Betatrophin and Insulin Resistance
Abstract
1. Identification, Structure, Localization, and Secretion of Betatrophin
1.1. Identification of Betatrophin
1.2. Structure of Betatrophin
1.3. Localization and Secretion of Betatrophin
2. Betatrophin and Metabolism
2.1. Betatrophin and Glucose Metabolism
2.2. Betatrophin and Lipid Metabolism
3. Betatrophin and Insulin Resistance
3.1. Betatrophin and Obesity
3.2. Betatrophin and T2D
3.3. Betatrophin and MetS
4. Influencing Factors for Betatrophin Expression
4.1. Nutrition
4.2. Insulin
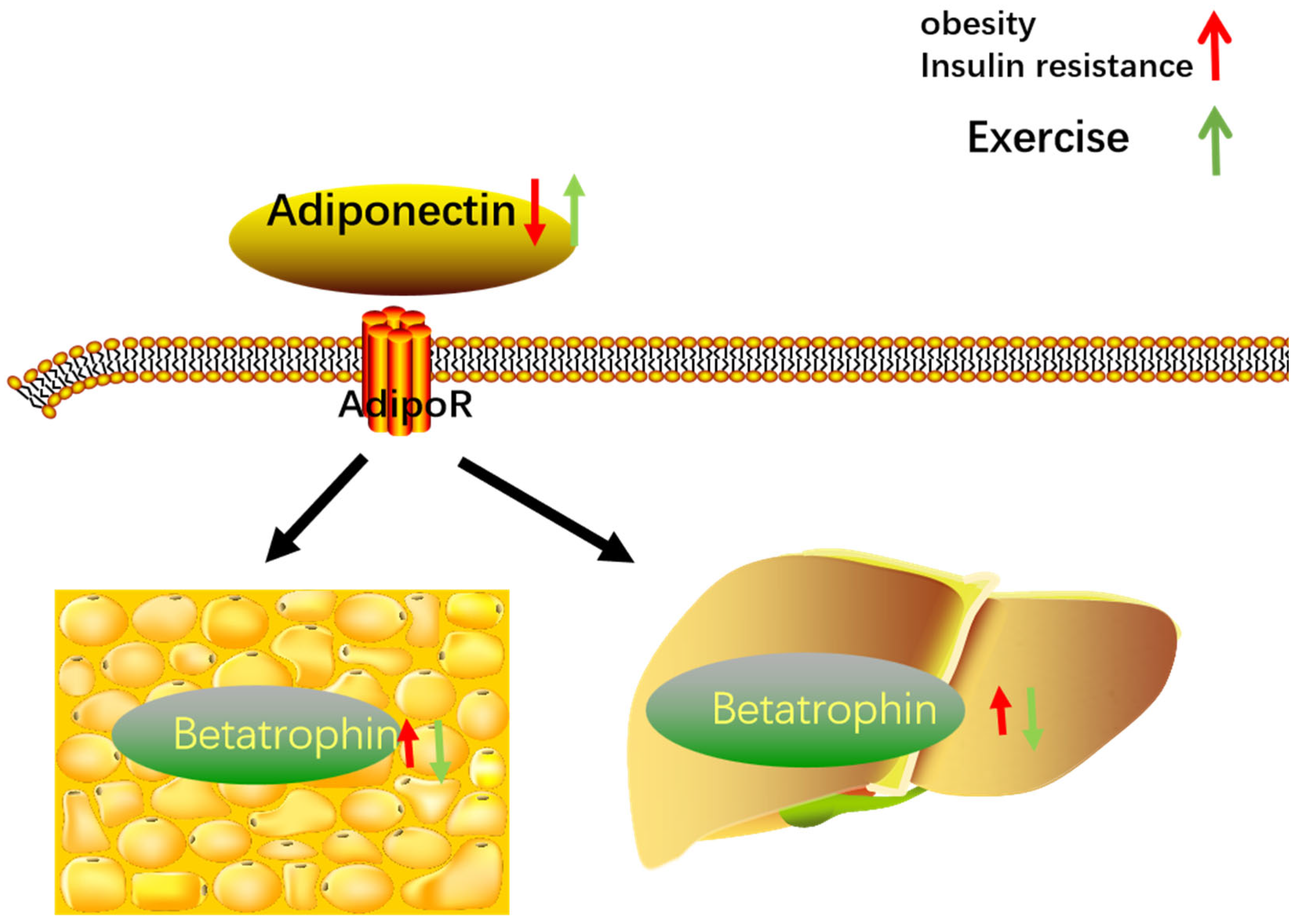
4.3. Adiponectin
4.4. Exercise
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tseng, Y.H.; Yeh, Y.H.; Chen, W.J.; Lin, K.H. Emerging regulation and function of betatrophin. Int. J. Mol. Sci. 2014, 15, 23640–23657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012, 424, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Park, J.S.; Melton, D.A. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell 2013, 153, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Itariu, B.K.; Kosi, L.; Fritzer-Szekeres, M.; Kautzky-Willer, A.; Stulnig, T.M.; Kiefer, F.W. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia 2014, 57, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, V.; Alexa, C.A.; Na, E.; Stevis, P.E.; Xin, Y.; Bonner-Weir, S.; Cohen, J.C.; Hobbs, H.H.; Murphy, A.J.; Yancopoulos, G.D.; et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell 2014, 159, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; le Lay, J.; Yu, M.; Naji, A.; Kaestner, K.H. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes 2014, 63, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Yu, C.H.; Li, Y.M. Betatrophin provides a new insight into diabetes treatment and lipid metabolism (Review). Biomed. Rep. 2014, 2, 447–451. [Google Scholar] [CrossRef][Green Version]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Hu, F.B.; Behbehani, K.; Elkum, N. Higher plasma betatrophin/ANGPTL8 level in Type 2 Diabetes subjects does not correlate with blood glucose or insulin resistance. Sci. Rep. 2015, 5, 10949. [Google Scholar] [CrossRef]
- Dong, X.Y.; Pang, X.W.; Yu, S.T.; Su, Y.R.; Wang, H.C.; Yin, Y.H.; Wang, Y.D.; Chen, W.F. Identification of genes differentially expressed in human hepatocellular carcinoma by a modified suppression subtractive hybridization method. Int. J. Cancer 2004, 112, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Kim, J.Y.; Smas, C.M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism, American journal of physiology. Endocrinol. Metab. 2012, 303, E334–E351. [Google Scholar]
- Stewart, A.F. Betatrophin versus bitter-trophin and the elephant in the room: Time for a new normal in β-cell regeneration research. Diabetes 2014, 63, 1198–1199. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Sriraman, D.; Cherian, P.; AlKhairi, I.; Elkum, N.; Behbehani, K.; Abubaker, J. Circulating ANGPTL8/Betatrophin Is Increased in Obesity and Reduced after Exercise Training. PLoS ONE 2016, 11, e0147367. [Google Scholar] [CrossRef]
- Chen, C.C.; Susanto, H.; Chuang, W.H.; Liu, T.Y.; Wang, C.H. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc. Diabetol. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef]
- Fu, Z.; Yao, F.; Abou-Samra, A.B.; Zhang, R. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem. Biophys. Res. Commun. 2013, 430, 1126–1131. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Tuomilehto, J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes Metab. Res. Rev. 2017, 33, e2919. [Google Scholar] [CrossRef]
- Clark, H.F.; Gurney, A.L.; Abaya, E.; Baker, K.; Baldwin, D.; Brush, J.; Chen, J.; Chow, B.; Chui, C.; Crowley, C.; et al. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: A bioinformatics assessment. Genome Res. 2003, 13, 2265–2270. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Ke, P.Y.; Liao, C.J.; Wu, S.M.; Chi, H.C.; Tsai, C.Y.; Chen, C.Y.; Lin, Y.H.; Lin, K.H. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 2014, 10, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Berhane, F.; Fite, A.; Seyoum, B.; Abou-Samra, A.B.; Zhang, R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 2014, 4, 5013. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.R.; Lam, C.J.; Bonnyman, C.W.; Chavez, J.; Rios, J.S.; Kushner, J.A. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia 2015, 58, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quagliarini, F.; Gusarova, V.; Gromada, J.; Valenzuela, D.M.; Cohen, J.C.; Hobbs, H.H. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 16109–16114. [Google Scholar] [CrossRef]
- Guo, X.R.; Wang, X.L.; Chen, Y.; Yuan, Y.H.; Chen, Y.M.; Ding, Y.; Fang, J.; Bian, L.J.; Li, D.S. ANGPTL8/betatrophin alleviates insulin resistance via the Akt-GSK3β or Akt-FoxO1 pathway in HepG2 cells. Exp. Cell Res. 2016, 345, 158–167. [Google Scholar]
- Wang, L. The effect of betatrophin on the expression of key genes of glucose metabolism was studied by mRNA synthesis in vitro. J. Hubei Med. Coll. 2016, 6, 531–535. [Google Scholar]
- Gómez-Ambrosi, J.; Pascual, E.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E2004–E2009. [Google Scholar] [CrossRef]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. S3), 14–23. [Google Scholar] [CrossRef]
- O’Rahilly, S. Human obesity and insulin resistance: Lessons from experiments of nature. Biochem. Soc. Trans. 2007, 35, 33–36. [Google Scholar] [CrossRef]
- Guo, K.; Lu, J.; Yu, H.; Zhao, F.; Pan, P.; Zhang, L.; Chen, H.; Bao, Y.; Jia, W. Serum betatrophin concentrations are significantly increased in overweight but not in obese or type 2 diabetic individuals. Obesity 2015, 23, 793–797. [Google Scholar] [CrossRef]
- Han, C.; Xia, X.; Liu, A.; Zhang, X.; Zhou, M.; Xiong, C.; Liu, X.; Sun, J.; Shi, X.; Shan, Z.; et al. Circulating Betatrophin Is Increased in Patients with Overt and Subclinical Hypothyroidism. BioMed Res. Int. 2016, 2016, 5090852. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Zulet, M.A.; Abete, I.; Amil, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Interplay of atherogenic factors, protein intake and betatrophin levels in obese-metabolic syndrome patients treated with hypocaloric diets. Int. J. Obes. 2016, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Kavalakatt, S.; Khadir, A.; Behbehani, K.; Alarouj, M.; Bennakhi, A.; et al. Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc. Diabetol. 2016, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Weir, G.C. New sources of pancreatic beta-cells. Nat. Biotechnol. 2005, 23, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Magnan, C.; Kargar, C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 2009, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Mizrachi, E.B.; Ocana, A.G.; Stewart, A.F. Human β-cell proliferation and intracellular signaling: Driving in the dark without a road map. Diabetes 2012, 61, 2205–2213. [Google Scholar] [CrossRef]
- Kugelberg, E. Diabetes: Betatrophin--inducing β-cell expansion to treat diabetes mellitus? Nat. Rev. Endocrinol. 2013, 9, 379. [Google Scholar] [CrossRef]
- Lickert, H. Betatrophin fuels β cell proliferation: First step toward regenerative therapy? Cell Metab. 2013, 18, 5–6. [Google Scholar] [CrossRef][Green Version]
- Espes, D.; Martinell, M.; Carlsson, P.O. Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int. J. Endocrinol. 2014, 2014, 323407. [Google Scholar] [CrossRef]
- Onalan, E.; Bozkurt, A.; Gursu, M.F.; Yakar, B.; Donder, E. Role of Betatrophin and Inflammation Markers in Type 2 Diabetes Mellitus, Prediabetes and Metabolic Syndrome. J. Coll. Physicians Surg. Pak. JCPSP 2022, 32, 303–307. [Google Scholar]
- Seyhanli, Z.; Seyhanli, A.; Aksun, S.; Pamuk, B.O. Evaluation of serum Angiopoietin-like protein 2 (ANGPTL-2), Angiopoietin-like protein 8 (ANGPTL-8), and high-sensitivity C-reactive protein (hs-CRP) levels in patients with gestational diabetes mellitus and normoglycemic pregnant women. The journal of maternal-fetal & neonatal medicine: The official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies. Int. Soc. Perinat. Obs. 2021, 2021, 1888919. [Google Scholar]
- Tokumoto, S.; Hamamoto, Y.; Fujimoto, K.; Yamaguchi, E.; Okamura, E.; Honjo, S.; Ikeda, H.; Wada, Y.; Hamasaki, A.; Koshiyama, H. Correlation of circulating betatrophin concentrations with insulin secretion capacity, evaluated by glucagon stimulation tests. Diabet. Med. A J. Br. Diabet. Assoc. 2015, 32, 653–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Z.; Deng, X.; Jia, J.; Zhao, L.; Wang, C.; Cai, Z.; Guo, C.; Yang, L.; Wang, D.; Ma, S.; et al. Angiopoietin-like protein 8 (betatrophin) inhibits hepatic gluconeogenesis through PI3K/Akt signaling pathway in diabetic mice. Metab. Clin. Exp. 2022, 126, 154921. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, D.; Li, L.; Li, Y.; Li, Q.; An, Z.; Sun, X.; Tian, H. Circulating Betatrophin in Patients with Type 2 Diabetes: A Meta-Analysis. J. Diabetes Res. 2016, 2016, 6194750. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.; Brachs, S.; Decker, A.M.; Brachs, M.; Leupelt, V.; von Schwartzenberg, R.J.; Ernert, A.; Bobbert, T.; Krude, H.; Spranger, J.; et al. Weight Loss Partially Restores Glucose-Driven Betatrophin Response in Humans. J. Clin. Endocrinol. Metab. 2016, 101, 4014–4020. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Y.; Han, C.; Liu, A.; Fan, C.; Wang, H.; Zhang, H.; Ding, S.; Teng, W.; Shan, Z. The Effects of Serum ANGPTL8/betatrophin on the Risk of Developing the Metabolic Syndrome—A Prospective Study. Sci. Rep. 2016, 6, 28431. [Google Scholar] [CrossRef]
- Zhai, Z. Comparison and correlation of serum betatrophin levels between Kazak and Han patients with hypertension in Xinjiang. J. Pract. Med. 2016, 32, 949–951. [Google Scholar]
- Qin, L.; Rehemuding, R.; Ainiwaer, A.; Ma, X. Correlation between betatrophin/angiogenin-likeprotein3/lipoprotein lipase pathway and severity of coronary artery disease in Kazakh patients with coronary heart disease. World J. Clin. Cases 2022, 10, 2095–2105. [Google Scholar] [CrossRef]
- Franck, N.; Gummesson, A.; Jernås, M.; Glad, C.; Svensson, P.A.; Guillot, G.; Rudemo, M.; Nyström, F.H.; Carlsson, L.M.; Olsson, B. Identification of adipocyte genes regulated by caloric intake. J. Clin. Endocrinol. Metab. 2011, 96, E413–E418. [Google Scholar] [CrossRef]
- Chen, Q. Study on the Correlation and Mechanism between Betatrophin and Insulin Resistance; Huazhong University of Science and Technology: Hangzhou, China, 2015. [Google Scholar]
- Tuhan, H.; Abacı, A.; Anık, A.; Çatlı, G.; Küme, T.; Çalan, Ö.G.; Acar, S.; Böber, E. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Res. Clin. Pract. 2016, 114, 37–42. [Google Scholar] [CrossRef]
- Sertogullarindan, B.; Komuroglu, A.U.; Ucler, R.; Gunbatar, H.; Sunnetcioglu, A.; Cokluk, E. Betatrophin association with serum triglyceride levels in obstructive sleep apnea patients. Ann. Thorac. Med. 2019, 14, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Mantzoros, C.S. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metab. Clin. Exp. 2015, 64, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Li, L.; Yin, J.; Liu, R.; Yang, G.; Liao, Y.; Wu, Q. Circulating ANGPTL8 Is Associated with the Presence of Metabolic Syndrome and Insulin Resistance in Polycystic Ovary Syndrome Young Women. Mediat. Inflamm. 2019, 2019, 6321427. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Fodor, J.G.; Adamo, K.B. Prevention of type 2 diabetes mellitus by changes in lifestyle. N. Engl. J. Med. 2001, 345, 696; author reply 696–697. [Google Scholar]
- Zheng, C.; Liu, Z. Vascular function, insulin action, and exercise: An intricate interplay. Trends Endocrinol. Metab. TEM 2015, 26, 297–304. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, G.; Wang, X.; Sun, J.; Gao, Z.; Zhou, T.; Yin, W.; Cai, R.; Ye, X.; Wang, Z. Effects of a diet with or without physical activity on angiopoietin-like protein 8 concentrations in overweight/obese patients with newly diagnosed type 2 diabetes: A randomized controlled trial. Endocr. J. 2019, 66, 89–105. [Google Scholar] [CrossRef]
- Rejeki, P.S.; Baskara, P.G.; Herawati, L.; Pranoto, A.; Setiawan, H.K.; Lesmana, R.; Halim, S. Moderate-intensity exercise decreases the circulating level of betatrophin and its correlation among markers of obesity in women. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Cao, S.; Wang, X. Betatrophin and Insulin Resistance. Metabolites 2022, 12, 925. https://doi.org/10.3390/metabo12100925
Guo Q, Cao S, Wang X. Betatrophin and Insulin Resistance. Metabolites. 2022; 12(10):925. https://doi.org/10.3390/metabo12100925
Chicago/Turabian StyleGuo, Qi, Shicheng Cao, and Xiaohong Wang. 2022. "Betatrophin and Insulin Resistance" Metabolites 12, no. 10: 925. https://doi.org/10.3390/metabo12100925
APA StyleGuo, Q., Cao, S., & Wang, X. (2022). Betatrophin and Insulin Resistance. Metabolites, 12(10), 925. https://doi.org/10.3390/metabo12100925







