The Impact of Maternal Folates on Brain Development and Function after Birth
Abstract
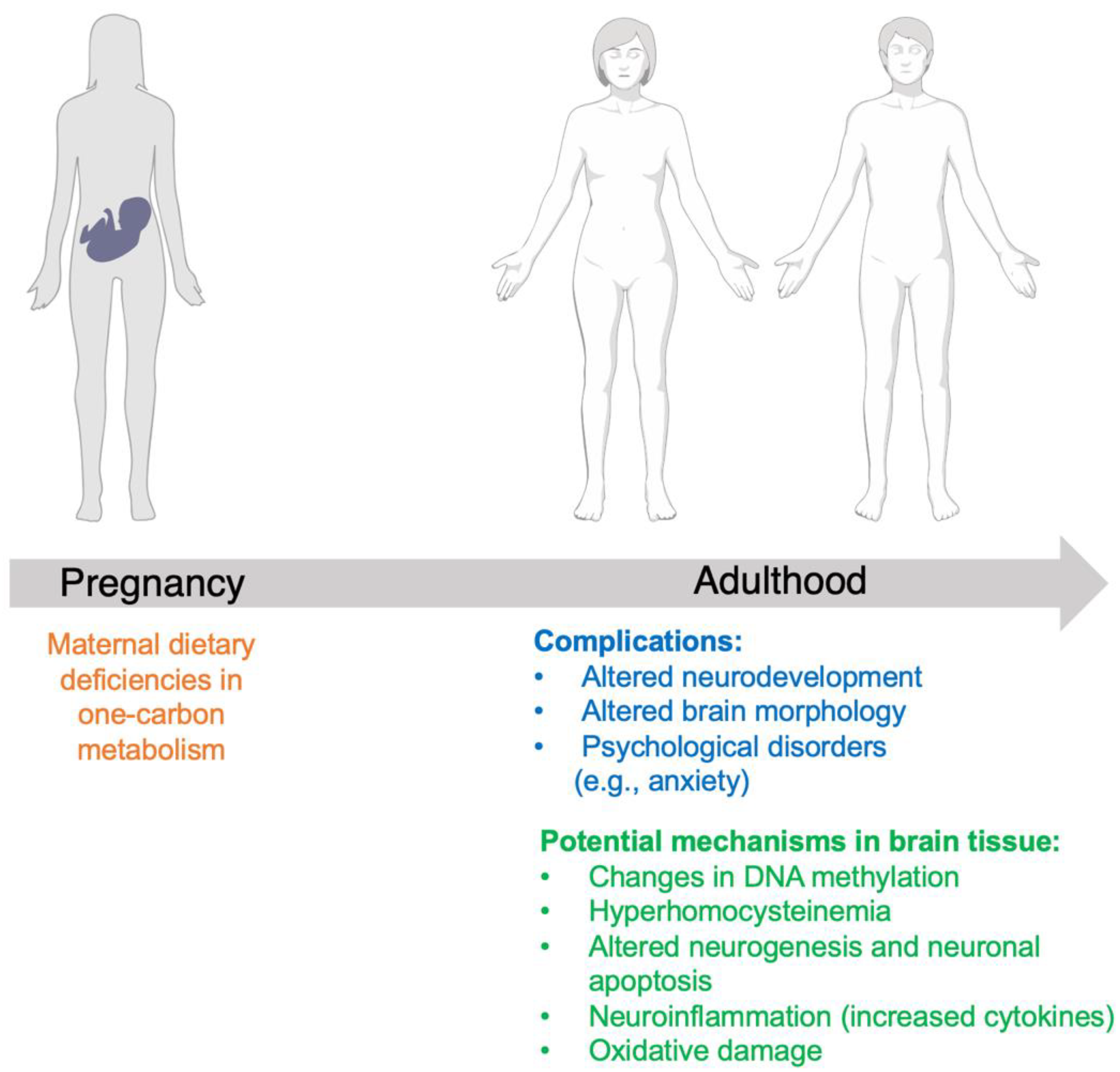
1. Introduction
2. The Impact of Maternal Folate Levels on Offspring Neurological Function in Clinical Populations
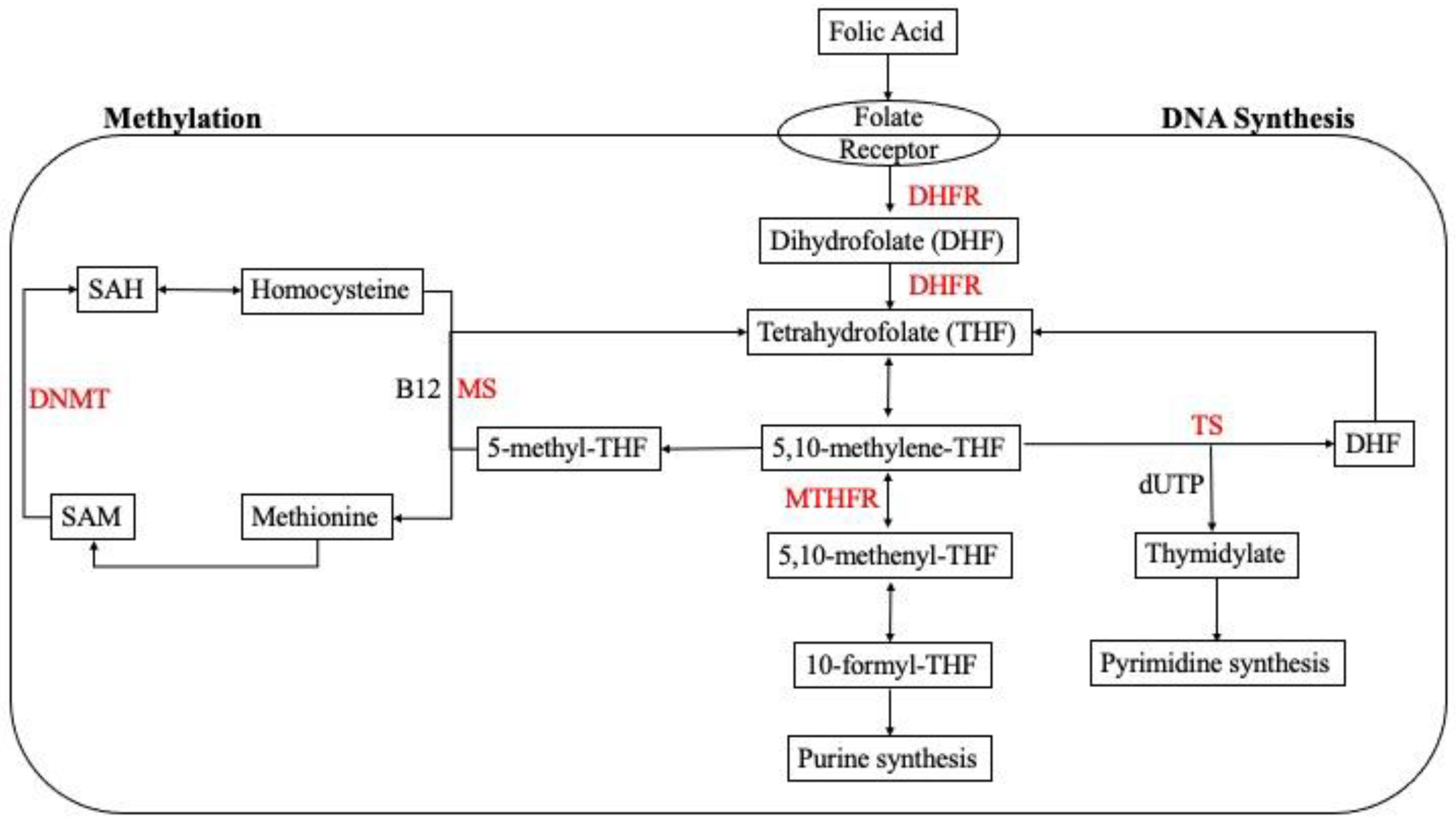
3. Folate Mechanism of Action
3.1. The Impact of Folate Deficiency on Cortical Morphology and Neurogenesis
3.2. Altered Neurogenesis and Apoptosis in the Fetal Brain
3.3. Neuroinflammatory Response to Folate Deficiency and Altered Behavior
3.4. Metabolic and Proteomic Changes in Response to Folic Acid Supplementation
3.5. The Role of Folate Deficiency in Epigenetic Modification: Methylation
3.6. Altered Neurodevelopment and Neurobehavior
3.7. MTHFR Deficiency and Hippocampal Structural Change
4. Discussion
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, Folic Acid and 5-Methyltetrahydrofolate Are Not the Same Thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y. Folic Acid Supplementation and Pregnancy: More Than Just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar] [PubMed]
- Merrell, B.J.; McMurry, J.P. Folic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stamm, R.A.; Houghton, L.A. Nutrient Intake Values for Folate during Pregnancy and Lactation Vary Widely around the World. Nutrients 2013, 5, 3920–3947. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Jialal, I. Folic Acid Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Breimer, L.H.; Nilsson, T.K. Has Folate a Role in the Developing Nervous System after Birth and Not Just during Embryogenesis and Gestation? Scand. J. Clin. Lab. Investig. 2012, 72, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Obermann-Borst, S.A.; Kremer, D.; Lindemans, J.; Siebel, C.; Steegers, E.A.; Slagboom, P.E.; Heijmans, B.T. Periconceptional Maternal Folic Acid Use of 400 Μg per Day Is Related to Increased Methylation of the IGF2 Gene in the Very Young Child. PLoS ONE 2009, 4, e7845. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal Use of Folic Acid Supplements during Pregnancy and Four-Year-Old Neurodevelopment in a Population-Based Birth Cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Schlotz, W.; Jones, A.; Phillips, D.I.W.; Gale, C.R.; Robinson, S.M.; Godfrey, K.M. Lower Maternal Folate Status in Early Pregnancy Is Associated with Childhood Hyperactivity and Peer Problems in Offspring. J. Child Psychol. Psychiatry 2010, 51, 594–602. [Google Scholar] [CrossRef]
- Roth, C.; Magnus, P.; Schjølberg, S.; Stoltenberg, C.; Surén, P.; McKeague, I.W.; Davey Smith, G.; Reichborn-Kjennerud, T.; Susser, E. Folic Acid Supplements in Pregnancy and Severe Language Delay in Children. JAMA 2011, 306, 1566–1573. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Irwin, R.E.; Walsh, C.P.; Pentieva, K. Maternal Folate Nutrition and Offspring Health: Evidence and Current Controversies. Proc. Nutr. Soc. 2019, 78, 208–220. [Google Scholar] [CrossRef]
- Chatzi, L.; Papadopoulou, E.; Koutra, K.; Roumeliotaki, T.; Georgiou, V.; Stratakis, N.; Lebentakou, V.; Karachaliou, M.; Vassilaki, M.; Kogevinas, M. Effect of High Doses of Folic Acid Supplementation in Early Pregnancy on Child Neurodevelopment at 18 Months of Age: The Mother-Child Cohort “Rhea” Study in Crete, Greece. Public Health Nutr. 2012, 15, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; den Dekker, H.T.; Felix, J.F.; Bohlin, J.; Ligthart, S.; Beckett, E.; Tiemeier, H.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; et al. Maternal Plasma Folate Impacts Differential DNA Methylation in an Epigenome-Wide Meta-Analysis of Newborns. Nat. Commun. 2016, 7, 10577. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.-A.; Cassidy, T.; McLaughlin, M.; Pentieva, K.; McNulty, H.; Walsh, C.P.; Lees-Murdock, D. Folic Acid Supplementation throughout Pregnancy: Psychological Developmental Benefits for Children. Acta Paediatr. 2018, 107, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, H.; Dowling, K.F.; Huntington, F.C.; Rodriguez-Thompson, A.; Soare, T.W.; Beard, L.M.; Lee, H.; Blossom, J.C.; Gollub, R.L.; Susser, E.; et al. Association of Prenatal Exposure to Population-Wide Folic Acid Fortification With Altered Cerebral Cortex Maturation in Youths. JAMA Psychiatry 2018, 75, 918–928. [Google Scholar] [CrossRef]
- Huang, X.; Ye, Y.; Li, Y.; Zhang, Y.; Zhang, Y.; Jiang, Y.; Chen, X.; Wang, L.; Yan, W. Maternal Folate Levels during Pregnancy and Children’s Neuropsychological Development at 2 Years of Age. Eur. J. Clin. Nutr. 2020, 74, 1585–1593. [Google Scholar] [CrossRef]
- Zou, R.; El Marroun, H.; Cecil, C.; Jaddoe, V.W.V.; Hillegers, M.; Tiemeier, H.; White, T. Maternal Folate Levels during Pregnancy and Offspring Brain Development in Late Childhood. Clin. Nutr. Edinb. Scotl. 2021, 40, 3391–3400. [Google Scholar] [CrossRef]
- Craciunescu, C.N.; Brown, E.C.; Mar, M.-H.; Albright, C.D.; Nadeau, M.R.; Zeisel, S.H. Folic Acid Deficiency during Late Gestation Decreases Progenitor Cell Proliferation and Increases Apoptosis in Fetal Mouse Brain. J. Nutr. 2004, 134, 162–166. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Achterfeldt, S.; Gorniak, J.P.; Halley-Hogg, K.J.A.; Oxley, D.; van Schooten, F.J.; Godschalk, R.W.L.; McKay, J.A.; Mathers, J.C. Maternal Folate Depletion and High-Fat Feeding from Weaning Affects DNA Methylation and DNA Repair in Brain of Adult Offspring. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 3323–3334. [Google Scholar] [CrossRef]
- Berrocal-Zaragoza, M.I.; Sequeira, J.M.; Murphy, M.M.; Fernandez-Ballart, J.D.; Baki, S.G.A.; Bergold, P.J.; Quadros, E.V. Folate Deficiency in Rat Pups during Weaning Causes Learning and Memory Deficits. Br. J. Nutr. 2014, 112, 1323–1332. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Deng, L.; Malysheva, O.; Caudill, M.A.; Rozen, R. MTHFR Deficiency or Reduced Intake of Folate or Choline in Pregnant Mice Results in Impaired Short-Term Memory and Increased Apoptosis in the Hippocampus of Wild-Type Offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Ly, A.; Ishiguro, L.; Kim, D.; Im, D.; Kim, S.-E.; Sohn, K.-J.; Croxford, R.; Kim, Y.-I. Maternal Folic Acid Supplementation Modulates DNA Methylation and Gene Expression in the Rat Offspring in a Gestation Period-Dependent and Organ-Specific Manner. J. Nutr. Biochem. 2016, 33, 103–110. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Ted Brown, W.; Junaid, M.A. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a Sex-Specific Manner in Mouse Offspring. J. Mol. Neurosci. 2016, 58, 277–286. [Google Scholar] [CrossRef]
- Bahous, R.H.; Jadavji, N.M.; Deng, L.; Cosín-Tomás, M.; Lu, J.; Malysheva, O.; Leung, K.-Y.; Ho, M.-K.; Pallás, M.; Kaliman, P.; et al. High Dietary Folate in Pregnant Mice Leads to Pseudo-MTHFR Deficiency and Altered Methyl Metabolism, with Embryonic Growth Delay and Short-Term Memory Impairment in Offspring. Hum. Mol. Genet. 2017, 26, 888–900. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, S.; Yan, J.; Wilson, J.X.; Huang, G. Maternal Folic Acid Supplementation During Pregnancy Improves Neurobehavioral Development in Rat Offspring. Mol. Neurobiol. 2018, 55, 2676–2684. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, D.; Wu, R.; Shi, R.; Shen, X.; Jin, N.; Gu, J.; Gu, J.-H.; Liu, F.; Chu, D. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Activates β-Catenin in the Brain of Male Mouse Offspring. Brain Res. Bull. 2022, 178, 133–143. [Google Scholar] [CrossRef]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.-Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Li, Y.; Sun, C. Integration of Metabolomics and Proteomics to Highlight Altered Neural Development Related Pathways in the Adult Offspring after Maternal Folic Acid Supplement. Clin. Nutr. Edinb. Scotl. 2021, 40, 476–487. [Google Scholar] [CrossRef]
- Garcez, M.L.; Mina, F.; Bellettini-Santos, T.; Ribeiro, F.M.; Ghisi Frassetto, A.Z.; Batista-Silva, H.; da Luz, A.P.; Schiavo, G.L.; Medeiros, E.B.; Zabot, G.C.; et al. Folic Acid Supplementation in the Gestational Phase of Female Rats Improves Age-Related Memory Impairment and Neuroinflammation in Their Adult and Aged Offspring. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 991–995. [Google Scholar] [CrossRef]
- Yang, X.; Sun, W.; Wu, Q.; Lin, H.; Lu, Z.; Shen, X.; Chen, Y.; Zhou, Y.; Huang, L.; Wu, F.; et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients 2021, 14, 66. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.; Roza, S.J.; Steegers, E.A.; Hofman, A.; Verhulst, F.C.; Jaddoe, V.W.; Tiemeier, H. Maternal Folate Status in Early Pregnancy and Child Emotional and Behavioral Problems: The Generation R Study. Am. J. Clin. Nutr. 2012, 95, 1413–1421. [Google Scholar] [CrossRef]
- Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; Ziller, M.J.; et al. Integrative Analysis of 111 Reference Human Epigenomes. Nature 2015, 518, 317–330. [Google Scholar] [CrossRef]
- Sandbacka, M.; Laivuori, H.; Freitas, É.; Halttunen, M.; Jokimaa, V.; Morin-Papunen, L.; Rosenberg, C.; Aittomäki, K. TBX6, LHX1 and Copy Number Variations in the Complex Genetics of Müllerian Aplasia. Orphanet J. Rare Dis. 2013, 8, 125. [Google Scholar] [CrossRef]
- Bedont, J.L.; LeGates, T.A.; Slat, E.A.; Byerly, M.S.; Wang, H.; Hu, J.; Rupp, A.C.; Qian, J.; Wong, G.W.; Herzog, E.D.; et al. Lhx1 Controls Terminal Differentiation and Circadian Function of the Suprachiasmatic Nucleus. Cell Rep. 2014, 7, 609–622. [Google Scholar] [CrossRef]
- van Es, J.H.; Kirkpatrick, C.; van de Wetering, M.; Molenaar, M.; Miles, A.; Kuipers, J.; Destrée, O.; Peifer, M.; Clevers, H. Identification of APC2, a Homologue of the Adenomatous Polyposis Coli Tumour Suppressor. Curr. Biol. CB 1999, 9, 105–108. [Google Scholar] [CrossRef]
- Xie, T.; Deng, L.; Mei, P.; Zhou, Y.; Wang, B.; Zhang, J.; Lin, J.; Wei, Y.; Zhang, X.; Xu, R. Genome-Wide Association Study Combining Pathway Analysis for Typical Sporadic Amyotrophic Lateral Sclerosis in Chinese Han Populations. Neurobiol. Aging 2014, 35, 1778.e9–1778.e23. [Google Scholar] [CrossRef]
- Goyette, P.; Pai, A.; Milos, R.; Frosst, P.; Tran, P.; Chen, Z.; Chan, M.; Rozen, R. Gene Structure of Human and Mouse Methylenetetrahydrofolate Reductase (MTHFR). Mamm. Genome Off. J. Int. Mamm. Genome Soc. 1998, 9, 652–656. [Google Scholar] [CrossRef]
- Moll, S.; Varga, E.A. Homocysteine and MTHFR Mutations. Circulation 2015, 132, e6–e9. [Google Scholar] [CrossRef]
- Botto, L.D.; Yang, Q. 5,10-Methylenetetrahydrofolate Reductase Gene Variants and Congenital Anomalies: A HuGE Review. Am. J. Epidemiol. 2000, 151, 862–877. [Google Scholar] [CrossRef]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of Continued Folic Acid Supplementation beyond the First Trimester of Pregnancy on Cognitive Performance in the Child: A Follow-up Study from a Randomized Controlled Trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef]
- Ranjbar Zahedani, M.; Eftekhari, M.H.; Nouri, M.; Alipour, S.; Hassanzadeh, J.; Fardaei, M. The Effect of Methyl Donor Supplementation on Body Composition, Homocysteine, Lipid Profile and Appetite Regulatory Hormones in Overweight and Obese Adults: A Randomized Placebo-Controlled Trial. Nutr. Food Sci. 2022. ahead of print. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Potter, J.D.; Ulrich, N. Folate Supplementation: Too Much of a Good Thing? Cancer Epidemiol. Biomark. Prev. 2006, 15, 189–193. [Google Scholar] [CrossRef]
| Citation | Study Question(s) Being Investigated | Study Population | Study Design | What Is the Setting of This Study | Describe the Main Findings | Limitations |
|---|---|---|---|---|---|---|
| Steegers-Theunissen [8] | Does periconceptional maternal folic acid effect IGF2 DMR methylation in offspring? Does this effect intrauterine growth? | Mother-child pairs chosen from an established study: ‘Haven’ | Mother and children between ages 12 and 18 months were enrolled into the study in the Netherlands between October 2003 and January 2007. | Unknown location in Rotterdam, The Netherlands | Mothers who used folic acid had children with a 4.5% increase in IGF2 DMR methylation, and IGF2 DMR methylation levels in children were associated with S-adenosylmethionine blood levels of the mother. An inverse independent relationship between methylation of IGF2 DMR and birth weight was reported. | Small sample size and recruitment of participants for from an already established birth cohort. |
| Julvez [9] | Does maternal folic acid supplementation alter neurodevelopment? | Mother-child pairs from an established birth cohort | The participants for this study were identified from a birth cohort in Spain. The study included women who had natal care from 12 weeks of gestation through birth, and the children were followed until 4 years of age. | Unknown specific location in Menorca, Spain | Folic acid supplementation in pregnancy improves cognition, motor skills, social skills, and attentiveness in 4-year-old children. These results were also adjusted for socioeconomic factors. | Researchers not able to validate self-reported folic acid supplementation and did not factor psychosocial variables. |
| Schlotz [10] | Is there an association between maternal folate status and brain growth and childhood behavioral problems? | Mother-child pairs from a previous nutrition during pregnancy and fetal growth study | The mothers and children (mean age 9 months and 8 years old) were recruited in the original study were in Southampton, UK. | Unknown location in Southampton, UK | Lower maternal folate status and total folate intake correlate with childhood hyperactivity and peer problem scores in offspring. Head circumference at birth is correlated with maternal folate status and that there was an inverse relationship with hyperactivity and peer problems. | Socioeconomic variable which may alter offspring neurodevelopment outside of dietary folic acid deficiency. |
| Roth [11] | Does prenatal folic acid supplementation affect the risk of developing severe language delay in offspring? | Mother-child pairs from the ‘Norwegian Mother and Child Study’ | Maternal folic acid use for this study was defined as supplementation 4 weeks before conception and 8 weeks after conception. Children aged 3 years old at time of assessment. | Unknown specific location in Norway | Folic acid supplementation from 4 weeks prior to conception to 8 weeks post-conception significantly reduces the risk of severe language delay when tested in children at age 3. Did not find a relationship between maternal folic acid supplementation and delay in motor skills at age 3. | Self-reporting by mothers via questionnaires on their children. |
| Steenweg-DeGraaf [12] | Does maternal folate status alter child emotional and behavioral issues? | Mother-child pairs from a population-based cohort: ‘Generation R Study’ | Participants from Generation R study. Mothers provided information on child emotional and behavioral problems at the age of 3. | Rotterdam, The Netherlands | Higher risk of emotional problems in offspring of mothers who were folate deficient but no effect on behavioral problems. The researchers report that homocysteine levels and MTHFR genotype were not associated with either behavioral or emotional problems. | Socioeconomic status and other factors of mothers enrolled varied and impact emotional and behavioral health of these children. |
| Chatzi [13] | Is there a relationship between high doses of folic acid supplementation in early pregnancy with offspring neurodevelopment at 18 months of age? | Mother-child cohort known as the ‘Rhea’ cohort | Women evaluated throughout the pregnancy with basic natal examinations and ultrasounds. Those who agreed to follow-up were recruited for the current study. | Heraklion, Crete, Greece | Researchers found that 5 mg supplementation of folic acid daily was associated with a 5-unit increase for receptive communication and a 3.5-unit increase for expressive communication when compared to no folic acid supplementation at all. Folic acid supplementation higher than 5 mg daily did not have a correlation with improved neurodevelopment. | The mothers self reported findings via telephone to interviewers who were assessing for offspring neurodevelopment. |
| Joubert [14] | Is there an association between maternal plasma folate during pregnancy and genome wide differential DNA methylation? | Newborns from 2 European Caucasian pregnancy cohorts: ‘Norwegian Mother and Child Cohort Study’ and the ‘Generation R study’ | Children had their cord blood analyzed for DNA methylation measurements and maternal plasma folate was measured at 18 weeks gestation. The second group assessed for this study is the Generation R population, assessed maternal folate, Vitamin B12, homocysteine, Vitamin D, and SNP measurements. The children were assessed for DNA methylation via evaluation of cord blood. | Norway and Netherlands | There is an association between maternal plasma folate levels and DNA methylation in cord blood at 443 CpGs. The researchers also performed sensitivity tests and noted that Vitamin B12 does not “confound” the folate methylation association that they observed. | The researchers noted that the sex of the children in the study was not expected to be associated with maternal plasma folate and was not included in the analyses. |
| Henry [15] | Does folic acid supplementation in the second and third trimester in pregnancy affect child psychosocial health (emotional intelligence and resilience)? | Mother-child pairs from: ‘Folic Acid Supplementation in the Second and Third Trimester study’ | Women that had taken folic acid supplementation throughout the first trimester of pregnancy and were recruited into the study at 14 weeks gestational age. Women were given either placebo or 400 μg/day of folic acid for 26 weeks. | Ireland | Mothers who received full-term supplementation of folic acid compared to mothers who only took supplementation through the end of the first trimester had children who scored significantly higher on the emotional intelligence and resilience tests. Maternal folate status at 36 weeks gestational age was a strong ‘predictor’ of emotional intelligence and resilience in offspring. | The sample size in this study was small, secondary to lack of funding as reported by the researchers. |
| Eryilmaz [16] | Does fetal folic acid exposure alter cortical maturation and psychiatric health in children? | Children from an observational clinical cohort study at Massachusetts General Hospital | Children recruited for this study were between 8 and 18 years of age at the time of the MRI scan, with birth dates between January 1993 and December 2001, as folic acid fortification was introduced to the US in 1996–1997. | USA | Folic acid exposure is associated with cortical thickness increase in the bilateral, frontal, and temporal regions, as well as delayed age-associated cortical thinning in the temporal and parietal regions. | MRI data collection was performed at different locations and with different MRI machines. This introduces a chance for error. |
| Huang [17] | Is there a relationship between maternal folate levels during pregnancy and children’s neuropsychological development at 2 years of age? | Children from ‘MKFOAD’ birth cohort in China | Subjects for this study were recruited from the MKFOAD cohort. The mothers had red blood cell and serum folate concentrations quantified at three different time periods while pregnant. | China | Maternal serum folate in late pregnancy was associated with children’s language development at age 2. The study also found that maternal serum folate in early pregnancy was inversely related to fine motor development in the children. | The researchers mentioned their own limitations in this study which includes testing a larger sample size. |
| Zou [18] | Is there a relationship between prenatal folate exposure and brain development in childhood? | Mother-child pairs from the ‘Generation R study’ | The participants in this study were recruited were from the Generation R study. For this study specifically, inclusion requirements involved neuroimaging and exclusions were made based on MRI findings. Children aged 9–11 years old. | Rotterdam Netherlands | Maternal folate deficiency (accounted for as levels less than 7 nmol/L during pregnancy) resulted in lower offspring total brain volume and less cerebral white matter in children 9–11 years old. They did not note any differences in cortical thickness. | The participants for this study are derived from an already established cohort, which may introduce variables. |
| Citation | Study Question(s) Being Investigated | Model System Used | Study Design | Main Findings | Limitations |
|---|---|---|---|---|---|
| Craciunescu [19] | Does maternal folate status during late gestation affect neurogenesis in the developing mouse brain? | Mouse | Pregnant mice at day 11 of gestation were assigned to either the folate-deficient group, control diet, or folate-supplemented group. The pregnant mice were euthanized on gestation day 17. Tissues were collected at end of the study. | Folate deficiency decreases the number of progenitor cells that divide in the developing mouse brain septum, caudate putamen, and the neocortex. In total, 106.2% more apoptotic cells were discovered with folate deficiency vs. in the control mouse brain. More calretinin-positive cells in the medial–septal diagonal band region of folate-deficient vs. control brains. Progenitor cells in the fetal brain are sensitive to folate status in late gestation. | Deficiency of other methyl donors was not excluded in this study, which may also contribute to the altered brain morphology. |
| Langie [20] | Does maternal folate depletion during pregnancy and lactation reduce DNA repair secondary to aberrant methylation and expression of base excision repair genes in the brain of adult mice offspring? Does postweaning exposure to high-fat diet enhance adverse effects of folate depletion? | Mouse | Adult female mice were assigned to a folate adequate group w/2 mg folic acid/kg diet or folate depleted group w/0.4 mg folic acid/kg diet. The diets were supplemented for 4 weeks before mating the male and female mice, and the diets continued through pregnancy and lactation. The pups were weaned at 22–25 days of age and assigned to either a control diet of 5% anhydrous milk or high-fat diet with 20% anhydrous milk fat (9 males and females in each group). At 5.5 months old, all animals were euthanized and tissue was analyzed. | Low-folate diet did not alter maternal body weight, litter size, or body weight of the offspring. High-fat diet postweaning resulted in increased body weight in the adult offspring but they did not attribute maternal folate supply to the adult offspring body mass. | The low sample size, which makes conclusions inconclusive and difficult to draw from. |
| Berrocal-Zarago [21] | What is the effect of a maternal folate-deficient diet on pup brain function? | Rats | Pregnant dams were fed a control diet. After birth, the dams and pups were separated into two groups, control and folic acid-deficient diets. The diets were started immediately after birth. At postnatal days 0 and 23, liver and brain tissue from dams and pups were collected for analysis. For the remaining pups, once they were separated they were continued on the control, low-folate, or high-folate diets. The pups were euthanized after behavioral testing, and the livers and brains were collected for analysis. | Developmental issues in the folate-deficient pups vs. the normal diet pups. There were also long-term memory and learning issues in the folate-deficient group. They found that folic acid supplementation in the post-weaning phase resulted in no change in the noted deficits. Maternal folate deficiency during weaning affects neurodevelopment of pups, even if folate status is sufficient in pregnancy. | The differences in myelination patterns in rats vs. humans in early life. |
| Jadavji [22] | Does maternal MTHFR deficiency alter offspring brain health? Does folate and choline dietary deficiency alter offspring brain function? | Mouse | Study 1: Maternal MTHFR deficiency. Female Mthfr+/+ and Mthfr+/− mice were fed a control diet with 2 mg/kg folic acid, along with other nutrients. Study 2: Dietary deficiency study. Dams were fed either a control diet, folic acid-, or choline-deficient diet. Male offspring underwent behavioral testing and tissue collection for analysis. | Short-term memory issues in the offspring who had maternal MTHFR deficiency with elevated levels of homocysteine in the mothers, but no elevation in the offspring. Offspring from Mthfr+/− mothers were noted to have increased cell death and growth in the hippocampus. For the maternal dietary deficiency study, there was also short-term memory issues and increased hippocampal cell death in the offspring of mothers who had folate and choline deficiency during pregnancy. There was noted to be increased neuron growth in the offspring of mothers who had choline deficiency during pregnancy. | A limitation of this study is the analysis of hippocampal and granular cell layers only. |
| Ly [23] | Which gestation period and organ is more sensitive to maternal folic acid on DNA methylation and gene expression in offspring? | Rats | Male and female rats were mated and maintained on a control diet. After confirmation of pregnancy, the dams were placed on 5 different folic acid diets: control, 2.5× the control diet for either 1st, 2nd, or 3rd week of gestation, or 2.5× the control throughout the entire pregnancy. Tissues were collected at the end of the study for analysis. | The brain is the most sensitive to maternal folic acid supplementation on global DNA methylation. The liver was sensitive to the effects of folic acid on gene expression in a gene-specific manner. | Other methyl donor deficiency is not considered in this study for altered DNA methylation status. |
| Barua [24] | Does excess maternal folic acid supplementation result in gene expression changes in the cerebral cortex of offspring? | Mouse | Female mice were divided into two groups prior to mating, and started on a control diet or excess folic acid. Tissues were collected at the end of study for analysis. | High maternal folic acid resulting in upregulation of the genes Nfix, Runx1, and Vgll2 in female offspring but downregulation in male offspring. There was also increased expression of Dnmt3b but no altered expression of Dnmt3a. Imprinted gene Dio3 was upregulated in male offspring dams with high maternal folic acid, but not female offspring. Imprinted genes H19 and Hist were upregulated in female offspring from dams with high maternal folic acid. They also found decreased expression of Auts2 (autism susceptible gene) in male offspring from dams with high maternal folic acid but increased expression in female offspring. Park2 was upregulated in both male and female mice from dams with high maternal folic acid. DNA methylation levels were unaltered with high maternal folic acid. | Focus on histological analysis of the cerebral cortex only for altered gene expression, there were no behavioral tests conducted. |
| Bahous [25] | Does high maternal folic acid intake alter brain function and/or metabolism in offspring? | Mice | Female mice were placed on control or high-folic acid diets. These mice were sacrificed at day 17.5 of gestation while another group containing mice from both diet groups was maintained on the diets through both pregnancy and lactation. In brain tissue (hippocampus), folate and choline metabolites were measured. | Smaller hippocampal and dentate gyrus thickness size in pups of the mothers on the high-folate diet, as well as memory impairment. In pups of the mothers who had the high-folate diet, there were also decreased levels of MTHFR, as well as phosphocholine and glycerophosphocholine in the liver and hippocampus. There were also decreased acetylcholine and Dnmt3a levels in the hippocampus and cortex. Altered morphology of the embryo brain with altered cortical layer development. | One additional variable that could have been tested for this study is varying concentrations of folic acid instead of just 2× the amount of the control diet. This would have possibly shown what the cut off is for negative impacts of excessive folic acid intake. |
| Wang [26] | Is folic acid supplementation throughout pregnancy beneficial for offspring neurobehavioral development? | Mice | Four diet groups were included in this study: folate normal diet, folate-deficient diet, folate-supplemented diet short period, folate-supplemented diet long period. At days 4 to 8 postnatal neurobehavioral testing was conducted. Hippocampal tissue was collected from 4-month-old male offspring of the normal diet and folate-deficient long diet. | Increased plasma homocysteine levels in the folate-deficient group vs. the folate normal group, as well as impaired neurobehavioral development in offspring. This was represented by delayed sensory-motor reflex development in infancy, impaired spatial learning, and memory ability in adolescence and adulthood. There were also changes to the ultrastructure of the hippocampus in adulthood. | Focus on the length of time folic acid was supplemented, instead of also including varying levels of folic acid to determine the outcome on neurodevelopment. |
| Wu [27] | Does excessive folic acid supplementation affect β-catenin phosphorylation and PP2Ac methylation in an adult brain? | Mice | At 8 months of age, mice were divided into two groups: the control group was fed food and water without folic acid, and the experimental group was fed 2× the amount of folic acid. The mice were then mated, and folic acid supplementation continued in the water supply until postnatal day 21. Seven male offspring were killed at postnatal day 21, and 5 male offspring at 5 months for brain analysis. | Levels of non-phosphorylated β-catenin were increased in the brains of weaning and adult folic acid-exposed offspring; demethylation of PP2Ac was decreased, and PI3K, Akt, and GSK3-β were stimulated in the folic acid-exposed brains only at weaning. Excess folic acid supplementation may increase β-catenin via inhibition of PP2Ac demethylation, and indicate that this may be a mechanism for the role of folic acid on neurodevelopment. | Only male mice were used and there was small sample size. |
| De Crescenzo [28] | What are the effects of maternal folate deficiency or excess folate on neurodevelopment in offspring? | Mouse | Histological data for this study were obtained from embryos at day 14.5, and pups at postnatal day 0 or 6 from each diet group (control, folic acid-deficient, folic acid-supplemented). Behavioral testing was carried out in 4–10-week-old mice, and brain tissue was collected for analysis. | Maternal folic acid deficiency or an excess of it can result in an increase in late-born neurons compared to early-born neurons in the cerebral cortex. Increased apoptosis of deep layer neurons in both folic acid-deficient and folic acid-excess mice. Altered neuronal morphology when compared to control mice. Behavioral testing showed that pups of folic acid-deficient dams did not perform as well as the control in object recognition. Pups born to folic acid-excess dams revealed higher anxiety during the open maze test. Behavioral findings were correlated to changes in cortex morphology. | This study was not clear on the sample size used for the several different outcomes assessed, including histological analysis. |
| Liu [29] | Does maternal folic acid supplementation alter offspring adult metabolism? | Rats | Does maternal folic acid supplementation alter offspring adult metabolism? | Metabolic changes with maternal folic acid supplementation vs. no supplementation. The metabolic changes are reflected by the presence of phospholipids, fatty acids, and amino acids that are associated with linoleic acid, docosahexaenoic acid, glycerophosphocholine, lysophosphatidylcholine, tryptophan, glycine, arachidonic acid, and ƴ-aminobutyric acid. Fifty-one proteins were also identified in the brains of the pups from the dams who received folic acid supplementations vs. the control diet without supplementation. Offspring of the dams who received folic acid supplementation had a better level of memory compared to the pups of the dams who did not have supplementation. | One thing to note for this study is the number of metabolites and proteins produced in the results. There are a lot of data present for what was tested, but this may make conclusions a bit more difficult to draw from. |
| Garcez [30] | What is the effect of folic acid supplementation in adult offspring? | Rats | The dams were divided into 5 diet groups, including 4 divisions within the control diet and the folate-deficient diet. These diets continued throughout pregnancy and lactation. Female offspring were divided into 8 groups based on the dam’s diet. Behavioral testing was carried out, followed by sacrificing the mice for analysis of the prefrontal cortex and hippocampus. | Age-related memory impairment was decreased in the rats who had maternal folate supplementation. Folic acid supplementation was also able to decrease levels of TNF-α and IL-1β in the rats, which are cytokines associated with aging. Folic acid deficiency resulted in decreased levels of IL-4 in the hippocampus of 2-month-old rats. Aging and maternal folate deficiency also resulted in decreased levels of brain derived neurotrophic factor in the hippocampus and nerve growth factor from the prefrontal cortex. Folate supplementation also prevented this. | Unclear presentation of sample sizes. |
| Yang [31] | Does excessive folic acid preconception, during pregnancy, or during lactation affect the brain of female adult mice? | Mouse | At 8 weeks of age, mice were divided either into the control group with no folic acid supplementation, or the experimental group with folic acid supplementation in their water supply. After one week, the mice were mated and the supplementation continued throughout pregnancy and lactation. At postnatal day 21, brain tissue was collected for analysis. Behavioral testing was performed on 2-month-old mice. | In adult male mice there was increased anxiety, decreased exploratory activity, and decreased motor coordination and spatial memory. RNA sequencing and qRT-PCR revealed increased transcription of the following genes: Tlr1, Sult1a1, Tph2, Acacb, Etnppl, Angplt4, and Apold1; and decreased transcription of Ppar-α in the folic acid-exposed female brains. Excess folic acid supplementation during pregnancy and during lactation altered the brain transcriptome in weaning female mice, and alters adult female mice behavior. Behaviors in presented study were different from previous work. | The estrus cycle was not monitored in female mice during experiments. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virdi, S.; Jadavji, N.M. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites 2022, 12, 876. https://doi.org/10.3390/metabo12090876
Virdi S, Jadavji NM. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites. 2022; 12(9):876. https://doi.org/10.3390/metabo12090876
Chicago/Turabian StyleVirdi, Sapna, and Nafisa M. Jadavji. 2022. "The Impact of Maternal Folates on Brain Development and Function after Birth" Metabolites 12, no. 9: 876. https://doi.org/10.3390/metabo12090876
APA StyleVirdi, S., & Jadavji, N. M. (2022). The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites, 12(9), 876. https://doi.org/10.3390/metabo12090876








