folate Intake on Risk of Breast Cancer: An Updated Meta-Analysis of Prospective Studies. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cui, Y.; Shen, L.; Sun, N.; Zhang, Y.; Li, J.; Xu, X.; Wang, B.; Xu, X.; Huo, Y.; et al. Folic Acid Supplementation and Cancer Risk: A Meta-Analysis of Randomized Controlled Trials. Int. J. Cancer 2013, 133, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Clarke, R.; Lewington, S.; Ebbing, M.; Halsey, J.; Lonn, E.; Armitage, J.; Manson, J.E.; Hankey, G.J.; Spence, J.D.; et al. Effects of Folic Acid Supplementation on Overall and Site-Specific Cancer Incidence during the Randomised Trials: Meta-Analyses of Data on 50,000 Individuals. Lancet 2013, 381, 1029–1036. [Google Scholar] [CrossRef]
- Wien, T.N.; Pike, E.; Wisløff, T.; Staff, A.; Smeland, S.; Klemp, M. Cancer Risk with Folic Acid Supplements: A Systematic Review and Meta-Analysis. BMJ Open 2012, 2, e000653. [Google Scholar] [CrossRef]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed Homocysteine Metabolism Is Associated with Cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the Association between the Methylenetetrahydrofolate Reductase (MTHFR) 677C>T Polymorphism and Blood Folate Concentrations: A Systematic Review and Meta-Analysis of Trials and Observational Studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef]
- Glade, M.J. Food, Nutrition, and the Prevention of Cancer: A Global Perspective. Am. Inst. Cancer Res. Cancer Res. Fund 1997, 15, 523–526. [Google Scholar] [CrossRef]
- Kim, Y.-I. Folate: A Magic Bullet or a Double Edged Sword for Colorectal Cancer Prevention? Gut 2006, 55, 1387–1389. [Google Scholar] [CrossRef]
- Giovannucci, E.; Stampfer, M.J.; Colditz, G.A.; Hunter, D.J.; Fuchs, C.; Rosner, B.A.; Speizer, F.E.; Willett, W.C. Multivitamin Use, Folate, and Colon Cancer in Women in the Nurses’ Health Study. Ann. Intern. Med. 1998, 129, 517–524. [Google Scholar] [CrossRef]
- Lamprecht, S.A.; Lipkin, M. Chemoprevention of Colon Cancer by Calcium, Vitamin D and Folate: Molecular Mechanisms. Nat. Rev. Cancer 2003, 3, 601–614. [Google Scholar] [CrossRef]
- Duthie, S.J.; Mavrommatis, Y.; Rucklidge, G.; Reid, M.; Duncan, G.; Moyer, M.P.; Pirie, L.P.; Bestwick, C.S. The Response of Human Colonocytes to Folate Deficiency in Vitro: Functional and Proteomic Analyses. J. Proteome Res. 2008, 7, 3254–3266. [Google Scholar] [CrossRef] [PubMed]
- Mentch, S.J.; Locasale, J.W. One Carbon Metabolism and Epigenetics: Understanding the Specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Schapira, M.; Arrowsmith, C.H.; Barsyte-Lovejoy, D. Protein Arginine Methylation: From Enigmatic Functions to Therapeutic Targeting. Nat. Rev. Drug Discov. 2021, 20, 509–530. [Google Scholar] [CrossRef]
- Ye, C.; Sutter, B.M.; Wang, Y.; Kuang, Z.; Tu, B.P. A Metabolic Function for Phospholipid and Histone Methylation. Mol. Cell 2017, 66, 180–193.e8. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.Q.; Hanse, E.A.; Habowski, A.N.; Li, H.; Ishak Gabra, M.B.; Yang, Y.; Lowman, X.H.; Ooi, A.M.; Liao, S.Y.; Edwards, R.A.; et al. α-Ketoglutarate Attenuates Wnt Signaling and Drives Differentiation in Colorectal Cancer. Nat. Cancer 2020, 1, 345–358. [Google Scholar] [CrossRef]
- Chabner, B.A.; DeVita, V.T.; Livingston, D.M.; Oliverio, V.T. Abnormalities of Tryptophan Metabolism and Plasma Pyridoxal Phosphate in Hodgkin’s Disease. N. Engl. J. Med. 1970, 282, 838–843. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhao, H.; Wang, F.; Bao, Y.; Guo, J.; Chang, S.; Wu, L.; Cheng, H.; Chen, S.; et al. Low Folate Concentration Impacts Mismatch Repair Deficiency in Neural Tube Defects. Epigenomics 2020, 12, 5–18. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Liu, S.-M.; Zhang, Y.-Z. Maternal Folic Acid Supplementation Mediates Offspring Health via DNA Methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Sunden, S.L.; Renduchintala, M.S.; Park, E.I.; Miklasz, S.D.; Garrow, T.A. Betaine-Homocysteine Methyltransferase Expression in Porcine and Human Tissues and Chromosomal Localization of the Human Gene. Arch. Biochem. Biophys. 1997, 345, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and Vitamin B-12 Status in Relation to Anemia, Macrocytosis, and Cognitive Impairment in Older Americans in the Age of Folic Acid Fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef]
- Van Amsterdam, J.; Opperhuizen, A.J.E. Masking of Vitamin B12 Deficiency Associated Neuropathy by Folic Acid; RIVM: Utrecht, The Netherlands, 2005; RIVM Rapport 340230002. [Google Scholar]
- Farber, S.; Cutler, E.C.; Hawkins, J.W.; Harrison, J.H.; Peirce, E.C.; Lenz, G.G. The Action of Pteroylglutamic Conjugates on Man. Science 1947, 106, 619–621. [Google Scholar] [CrossRef] [PubMed]
- ZHANG, X.-D.; LI, Y.-T.; YANG, S.-Y.; LI, W. Meta-Analysis on MTHFR Polymorphism and Lung Cancer Susceptibility in East Asian Populations. Biomed. Rep. 2013, 1, 440–446. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Z.; Li, G.; Pillow, P.C.; Hernandez, L.M.; Spitz, M.R.; Wei, Q. Sex Differences in Risk of Lung Cancer Associated with Methylene-Tetrahydrofolate Reductase Polymorphisms. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1477–1484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heine-Bröring, R.C.; Winkels, R.M.; Renkema, J.M.S.; Kragt, L.; van Orten-Luiten, A.-C.B.; Tigchelaar, E.F.; Chan, D.S.M.; Norat, T.; Kampman, E. Dietary Supplement Use and Colorectal Cancer Risk: A Systematic Review and Meta-Analyses of Prospective Cohort Studies. Int. J. Cancer 2015, 136, 2388–2401. [Google Scholar] [CrossRef] [PubMed]
- Cravo, M.L.; Mason, J.B.; Dayal, Y.; Hutchinson, M.; Smith, D.; Selhub, J.; Rosenberg, I.H. Folate Deficiency Enhances the Development of Colonic Neoplasia in Dimethylhydrazine-Treated Rats. Cancer Res. 1992, 52, 5002–5006. [Google Scholar] [PubMed]
- Kim, Y.I.; Pogribny, I.P.; Salomon, R.N.; Choi, S.W.; Smith, D.E.; James, S.J.; Mason, J.B. Exon-Specific DNA Hypomethylation of the P53 Gene of Rat Colon Induced by Dimethylhydrazine. Modulation by Dietary Folate. Am. J. Pathol. 1996, 149, 1129–1137. [Google Scholar] [PubMed]
- Weinstein, S.J.; Albanes, D.; Selhub, J.; Graubard, B.; Lim, U.; Taylor, P.R.; Virtamo, J.; Stolzenberg-Solomon, R. One-Carbon Metabolism Biomarkers and Risk of Colon and Rectal Cancers. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3233–3240. [Google Scholar] [CrossRef]
- Shrubsole, M.J.; Yang, G.; Gao, Y.-T.; Chow, W.H.; Shu, X.O.; Cai, Q.; Rothman, N.; Gao, J.; Wagner, C.; Zheng, W. Dietary B Vitamin and Methionine Intakes and Plasma Folate Are Not Associated with Colorectal Cancer Risk in Chinese Women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1003–1006. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Q.; Yang, J. The Effect of Folate Intake on Ovarian Cancer Risk: A Meta-Analysis of Observational Studies. Medicine (Baltimore) 2021, 100, e22605. [Google Scholar] [CrossRef]
- Sakai, H.; Kawakami, H.; Teramura, T.; Onodera, Y.; Somers, E.; Furuuchi, K.; Uenaka, T.; Kato, R.; Nakagawa, K. Folate Receptor α Increases Chemotherapy Resistance through Stabilizing MDM2 in Cooperation with PHB2 That Is Overcome by MORAb-202 in Gastric Cancer. Clin. Transl. Med. 2021, 11, e454. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, Y.; Huang, J.-Y.; Zhang, A.-Q.; Zhou, Y.-H.; Wang, J.-N. Folate Intake, Serum Folate Levels, and Prostate Cancer Risk: A Meta-Analysis of Prospective Studies. BMC Public Health 2014, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, X.; Wu, W.; Guo, Y.; Cui, W. Elevated Homocysteine Level and Folate Deficiency Associated with Increased Overall Risk of Carcinogenesis: Meta-Analysis of 83 Case-Control Studies Involving 35,758 Individuals. PLoS ONE 2015, 10, e0123423. [Google Scholar] [CrossRef] [PubMed]
- Morscher, R.J.; Ducker, G.S.; Li, S.H.-J.; Mayer, J.A.; Gitai, Z.; Sperl, W.; Rabinowitz, J.D. Mitochondrial Translation Requires Folate-Dependent TRNA Methylation. Nature 2018, 554, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Affronti, H.C.; Long, M.D.; Rosario, S.R.; Gillard, B.M.; Karasik, E.; Boerlin, C.S.; Pellerite, A.J.; Foster, B.A.; Attwood, K.; Pili, R.; et al. Dietary Folate Levels Alter the Kinetics and Molecular Mechanism of Prostate Cancer Recurrence in the CWR22 Model. Oncotarget 2017, 8, 103758–103774. [Google Scholar] [CrossRef]
- CDC. MTHFR Gene and Folic Acid. Available online: https://www.cdc.gov/ncbddd/folicacid/mthfr-gene-and-folic-acid.html (accessed on 7 September 2022).
- Varela-Rey, M.; Woodhoo, A.; Martinez-Chantar, M.-L.; Mato, J.M.; Lu, S.C. Alcohol, DNA Methylation, and Cancer. Alcohol Res. Curr. Rev. 2013, 35, 25–35. [Google Scholar]
- Xie, R.; Jia, D.; Gao, C.; Zhou, J.; Sui, H.; Wei, X.; Zhang, T.; Han, Y.; Shi, J.; Bai, Y. Homocysteine Induces Procoagulant Activity of Red Blood Cells via Phosphatidylserine Exposure and Microparticles Generation. Amino Acids 2014, 46, 1997–2004. [Google Scholar] [CrossRef]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine Induces the Expression of C-Reactive Protein via NMDAr-ROS-MAPK-NF-ΚB Signal Pathway in Rat Vascular Smooth Muscle Cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Cravo, M.; Fidalgo, P.; Pereira, A.D.; Gouveia-Oliveira, A.; Chaves, P.; Selhub, J.; Mason, J.B.; Mira, F.C.; Leitao, C.N. DNA Methylation as an Intermediate Biomarker in Colorectal Cancer: Modulation by Folic Acid Supplementation. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 1994, 3, 473–479. [Google Scholar] [CrossRef]
- Choi, S.-W.; Mason, J.B. Folate Status: Effects on Pathways of Colorectal Carcinogenesis. J. Nutr. 2002, 132, 2413S–2418S. [Google Scholar] [CrossRef]
- Kim, Y.I. Folate and Carcinogenesis: Evidence, Mechanisms, and Implications. J. Nutr. Biochem. 1999, 10, 66–88. [Google Scholar] [CrossRef]
- Kim, Y.-I. Nutritional Epigenetics: Impact of Folate Deficiency on DNA Methylation and Colon Cancer Susceptibility. J. Nutr. 2005, 135, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Pogribny, I.P.; Basnakian, A.G.; Miller, J.W.; Selhub, J.; James, S.J.; Mason, J.B. Folate Deficiency in Rats Induces DNA Strand Breaks and Hypomethylation within the P53 Tumor Suppressor Gene. Am. J. Clin. Nutr. 1997, 65, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ni, J.; Zhu, Y.; Zhou, T.; Ma, X.; Xue, J.; Wang, X. Folate Deficiency Induces Mitotic Aberrations and Chromosomal Instability by Compromising the Spindle Assembly Checkpoint in Cultured Human Colon Cells. Mutagenesis 2017, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/graphic-isotype (accessed on 11 September 2022).
- Kurosaki, A.; Hasegawa, K.; Kato, T.; Abe, K.; Hanaoka, T.; Miyara, A.; O’Shannessy, D.J.; Somers, E.B.; Yasuda, M.; Sekino, T.; et al. Serum Folate Receptor Alpha as a Biomarker for Ovarian Cancer: Implications for Diagnosis, Prognosis and Predicting Its Local Tumor Expression. Int. J. Cancer 2016, 138, 1994–2002. [Google Scholar] [CrossRef]
- Ni, Y.; Du, J.; Yin, X.; Lu, M. Folate Intake, Serum Folate, and Risk of Esophageal Cancer: A Systematic Review and Dose-Response Meta-Analysis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2019, 28, 173–180. [Google Scholar] [CrossRef]
- Rao, D.N.; Sanghvi, L.D.; Desai, P.B. Epidemiology of Esophageal Cancer. Semin. Surg. Oncol. 1989, 5, 351–354. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in Targeting the Folate Receptor in the Treatment/Imaging of Cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the Folate Receptor for Active Targeting of Cancer Nanotherapeutics. Nano Rev. 2012, 3. [Google Scholar] [CrossRef]
- Mahmoud, K.; Swidan, S.; El-Nabarawi, M.; Teaima, M. Lipid Based Nanoparticles as a Novel Treatment Modality for Hepatocellular Carcinoma: A Comprehensive Review on Targeting and Recent Advances. J. Nanobiotechnol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Alkan, A.; Mızrak, D.; Utkan, G. Lower Folate Levels in Gastric Cancer: Is It a Cause or a Result? World J. Gastroenterol. 2015, 21, 4101–4102. [Google Scholar] [CrossRef] [PubMed]
- Schipper, R.G.; Romijn, J.C.; Cuijpers, V.M.J.I.; Verhofstad, A.a.J. Polyamines and Prostatic Cancer. Biochem. Soc. Trans. 2003, 31, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.M.; Ruiz-Echevarría, M.J. One-Carbon Metabolism in Prostate Cancer: The Role of Androgen Signaling. Int. J. Mol. Sci. 2016, 17, 1208. [Google Scholar] [CrossRef]
- Bistulfi, G.; Diegelman, P.; Foster, B.A.; Kramer, D.L.; Porter, C.W.; Smiraglia, D.J. Polyamine Biosynthesis Impacts Cellular Folate Requirements Necessary to Maintain S-Adenosylmethionine and Nucleotide Pools. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2888–2897. [Google Scholar] [CrossRef]
- Folic Acid. Available online: https://www.breastcancer.org/managing-life/diet-nutrition/dietary-supplements/known/folic-acid (accessed on 7 September 2022).
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; van den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Holmberg, L.; Howe, G.R.; Marshall, J.R.; et al. Alcohol and Breast Cancer in Women: A Pooled Analysis of Cohort Studies. JAMA 1998, 279, 535–540. [Google Scholar] [CrossRef]
- Hillman, R.S.; Steinberg, S.E. The Effects of Alcohol on Folate Metabolism. Annu. Rev. Med. 1982, 33, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Gyürgy, P.; Eckardt, R.E. Vitamin B6 and Skin Lesions in Rats. Nature 1939, 144, 512. [Google Scholar] [CrossRef]
- Simpson, J.L.; Bailey, L.B.; Pietrzik, K.; Shane, B.; Holzgreve, W. Micronutrients and Women of Reproductive Potential: Required Dietary Intake and Consequences of Dietary Deficiency or Excess. Part I—Folate, Vitamin B12, Vitamin B6. J. Matern. Fetal Neonatal Med. 2010, 23, 1323–1343. [Google Scholar] [CrossRef] [PubMed]
- Manore, M.M. Effect of Physical Activity on Thiamine, Riboflavin, and Vitamin B-6 Requirements. Am. J. Clin. Nutr. 2000, 72, 598S–606S. [Google Scholar] [CrossRef]
- Raczynski, G.; Szczepanska, B. Longitudinal Studies on Vitamin B-1 and B-6 Status in Polish Elite Athletes. Biol. Sport 1993, 10, 189–194. [Google Scholar]
- Vakur Bor, M.; Fedosov, S.N.; Laursen, N.B.; Nexø, E. Recombinant Human Intrinsic Factor Expressed in Plants Is Suitable for Use in Measurement of Vitamin B12. Clin. Chem. 2003, 49, 2081–2083. [Google Scholar] [CrossRef][Green Version]
- Leklem, J.E.; Hollenbeck, C.B. Acute Ingestion of Glucose Decreases Plasma Pyridoxal 5’-Phosphate and Total Vitamin B-6 Concentration. Am. J. Clin. Nutr. 1990, 51, 832–836. [Google Scholar] [CrossRef]
- Schaeffer, M.C.; Sampson, D.A.; Skala, J.H.; Gietzen, D.W.; Grier, R.E. Evaluation of Vitamin B-6 Status and Function of Rats Fed Excess Pyridoxine. J. Nutr. 1989, 119, 1392–1398. [Google Scholar] [CrossRef]
- Leklem, J.E. Vitamin B-6: A Status Report. J. Nutr. 1990, 120, 1503–1507. [Google Scholar] [CrossRef]
- Ames, B.N.; Elson-Schwab, I.; Silver, E.A. High-Dose Vitamin Therapy Stimulates Variant Enzymes with Decreased Coenzyme Binding Affinity (Increased K(m)): Relevance to Genetic Disease and Polymorphisms. Am. J. Clin. Nutr. 2002, 75, 616–658. [Google Scholar] [CrossRef]
- Sherwood, S.R.A. Chapter 9—Methods for Assessment of Vitamin B6. In Laboratory Assessment of Vitamin Status; Harrington, D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 181–191. ISBN 9780128130506. [Google Scholar]
- Bisp, M.R.; Vakur Bor, M.; Heinsvig, E.-M.; Kall, M.A.; Nexø, E. Determination of Vitamin B6 Vitamers and Pyridoxic Acid in Plasma: Development and Evaluation of a High-Performance Liquid Chromatographic Assay. Anal. Biochem. 2002, 305, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.J.; Brain, A.; Reynolds, T.M.; Penny, M.; Holland, S. Relationship between Serum Alkaline Phosphatase and Pyridoxal-5′-Phosphate Levels in Hypophosphatasia. Clin. Sci. 1998, 94, 203–206. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Daub, H.; Olsen, J.V.; Bairlein, M.; Gnad, F.; Oppermann, F.S.; Körner, R.; Greff, Z.; Kéri, G.; Stemmann, O.; Mann, M. Kinase-Selective Enrichment Enables Quantitative Phosphoproteomics of the Kinome across the Cell Cycle. Mol. Cell 2008, 31, 438–448. [Google Scholar] [CrossRef]
- Akimov, V.; Barrio-Hernandez, I.; Hansen, S.V.F.; Hallenborg, P.; Pedersen, A.-K.; Bekker-Jensen, D.B.; Puglia, M.; Christensen, S.D.K.; Vanselow, J.T.; Nielsen, M.M.; et al. UbiSite Approach for Comprehensive Mapping of Lysine and N-Terminal Ubiquitination Sites. Nat. Struct. Mol. Biol. 2018, 25, 631–640. [Google Scholar] [CrossRef]
- Chen, C.-C.; Li, B.; Millman, S.E.; Chen, C.; Li, X.; Morris, J.P.; Mayle, A.; Ho, Y.-J.; Loizou, E.; Liu, H.; et al. Vitamin B6 Addiction in Acute Myeloid Leukemia. Cancer Cell 2020, 37, 71–84.e7. [Google Scholar] [CrossRef] [PubMed]
- Darin, N.; Reid, E.; Prunetti, L.; Samuelsson, L.; Husain, R.A.; Wilson, M.; El Yacoubi, B.; Footitt, E.; Chong, W.K.; Wilson, L.C.; et al. Mutations in PROSC Disrupt Cellular Pyridoxal Phosphate Homeostasis and Cause Vitamin-B6-Dependent Epilepsy. Am. J. Hum. Genet. 2016, 99, 1325–1337. [Google Scholar] [CrossRef]
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. A Vitamin B-6 Antagonist from Flaxseed Perturbs Amino Acid Metabolism in Moderately Vitamin B-6-Deficient Male Rats. J. Nutr. 2016, 146, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yasuhara, Y.; Yasuda, K. Effects of Cooking Methods on the Retention of Vitamin B6 in Foods, and the Approximate Cooking Loss in Daily Meals. J. Horne Econ. Jpn. 2001, 52, 1187–1197. [Google Scholar]
- Zintzaras, E. Methylenetetrahydrofolate Reductase Gene and Susceptibility to Breast Cancer: A Meta-Analysis. Clin. Genet. 2006, 69, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hoyumpa, A.M. Mechanisms of Vitamin Deficiencies in Alcoholism. Alcohol. Clin. Exp. Res. 1986, 10, 573–581. [Google Scholar] [CrossRef]
- Rojo-Sebastián, A.; González-Robles, C.; García de Yébenes, J. Vitamin B6 Deficiency in Patients with Parkinson Disease Treated with Levodopa/Carbidopa. Clin. Neuropharmacol. 2020, 43, 151–157. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. Monitoring Micronutrients in Cigarette Smokers. Clin. Chim. Acta 2007, 377, 14–38. [Google Scholar] [CrossRef]
- Marszałł, M.L.; Makarowski, R.; Hinc, S.; Kłos, M.; Czarnowski, W. [Hiperhomocysteinemia in active and passive smokers and the levels of folate and vitamin B6 in plasma]. Przegl. Lek. 2008, 65, 486–490. [Google Scholar] [PubMed]
- Guilarte, T.R. Vitamin B6 and Cognitive Development: Recent Research Findings from Human and Animal Studies. Nutr. Rev. 1993, 51, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lainé-Cessac, P.; Cailleux, A.; Allain, P. Mechanisms of the Inhibition of Human Erythrocyte Pyridoxal Kinase by Drugs. Biochem. Pharmacol. 1997, 54, 863–870. [Google Scholar] [CrossRef]
- Mccormick, D.B.; Snell, E.E. Pyridoxal Phosphokinases. II. Effects of Inhibitors. J. Biol. Chem. 1961, 236, 2085–2088. [Google Scholar] [CrossRef]
- Brown, M.J.; Ameer, M.A.; Beier, K. Vitamin B6 Deficiency; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://ncbi.nih.gov/books/NBK470579 (accessed on 10 August 2022).
- Inubushi, T.; Takasawa, T.; Tuboi, Y.; Watanabe, N.; Aki, K.; Katunuma, N. Changes of Glucose Metabolism and Skin-Collagen Neogenesis in Vitamin B6 Deficiency. BioFactors 2005, 23, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Vilter, R.W.; Mueller, J.F.; Glazer, H.S.; Jarrold, T.; Abraham, J.; Thompson, C.; Hawkins, V.R. The Effect of Vitamin B6 Deficiency Induced by Desoxypyridoxine in Human Beings. J. Lab. Clin. Med. 1953, 42, 335–357. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Cheng, C.-H.; Hsu, C.-L.; Lee, W.-J.; Huang, S.-C.; Huang, Y.-C. Role of Vitamin B6 Status on Antioxidant Defenses, Glutathione, and Related Enzyme Activities in Mice with Homocysteine-Induced Oxidative Stress. Food Nutr. Res. 2015, 59, 10–3402. [Google Scholar] [CrossRef]
- Zhang, P.; Suda, T.; Suidasari, S.; Kumrungsee, T.; Yanaka, N.; Kato, N. Chapter 15 - Novel Preventive Mechanisms of Vitamin B6 against Inflammation, Inflammasome, and Chronic Diseases. In Molecular Nutrition; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 283–299. ISBN 9780128119075. [Google Scholar]
- Wei, D.-H.; Mao, Q.-Q. Vitamin B6, Vitamin B12 and Methionine and Risk of Pancreatic Cancer: A Meta-Analysis. Nutr. J. 2020, 19, 111. [Google Scholar] [CrossRef]
- Mehrotra, R.; Zhang, M.; Li, Y. Chapter 25 - Nutrition and Anemia in End-Stage Renal Disease. In Nutritional Management of Renal Disease, 3rd ed.; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 383–391. ISBN 9780123919342. [Google Scholar]
- Yasuda, H.; Hatano, T.; Honda, T.; Tsutsui, M.; Hattori, N.; Ando, M.; Komatsu, N. Vitamin B6 Deficiency Anemia Attributed to Levodopa/Carbidopa Intestinal Gel Therapy for Parkinson’s Disease: A Diagnostic Pitfall for Myelodysplastic Syndrome with Ring Sideroblasts. Intern. Med. 2022, 9577–9622. [Google Scholar] [CrossRef]
- Calori, I.R.; Gusmão, L.A.; Tedesco, A.C. B6 Vitamers as Generators and Scavengers of Reactive Oxygen Species. J. Photochem. Photobiol. 2021, 7, 100041. [Google Scholar] [CrossRef]
- Matxain, J.M.; Ristilä, M.; Strid, A.; Eriksson, L.A. Theoretical Study of the Antioxidant Properties of Pyridoxine. J. Phys. Chem. A 2006, 110, 13068–13072. [Google Scholar] [CrossRef] [PubMed]
- Windebank, A.J.; Low, P.A.; Blexrud, M.D.; Schmelzer, J.D.; Schaumburg, H.H. Pyridoxine Neuropathy in Rats: Specific Degeneration of Sensory Axons. Neurology 1985, 35, 1617–1622. [Google Scholar] [CrossRef]
- Meyer, H.E.; Willett, W.C.; Fung, T.T.; Holvik, K.; Feskanich, D. Association of High Intakes of Vitamins B6 and B12 From Food and Supplements with Risk of Hip Fracture Among Postmenopausal Women in the Nurses’ Health Study. JAMA Netw. Open 2019, 2, e193591. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Wan, L.; Song, X.; Yuan, D.; Zhang, S.; Wu, D.; Jiang, J. Vitamin B6 as a Novel Risk Biomarker of Fractured Ankles. Medicine 2021, 100, e27442. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Pellagra and Alcoholism: A Biochemical Perspective. Alcohol Alcohol. 2014, 49, 238–250. [Google Scholar] [CrossRef]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef]
- Pais, R.C.; Vanous, E.; Hollins, B.; Faraj, B.A.; Davis, R.; Camp, V.M.; Ragab, A.H. Abnormal Vitamin B6 Status in Childhood Leukemia. Cancer 1990, 66, 2421–2428. [Google Scholar] [CrossRef]
- Gylling, B.; Myte, R.; Schneede, J.; Hallmans, G.; Häggström, J.; Johansson, I.; Ulvik, A.; Ueland, P.M.; Van Guelpen, B.; Palmqvist, R. Vitamin B-6 and Colorectal Cancer Risk: A Prospective Population-Based Study Using 3 Distinct Plasma Markers of Vitamin B-6 Status. Am. J. Clin. Nutr. 2017, 105, 897–904. [Google Scholar] [CrossRef]
- Mills, P.B.; Camuzeaux, S.S.M.; Footitt, E.J.; Mills, K.A.; Gissen, P.; Fisher, L.; Das, K.B.; Varadkar, S.M.; Zuberi, S.; McWilliam, R.; et al. Epilepsy Due to PNPO Mutations: Genotype, Environment and Treatment Affect Presentation and Outcome. Brain 2014, 137, 1350–1360. [Google Scholar] [CrossRef]
- Guo, R.; Liang, J.H.; Zhang, Y.; Lutchenkov, M.; Li, Z.; Wang, Y.; Trujillo-Alonso, V.; Puri, R.; Giulino-Roth, L.; Gewurz, B.E. Methionine Metabolism Controls the B Cell EBV Epigenome and Viral Latency. Cell Metab. 2022, 34, 1280–1297. [Google Scholar] [CrossRef]
- Zhang, P.; Suidasari, S.; Hasegawa, T.; Yanaka, N.; Kato, N. Vitamin B6 Activates P53 and Elevates P21 Gene Expression in Cancer Cells and the Mouse Colon. Oncol. Rep. 2014, 31, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Senovilla, L.; Olaussen, K.A.; Pinna, G.; Eisenberg, T.; Goubar, A.; Martins, I.; Michels, J.; Kratassiouk, G.; et al. Prognostic Impact of Vitamin B6 Metabolism in Lung Cancer. Cell Rep. 2012, 2, 257–269. [Google Scholar] [CrossRef] [PubMed]
- The Lung Cancer Cohort Consortium Circulating Folate, Vitamin B6, and Methionine in Relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3). JNCI J. Natl. Cancer Inst. 2018, 110, 57–67. [CrossRef]
- Brasky, T.M.; White, E.; Chen, C.-L. Long-Term, Supplemental, One-Carbon Metabolism–Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J. Clin. Oncol. 2017, 35, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2017, 109, djw230. [Google Scholar] [CrossRef]
- Taş, S.; Sarandöl, E.; Dirican, M. Vitamin B6 Supplementation Improves Oxidative Stress and Enhances Serum Paraoxonase/Arylesterase Activities in Streptozotocin-Induced Diabetic Rats. Sci. World J. 2014, 2014, 351598. [Google Scholar] [CrossRef]
- Mills, P.B.; Surtees, R.A.H.; Champion, M.P.; Beesley, C.E.; Dalton, N.; Scambler, P.J.; Heales, S.J.R.; Briddon, A.; Scheimberg, I.; Hoffmann, G.F.; et al. Neonatal Epileptic Encephalopathy Caused by Mutations in the PNPO Gene Encoding Pyridox(Am)Ine 5′-Phosphate Oxidase. Hum. Mol. Genet. 2005, 14, 1077–1086. [Google Scholar] [CrossRef]
- Chelban, V.; Wilson, M.P.; Warman Chardon, J.; Vandrovcova, J.; Zanetti, M.N.; Zamba-Papanicolaou, E.; Efthymiou, S.; Pope, S.; Conte, M.R.; Abis, G.; et al. PDXK Mutations Cause Polyneuropathy Responsive to Pyridoxal 5′-phosphate Supplementation. Ann. Neurol. 2019, 86, 225–240. [Google Scholar] [CrossRef]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Vitamin B6 Intake, Alcohol Consumption, and Colorectal Cancer: A Longitudinal Population-Based Cohort of Women. Gastroenterology 2005, 128, 1830–1837. [Google Scholar] [CrossRef]
- Lu, M.; Sanderson, S.M.; Zessin, A.; Ashcraft, K.A.; Jones, L.W.; Dewhirst, M.W.; Locasale, J.W.; Hsu, D.S. Exercise Inhibits Tumor Growth and Central Carbon Metabolism in Patient-Derived Xenograft Models of Colorectal Cancer. Cancer Metab. 2018, 6, 14. [Google Scholar] [CrossRef]
- Hartman, T.J.; Woodson, K.; Stolzenberg-Solomon, R.; Virtamo, J.; Selhub, J.; Barrett, M.J.; Albanes, D. Association of the B-Vitamins Pyridoxal 5′-Phosphate (B6), B12, and Folate with Lung Cancer Risk in Older Men. Am. J. Epidemiol. 2001, 153, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Shrubsole, M.J.; Jin, F.; Dai, Q.; Shu, X.O.; Potter, J.D.; Hebert, J.R.; Gao, Y.T.; Zheng, W. Dietary Folate Intake and Breast Cancer Risk: Results from the Shanghai Breast Cancer Study. Cancer Res. 2001, 61, 7136–7141. [Google Scholar] [PubMed]
- Shrubsole, M.J.; Gao, Y.-T.; Cai, Q.; Shu, X.O.; Dai, Q.; Hébert, J.R.; Jin, F.; Zheng, W. MTHFR Polymorphisms, Dietary Folate Intake, and Breast Cancer Risk: Results from the Shanghai Breast Cancer Study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 190–196. [Google Scholar] [CrossRef]
- Vidal, A.C.; Grant, D.J.; Williams, C.D.; Masko, E.; Allott, E.H.; Shuler, K.; McPhail, M.; Gaines, A.; Calloway, E.; Gerber, L.; et al. Associations between Intake of Folate, Methionine, and Vitamins B-12, B-6 and Prostate Cancer Risk in American Veterans. J. Cancer Epidemiol. 2012, 2012, 957467. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.A.; Silcocks, P.B.; Davey, G.K.; Appleby, P.N.; Bishop, D.T. A Case-Control Study of Diet and Prostate Cancer. Br. J. Cancer 1997, 76, 678–687. [Google Scholar] [CrossRef] [PubMed]
- DiSorbo, D.M.; Wagner, R.; Nathanson, L. In Vivo and in Vitro Inhibition of B16 Melanoma Growth by Vitamin B6. Nutr. Cancer 1985, 7, 43–52. [Google Scholar] [CrossRef]
- Matsuo, T.; Fujiwara, A.; Nakamura, K.; Sadzuka, Y. The Effects of Vitamin B6 Compounds on Cell Proliferation and Melanogenesis in B16F10 Melanoma Cells. Oncol. Lett. 2018, 15, 5181–5184. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. The Effects of B and D Vitamins in Renal Cell Cancer. Available online: https://www.wcrf.org/researchwefund/evaluating-effects-b-d-vitamins-renal-cancer-large-european-prospective-studies/ (accessed on 7 September 2022).
- Muller, D.C.; Johansson, M.; Zaridze, D.; Moukeria, A.; Janout, V.; Holcatova, I.; Navratilova, M.; Mates, D.; Midttun, Ø.; Ueland, P.M.; et al. Circulating Concentrations of Vitamin B6 and Kidney Cancer Prognosis: A Prospective Case-Cohort Study. PLoS ONE 2015, 10, e0140677. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Huang, J.Y.; Butler, L.M.; Wang, R.; Jin, A.; Koh, W.-P.; Yuan, J.-M. Dietary Intake of One-Carbon Metabolism–Related Nutrients and Pancreatic Cancer Risk: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 417–424. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Nie, D.Y.; Ba-alawi, W.; Ji, Y.; Zhang, Z.; Cruickshank, J.; Haight, J.; Ciamponi, F.E.; Chen, J.; Duan, S.; et al. PRMT Inhibition Induces a Viral Mimicry Response in Triple-Negative Breast Cancer. Nat. Chem. Biol. 2022, 18, 821–830. [Google Scholar] [CrossRef]
- Bender, D.A. Novel Functions of Vitamin B6. Proc. Nutr. Soc. 1994, 53, 625–630. [Google Scholar] [CrossRef] [PubMed]
- The ABCs of Vitamins for Kidney Patients . Available online: https://www.davita.com/diet-nutrition/articles/basics/the-abcs-of-vitamins-for-kidney-patients (accessed on 7 September 2022).
- Dankner, R.; Boffetta, P.; Balicer, R.D.; Boker, L.K.; Sadeh, M.; Berlin, A.; Olmer, L.; Goldfracht, M.; Freedman, L.S. Time-Dependent Risk of Cancer After a Diabetes Diagnosis in a Cohort of 2.3 Million Adults. Am. J. Epidemiol. 2016, 183, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Wachstein, M. Evidence for a Relative Vitamin B6 Deficiency in Pregnancy and Some Disease States. Vitam. Horm. 1964, 22, 705–719. [Google Scholar] [CrossRef]
- Fuso, A.; Cavallaro, R.A.; Zampelli, A.; D’Anselmi, F.; Piscopo, P.; Confaloni, A.; Scarpa, S. Gamma-Secretase Is Differentially Modulated by Alterations of Homocysteine Cycle in Neuroblastoma and Glioblastoma Cells. J. Alzheimers Dis. JAD 2007, 11, 275–290. [Google Scholar] [CrossRef]
- Storvick, C.A.; Peters, J.M. Methods for the Determination of Vitamin B6 in Biological Materials. Vitam. Horm. 1964, 22, 833–854. [Google Scholar] [CrossRef]
- Bird, O.D.; McGlohon, V.M.; Vaitkus, J.W. A Microbiological Assay System for Naturally Occurring Folates. Can. J. Microbiol. 1969, 15, 465–472. [Google Scholar] [CrossRef]
- Sobczyńska-Malefora, A. Chapter 11—Methods for Assessment of Folate (Vitamin B9). In Laboratory Assessment of Vitamin Status; Harrington, D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 219–264. ISBN 9780128130506. [Google Scholar]
- Shamir, M.; Bar-On, Y.; Phillips, R.; Milo, R. SnapShot: Timescales in Cell Biology. Cell 2016, 164, 1302.e1. [Google Scholar] [CrossRef]
- Cantor, J.R.; Abu-Remaileh, M.; Kanarek, N.; Freinkman, E.; Gao, X.; Louissaint, A.; Lewis, C.A.; Sabatini, D.M. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 2017, 169, 258–272.e17. [Google Scholar] [CrossRef]
- Chen, L.; Ducker, G.S.; Lu, W.; Teng, X.; Rabinowitz, J.D. An LC-MS Chemical Derivatization Method for the Measurement of Five Different One-Carbon States of Cellular Tetrahydrofolate. Anal. Bioanal. Chem. 2017, 409, 5955–5964. [Google Scholar] [CrossRef] [PubMed]
- Schittmayer, M.; Birner-Gruenberger, R.; Zamboni, N. Quantification of Cellular Folate Species by LC-MS after Stabilization by Derivatization. Anal. Chem. 2018, 90, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S. Multiplex Measurement of Serum Folate Vitamers by UPLC-MS/MS. Methods Mol. Biol. 2017, 1546, 245–256. [Google Scholar] [CrossRef]
- Garrett, M.; Sperry, J.; Braas, D.; Yan, W.; Le, T.M.; Mottahedeh, J.; Ludwig, K.; Eskin, A.; Qin, Y.; Levy, R.; et al. Metabolic Characterization of Isocitrate Dehydrogenase (IDH) Mutant and IDH Wildtype Gliomaspheres Uncovers Cell Type-Specific Vulnerabilities. Cancer Metab. 2018, 6, 4. [Google Scholar] [CrossRef]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Bae, H.; Lam, K.; Jang, C. Metabolic Flux between Organs Measured by Arteriovenous Metabolite Gradients. Exp. Mol. Med. 2022, 1354–1366. [Google Scholar] [CrossRef]
- Van Outersterp, R.E.; Engelke, U.F.H.; Merx, J.; Berden, G.; Paul, M.; Thomulka, T.; Berkessel, A.; Huigen, M.C.D.G.; Kluijtmans, L.A.J.; Mecinović, J.; et al. Metabolite Identification Using Infrared Ion Spectroscopy—Novel Biomarkers for Pyridoxine-Dependent Epilepsy. Anal. Chem. 2021, 93, 15340–15348. [Google Scholar] [CrossRef]
- Van der Ham, M.; Albersen, M.; de Koning, T.J.; Visser, G.; Middendorp, A.; Bosma, M.; Verhoeven-Duif, N.M.; de Sain-van der Velden, M.G.M. Quantification of Vitamin B6 Vitamers in Human Cerebrospinal Fluid by Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chim. Acta 2012, 712, 108–114. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Franco, C.N.; Noe, M.M.; Albrecht, L.V. Metabolism and Endocrine Disorders: What Wnt Wrong? Front. Endocrinol. 2022, 13, 887037. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Azari, F.S.; Bernstein, E.; Nadeem, B.; Chang, A.; Segil, A.; Sullivan, N.; Encarnado, E.; Desphande, C.; Kucharczuk, J.C.; et al. Targeted Detection of Cancer Cells during Biopsy Allows Real-Time Diagnosis of Pulmonary Nodules. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4194–4204. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Hebenbrock, M.; Kool, E.T. A Fluorescent Hydrazone Exchange Probe of Pyridoxal Phosphate for the Assessment of Vitamin B6 Status. Chem. Commun. 2019, 56, 317–320. [Google Scholar] [CrossRef]
- Brun, E.M.S.P.-T.; Calvert, N.D.; Suchý, M.; Kirby, A.; Melkus, G.; Garipov, R.; Addison, C.L.; Shuhendler, A.J. Mapping Vitamin B6 Metabolism by HydrazoCEST Magnetic Resonance Imaging. Chem. Commun. 2021, 57, 10867–10870. [Google Scholar] [CrossRef]
- Nahvi, A.; Sudarsan, N.; Ebert, M.S.; Zou, X.; Brown, K.L.; Breaker, R.R. Genetic Control by a Metabolite Binding MRNA. Chem. Biol. 2002, 9, 1043–1049. [Google Scholar] [CrossRef]
- Li, X.; Mo, L.; Litke, J.L.; Dey, S.K.; Suter, S.R.; Jaffrey, S.R. Imaging Intracellular S-Adenosyl Methionine Dynamics in Live Mammalian Cells with a Genetically Encoded Red Fluorescent RNA-Based Sensor. J. Am. Chem. Soc. 2020, 142, 14117–14124. [Google Scholar] [CrossRef] [PubMed]
- Bery, N.; Cruz-Migoni, A.; Bataille, C.J.; Quevedo, C.E.; Tulmin, H.; Miller, A.; Russell, A.; Phillips, S.E.; Carr, S.B.; Rabbitts, T.H. BRET-Based RAS Biosensors That Show a Novel Small Molecule Is an Inhibitor of RAS-Effector Protein-Protein Interactions. eLife 2018, 7, e37122. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Nguyen-Duc, T.; Song, W.; Jaffrey, S.R. Fluorescence Imaging of Cellular Metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ishibe-Murakami, S.; Patel, D.J.; Serganov, A. Long-Range Pseudoknot Interactions Dictate the Regulatory Response in the Tetrahydrofolate Riboswitch. Proc. Natl. Acad. Sci. USA 2011, 108, 14801–14806. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, G.; Zhang, Y.; Chen, T.; Feng, S.; Cai, R.; Lu, C. The Second Class of Tetrahydrofolate (THF-II) Riboswitches Recognizes the Tetrahydrofolic Acid Ligand via Local Conformation Changes. Int. J. Mol. Sci. 2022, 23, 5903. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Litke, J.L.; Jaffrey, S.R. Imaging Metabolite Dynamics in Living Cells Using a Spinach-Based Riboswitch. Proc. Natl. Acad. Sci. USA 2015, 112, E2756–E2765. [Google Scholar] [CrossRef]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch Diversity and Distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef]
- Liu, X.-H.; Li, B.-R.; Ying, Z.-M.; Tang, L.-J.; Wang, F.; Jiang, J.-H. Small-Molecule-Mediated Split-Aptamer Assembly for Inducible CRISPR-DCas9 Transcription Activation. ACS Chem. Biol. 2022, 17, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine Catabolism Is a Major Determinant of Methotrexate Sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef]
- Puig, L. Methotrexate: New Therapeutic Approaches. Actas Dermo-Sifiliográficas Engl. Ed. 2014, 105, 583–589. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Gorlick, R.; Goker, E.; Trippett, T.; Waltham, M.; Banerjee, D.; Bertino, J.R. Intrinsic and Acquired Resistance to Methotrexate in Acute Leukemia. N. Engl. J. Med. 1996, 335, 1041–1048. [Google Scholar] [CrossRef]
- Huennekens, F.M. The Methotrexate Story: A Paradigm for Development of Cancer Chemotherapeutic Agents. Adv. Enzyme Regul. 1994, 34, 397–419. [Google Scholar] [CrossRef]
- Petrova, B.; Kanarek, N. Potential Benefits and Pitfalls of Histidine Supplementation for Cancer Therapy Enhancement. J. Nutr. 2020, 150, 2580S–2587S. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.R.; Darnell, A.M.; Reilly, M.F.; Kunchok, T.; Joesch-Cohen, L.; Rosenberg, D.; Ali, A.; Rees, M.G.; Roth, J.A.; Lewis, C.A.; et al. Methionine Synthase Is Essential for Cancer Cell Proliferation in Physiological Folate Environments. Nat. Metab. 2021, 3, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Ghergurovich, J.M.; Xu, X.; Wang, J.Z.; Yang, L.; Ryseck, R.-P.; Wang, L.; Rabinowitz, J.D. Methionine Synthase Supports Tumour Tetrahydrofolate Pools. Nat. Metab. 2021, 3, 1512–1520. [Google Scholar] [CrossRef]
- Duschinsky, R.; Edward, P.; Heidelberger, C. The Synthesis of 5-Fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Izzo, L.T.; Affronti, H.C.; Wellen, K.E. The Bidirectional Relationship Between Cancer Epigenetics and Metabolism. Annu. Rev. Cancer Biol. 2021, 5, 235–257. [Google Scholar] [CrossRef]


| Enzymes | Function | PTM | Regulation | Cofactor | Ref. |
|---|---|---|---|---|---|
| BHMT | Methionine from betaine and homocysteine | Acetylation (K232,283) Phosphorylation (T45, Y363, S366) | Homocysteine: Methionine | Zn2+ | [18,19] |
| DHFR | DHF reduction to THF | Acetylation (R33, K174) Phosphorylation (S145, Y183) R-monomethylation (R29) Ubiquitylation (K47, K153) | THF | NADPH | [20,21,22,23] |
| MAT | SAM synthesis | R-monomethylation (R264) Phosphorylation (S114, Y296) Ubiquitylation (K351) | SAM | ATP, H2O, methionine | [23,24,25] |
| MS | Methionine synthesis | Phosphorylation (T1264) | Methionine | Cobalamin, Zn2+ | [26] |
| MTHFD | Tetrahydrofolate interconversion | R-monomethylation (R37, R324, R495) | THF | ATP, NADPH, H2O | [27] |
| MTHFR | 5-MTHF synthesis | Phosphorylation (S9, 10, 19, 20, 21, 23, 25, 26, 29, 30, 103, 394; T34, 94, 451; Y90) | 5-MTHF | FAD | [28,29] |
| SHMT | 5,10-MTHF and glycine synthesis | Acetylation (K271) Phosphorylation (Y34) | THF | Serine | [30] |
| PDXK | PLP synthesis | Acetylation (K76) Phosphorylation (S59, 164, 213, 285) | PLP | ATP | [31] |
| PNPO | PLP synthesis | Phosphorylation (S231, T238) | PLP | O2 | [32,33] |
| TS | DHF synthesis | Phosphorylation (S114, Y153) Ubiquitylation (K169, K308) | dTMP | - | [23,34] |
| Cancer | B9 | Model | Readout | Cellular and Tissue Response | Ref. |
|---|---|---|---|---|---|
| Lung | Up | Meta-analysis | Cancer incidence |
| [60] |
| Down | Case-control study | Cancer incidence | MTHFR C677TT genotype correlated with decreased risk in women | [61] | |
| Colon | Down |
|
|
| [62,63,64] |
| None |
|
|
| [65,66] | |
| Ovarian | None | Meta-analysis | Dietary and total folate intake | No association between folate and risk | [67] |
| Up | Tumor biopsy | p53 and MDM2 tissue expression | Folate receptor (FR) increases chemotherapy resistance by stabilizing MDM2 | [68,69] | |
| Down | Meta-analysis | Dietary folate intake | Inverse association between folate and risk | [70] | |
| Pancreatic | None | Meta-analysis | Dietary folate intake | Inconsistent results linking dietary folate intake with risk | [71] |
| Down | Meta-analysis | Dietary folate intake |
| [71,72,73] | |
| Prostate | None | Meta-analysis | Serum folate levels | No association between folate and risk | [74,75] |
| Up |
| Serum folate levels |
| [76,77] | |
| Down | Case-control study | Serum folate, homocysteine, and B12 levels and 5,10-MTHFR polymorphism | Low folate and high homocysteine associated with increased risk | [78] | |
| Breast | None | Meta-analysis | Dietary folate intake | No association between folate and risk | [72,79,80] |
| Down/Up | Systematic review | Serum folate levels | Dietary intake between 153–400 ug/day correlated with reduced risk. More pronounced in women with high alcohol consumption | [79] |
| Cancer | B6 | Model | Readout | Cellular and Tissue Response | Ref. |
|---|---|---|---|---|---|
| AML | Up | CRISPR-Cas9 screen | Cancer incidence | AML addiction to PLP; PDXK disruption inhibited AML proliferation | [136] |
| Down | Clinical | Serum PLP levels | Low vitamin B6 levels associated with increased cancer risk | [166] | |
| Colon | Down |
|
|
| [167,168,169] |
| Down | Xenograft mouse model | Tumor volume | Vitamin B6 elevated in exercising mice associated with slowed tumor growth | [170] | |
| Lung | Down |
|
|
| [171,172] |
| Up |
|
|
| [173,174] | |
| None | Systematic review | Dietary PLP intake and serum or blood PLP levels | No association between vitamin B6 and lung tumor sites | [175] | |
| Breast | Up | Population-based case-control study | Dietary PLP intake and serum PLP levels | Breast cancer patients displayed higher serum vitamin B6 levels | [176] |
| Down | Population-based case-control study | Dietary PLP intake and serum PLP levels | Vitamin B6 increase folate’s chemoprotective effect, lowering breast cancer risk | [176] | |
| None | Population-based case-control study | Dietary PLP intake and serum PLP levels; PCR-RFLP-based assay | No association between high vitamin B6 intake or serum levels with cancer risk | [177] | |
| Pancreatic | Down |
|
|
| [32,178] |
| Prostate | None |
|
| No association between PLP and cancer risk | [175,179] |
| Down | Case-control study | Dietary PLP intake | Low vitamin B6 levels associated with increased cancer risk; organ sensitivity to hormone action increased with low levels of vitamin B6 | [180] | |
| Skin | Down |
|
|
| [181,182] |
| Kidney | Down | Case-cohort study | Plasma PLP levels | High vitamin B6 levels associated with decreased risk of cancer and better prognosis | [183,184] |
| None | Meta-analysis | Dietary PLP intake | No association between vitamin B6 intake and kidney tumors | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, C.N.; Seabrook, L.J.; Nguyen, S.T.; Leonard, J.T.; Albrecht, L.V. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites 2022, 12, 961. https://doi.org/10.3390/metabo12100961
Franco CN, Seabrook LJ, Nguyen ST, Leonard JT, Albrecht LV. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites. 2022; 12(10):961. https://doi.org/10.3390/metabo12100961
Chicago/Turabian StyleFranco, Carolina N., Laurence J. Seabrook, Steven T. Nguyen, Jack T. Leonard, and Lauren V. Albrecht. 2022. "Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond" Metabolites 12, no. 10: 961. https://doi.org/10.3390/metabo12100961
APA StyleFranco, C. N., Seabrook, L. J., Nguyen, S. T., Leonard, J. T., & Albrecht, L. V. (2022). Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites, 12(10), 961. https://doi.org/10.3390/metabo12100961









