Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Trial
2.3. Sample Collection and Chemical Analysis
2.4. Intestinal Histology Observation
2.5. Intestinal Microbiota Analysis
2.6. RNA Extraction and Gene Expression
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Proximate Composition
3.2. Intestinal Antioxidant Capacity
3.3. Plasma Components
3.4. Intestinal Digestive Enzyme Activity
3.5. Intestinal Permeability
3.6. Intestinal Histomorphology
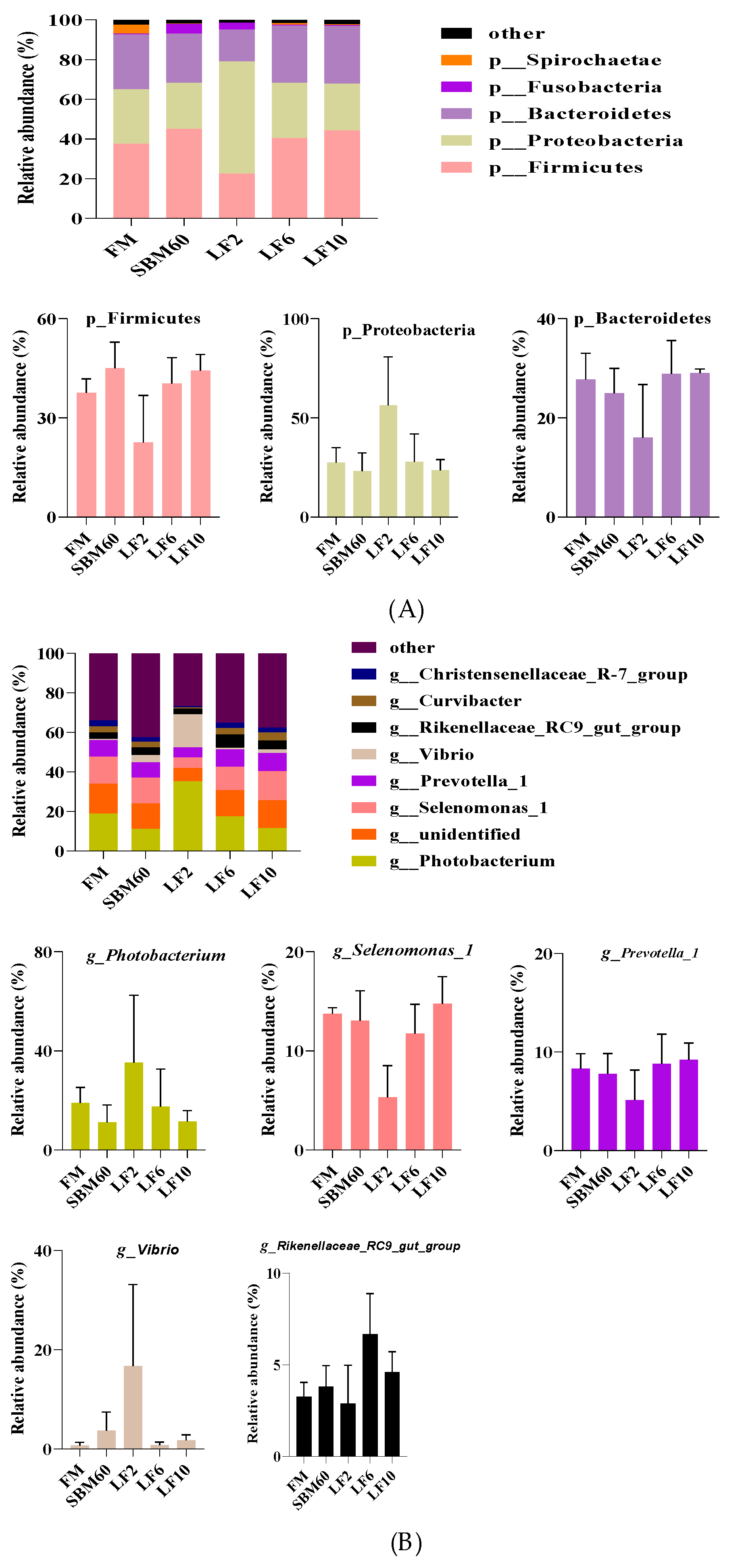
3.7. Abundance and Difference in Intestinal Microbiota
3.8. Expression of Intestinal Inflammatory Factor Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Wang, Y.; Wang, X.; Ye, J. Growth performance, plasma components, and intestinal barrier in grouper (Epinephelus coioides) are altered by dietary fish meal replacement with extruded soybean meal. Aquac. Rep. 2021, 21, 100863. [Google Scholar] [CrossRef]
- Ghosh, K.; Ray, A.K.; Ringø, E. Applications of plant ingredients for tropical and subtropical freshwater finfish: Possibilities and challenges. Rev. Aquac. 2019, 11, 793–815. [Google Scholar] [CrossRef]
- Shao, J.; Zhao, W.; Liu, X.; Wang, L. Growth performance, digestive enzymes, and TOR signaling pathway of Litopenaeus vannamei are not significantly affected by dietary protein hydrolysates in practical conditions. Front. Physiol. 2018, 9, 998. [Google Scholar] [CrossRef]
- Bravo-Tello, K.; Ehrenfeld, N.; Solís, C.J.; Ulloa, P.E.; Hedrera, M.; Pizarro-Guajardo, M.; Paredes-Sabja, D.; Feijóo, C.G. Effect of microalgae on intestinal inflammation triggered by soybean meal and bacterial infection in zebrafish. PLoS ONE 2017, 12, e0187696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhang, C.; Song, K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquac. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Huang, H.; Zhong, H. Effects of the replacement of fishmeal by soy protein concentrate on growth performance, apparent digestibility, and retention of protein and amino acid in juvenile pearl gentian grouper. PLoS ONE 2019, 14, e0222780. [Google Scholar] [CrossRef]
- Dam, C.T.M.; Ventura, T.; Booth, M.; Pirozzi, I.; Salini, M.; Smullen, R.; Elizur, A. Intestinal transcriptome analysis highlights key differentially expressed genes involved in nutrient metabolism and digestion in yellowtail kingfish (Seriola lalandi) fed terrestrial animal and plant proteins. Genes 2020, 11, 621. [Google Scholar] [CrossRef]
- Bai, N.; Gu, M.; Xu, X.; Xu, B.; Krogdahl, Å. Protective effects of mannan oligosaccharides on turbot Scophthalmus maximus suffering from soy enteropathy. Aquaculture 2017, 476, 141–151. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida utilis and Chlorella vulgaris counteract intestinal inflammation in Atlantic salmon (Salmo salar L.). PLoS ONE 2013, 8, e83213. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Hetland, D.L.; Skrede, A.; Øverland, M.; Mydland, L.T.; Landsverk, T. Prevention of soya-induced enteritis in Atlantic salmon (Salmo salar) by bacteria grown on natural gas is dose dependent and related to epithelial MHC II reactivity and CD8α+ intraepithelial lymphocytes. Br. J. Nutr. 2013, 109, 1062–1070. [Google Scholar] [CrossRef]
- Wu, N.; Xu, X.; Wang, B.; Li, X.; Cheng, Y.; Li, M.; Xia, X.; Zhang, Y. Anti-foodborne enteritis effect of galantamine potentially via acetylcholine anti-inflammatory pathway in fish. Fish Shellfish Immunol. 2020, 97, 204–215. [Google Scholar] [CrossRef]
- Xie, J.; Li, M.; Ye, W.; Shan, J.; Zhao, X.; Duan, Y.; Liu, Y.; Unger, B.H.; Cheng, Y.; Zhang, W.; et al. Sinomenine hydrochloride ameliorates fish foodborne enteritis via α7nAchR-mediated anti-inflammatory effect whilst altering microbiota composition. Front. Immunol. 2021, 12, 766845. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef] [PubMed]
- Luna Castro, S.; Ceballos Olvera, I.; Benavides González, F.; Blanco Martínez, Z.; Sánchez Martínez, G.; Vázquez Sauceda, M.D.L.L.; de la Garza, M. Bovine lactoferrin in fish culture: Current research and future directions. Aquac. Res. 2022, 53, 735–745. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Solís, C.J.; De La Paz, J.F.; Alaurent, T.G.S.; Caruffo, M.; Hernández, A.J.; Dantagnan, P.; Feijóo, C.G. Lactoferrin decreases the intestinal inflammation triggered by a soybean meal-based diet in zebrafish. J. Immunol. Res. 2016, 2016, 1639720. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, T.; Xu, Z.; Feng, J.; Wang, Z. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2007, 135, 263–272. [Google Scholar] [CrossRef]
- Li, M.; Fang, C.; Zhang, K.; Li, Y.; Zhang, R. Effects of lactoferrin supplementation on growth performance, intestinal microflora and mucosal morphology of early weaned piglets. Chin. J. Anim. Nutr. 2012, 24, 111–116. [Google Scholar]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Niu, X.; Qian, X.; Feng, H.; Yi, K.; Li, D.; Chen, W.; Ye, J. Growth and metabolic responses of grouper juveniles (Epinephelus coioides) fed diets containing varying levels of leucine. Aquaculture 2021, 534, 736281. [Google Scholar] [CrossRef]
- Shapawi, R.; Abdullah, F.C.; Senoo, S.; Mustafa, S. Nutrition, growth and resilience of tiger grouper (Epinephelus fuscoguttatus) × giant grouper (Epinephelus lanceolatus) hybrid—A review. Rev. Aquac. 2019, 11, 1285–1296. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Bai, F.; Niu, X.; Wang, X.; Ye, J. Growth performance, biochemical composition and expression of lipid metabolism related genes in groupers (Epinephelus coioides) are altered by dietary taurine. Aquac. Nutr. 2021, 27, 2690–2702. [Google Scholar] [CrossRef]
- Zhuo, L.; Chen, C.; Lin, Y. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef]
- Ye, J.; Liu, X.; Wang, Z.; Wang, K. Effect of partial fish meal replacement by soybean meal on the growth performance and biochemical indices of juvenile Japanese flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995.
- Hu, H.; Liu, J.; Li, Y.; Zhang, Y.; Mai, K.; Ai, Q.; Shao, M.; Yang, P. Effects of dietary daidzein on growth performance, activities of digestive enzymes, anti-oxidative ability and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). J. Fish. China 2014, 38, 1503–1513. [Google Scholar]
- Anguiano, M.; Pohlenz, C.; Buentello, A.; Gatlin, D.M. The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × M. saxatilis). Br. J. Nutr. 2013, 109, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hanaki, K.; Ike, F.; Kajita, A.; Yasuno, W.; Yanagiba, M.; Goto, M.; Sakai, K.; Ami, Y.; Kyuwa, S. A broadly reactive one-step SYBR Green I real-time RT-PCR assay for rapid detection of murine norovirus. PLoS ONE 2014, 9, e98108. [Google Scholar] [CrossRef]
- Jahan, H.; Tumpa, I.J.; Qasem, W.A.; Moniruzzaman, M.; Pervin, M.A.; Akter, R.; Omri, A.; Min, T.; Hossain, Z. Evaluation of the partial replacement of dietary fish meal with fermented or untreated soybean meal in juvenile silver barb, Barbonymus gonionotus. Front. Nutr. 2021, 8, 733402. [Google Scholar] [CrossRef]
- Yang, H.; Bian, Y.; Huang, L.; Lan, Q.; Ma, L.; Li, X.; Leng, X. Effects of replacing fish meal with fermented soybean meal on the growth performance, intestinal microbiota, morphology and disease resistance of largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 22, 100954. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Poolsawat, L.; Guo, Z.; Yao, W.; Zhang, C.; Leng, X. Effects of fish meal replaced by fermented soybean meal on growth performance, intestinal histology and microbiota of largemouth bass (Micropterus salmoides). Aquac. Nutr. 2020, 26, 1058–1071. [Google Scholar] [CrossRef]
- Liang, X.F.; Hu, L.; Dong, Y.C.; Wu, X.F.; Qin, Y.C.; Zheng, Y.H.; Shi, D.D.; Xue, M. Substitution of fish meal by fermented soybean meal affects the growth performance and flesh quality of Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2017, 229, 1–12. [Google Scholar] [CrossRef]
- Lim, S.-J.; Kim, S.-S.; Ko, G.-Y.; Song, J.-W.; Oh, D.-H.; Kim, J.-D.; Kim, J.-U.; Lee, K.-J. Fish meal replacement by soybean meal in diets for tiger puffer, Takifugu rubripes. Aquaculture 2011, 313, 165–170. [Google Scholar] [CrossRef]
- Abdel-Warith, A.A.; Younis, E.M.; Al-Asgah, N.A.; Mahboob, S. Effect of replacing fish meal by full fat soybean meal on growth performance, feed utilization and gastrointestinal enzymes in diets for African catfish Clarias gariepinus. Braz. J. Biol. 2020, 80, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Prenner, M.L.; Prgomet, C.; Sauerwein, H.; Pfaffl, M.W.; Brož, J.; Schwarz, F.J. Effects of lactoferrin feeding on growth, feed intake and health of calves. Arch. Anim. Nutr. 2007, 61, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005. [Google Scholar] [CrossRef]
- Olyayee, M.; Javanmard, A.; Janmohammadi, H.; Kianfar, R.; Alijani, S.; Mir Ghelenj, S.A. Supplementation of broiler chicken diets with bovine lactoferrin improves growth performance, histological parameters of jejunum and immune-related gene expression. J. Anim. Physiol. Anim. Nutr. 2022. [Google Scholar] [CrossRef]
- Esmaeili, A.; Sotoudeh, E.; Morshedi, V.; Bagheri, D.; Dorafshan, S. Effects of dietary supplementation of bovine lactoferrin on antioxidant status, immune response and disease resistance of yellowfin sea bream (Acanthopagrus latus) against Vibrio harveyi. Fish Shellfish Immunol. 2019, 93, 917–923. [Google Scholar] [CrossRef]
- Badawy, T.E.; Al-Kenawy, D. Assessment of immune response supplemental immunoton and bovine lactoferrin as alternatives to antibiotics in Nile tilapia (Oreochromis niloticus). Assessment 2013, 8, 341–356. [Google Scholar]
- Kakuta, I. Effects of orally administrated bovine lactoferrin on growth and blood properties of goldfish. Aquac. Sci. 1996, 44, 419–426. [Google Scholar]
- Lygren, B.; Sveier, H.; Hjeltnes, B.; Waagbø, R. Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin C fed to Atlantic salmon (Salmo salar) for a short-term period. Fish Shellfish Immunol. 1999, 9, 95–107. [Google Scholar] [CrossRef]
- Welker, T.L.; Lim, C.; Yildirim-Aksoy, M.; Klesius, P.H. Growth, immune function, and disease and stress resistance of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of bovine lactoferrin. Aquaculture 2007, 262, 156–162. [Google Scholar] [CrossRef]
- Esteban, M.A.; Rodríguez, A.; Cuesta, A.; Meseguer, J. Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparus auratus L.). Fish Shellfish Immunol. 2005, 18, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Moradian, A.M.; Dorafshan, S.; Heyrati, F.P.; Ebrahimi, E. Effects of dietary bovine lactoferrin on growth, haemato-biochemical parameters, immune functions and tolerance to air exposure stress in the African cichlid Sciaenochromis fryeri. Aquac. Nutr. 2017, 24, 392–399. [Google Scholar] [CrossRef]
- Yokoyama, S.; Koshio, S.; Takakura, N.; Oshida, K.; Ishikawa, M.; Gallardo-Cigarroa, F.J.; Catacutan, M.R.; Teshima, S. Effect of dietary bovine lactoferrin on growth response, tolerance to air exposure and low salinity stress conditions in orange spotted grouper Epinephelus coioides. Aquaculture 2006, 255, 507–513. [Google Scholar] [CrossRef]
- Falahatkar, B.; Eslamloo, K.; Yokoyama, S. Suppression of stress responses in siberian sturgeon, Acipenser baeri, juveniles by the dietary administration of bovine lactoferrin. J. World Aquac. Soc. 2014, 45, 699–708. [Google Scholar] [CrossRef]
- Lee, S.; Mohammadi Azarm, H.; Chang, K.H. Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture 2016, 459, 110–116. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Chen, S.; Zhuojia, L.; Huang, Z.; Niu, J.; Wu, K.; Lu, X. Replacement of fish meal with fermented soybean meal in practical diets for pompano Trachinotus ovatus. Aquac. Res. 2012, 44, 151–156. [Google Scholar] [CrossRef]
- García-Ortega, A.; Kissinger, K.R.; Trushenski, J.T. Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture 2016, 452, 1–8. [Google Scholar] [CrossRef]
- Morshedi, V.; Agh, N.; Noori, F.; Jafari, F.; Ghasemi, A.; Mozanzadeh, M.T. Effects of single and combined supplementation of dietary probiotic with bovine lactoferrin and xylooligosaccharide on hemato-immunological and digestive enzymes of silvery-black porgy (Sparidentex hasta) fingerlings. Ann. Anim. Sci. 2020, 20, 137–155. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, W.; Sun, Y.; Liu, X.; Liu, M.; Cheng, Y. Juvenile Procambarus clarkii farmed using biofloc technology or commercial feed in zero-water exchange indoor tanks: A comparison of growth performance, enzyme activity and proximate composition. Aquac. Res. 2019, 50, 1834–1843. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Rombenso, A.N.; Guerrero-Rentería, Y.; Nomura-Solís, M.; Gomez-Calle, V.; Lazo, J.P.; Mata-Sotres, J.A. Enteritis induction by soybean meal in Totoaba macdonaldi diets: Effects on growth performance, digestive capacity, immune response and distal intestine integrity. Aquaculture 2018, 495, 78–89. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Lin, S.; Luo, L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim. Feed Sci. Technol. 2011, 168, 80–87. [Google Scholar] [CrossRef]
- Dias, J.; Alvarez, M.J.; Arzel, J.; Corraze, G.; Diez, A.; Bautista, J.M.; Kaushik, S.J. Dietary protein source affects lipid metabolism in the European seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 19–31. [Google Scholar] [CrossRef]
- Santigosa, E.; Sánchez, J.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mozanzadeh, M.T.; Marammazi, J.G.; Safari, O.; Gisbert, E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar] [CrossRef]
- Morshedi, V.; Agh, N.; Marammazi, J.G.; Noori, F.; Mohammadian, T.; Mozanzadeh, M.T. Combined effects of dietary bovine lactoferrin, lactobacillus plantarum, and xylooligosaccharide on hemato-immunological and digestive enzymes of silvery-black porgy (Sparidentex hasta) fingerlings. Comp. Clin. Pathol. 2019, 28, 731–736. [Google Scholar] [CrossRef]
- Pagheh, E.; Marammazi, J.G.; Agh, N.; Nouri, F.; Sepahdari, A.; Gisbert, E.; Mozanzadeh, M.T. Growth performance, hemato-immunological responses, and digestive enzyme activities in silvery-black porgy (Sparidentex hasta) fed dietary bovine lactoferrin. Probiotics Antimicrob. Proteins 2017, 10, 399–407. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Zhao, J.; Wang, J.; Dai, Y.; Zhao, Y.; Meng, Q.; Li, N. Supplementation transgenic cow’s milk containing recombinant human lactoferrin enhances systematic and intestinal immune responses in piglets. Mol. Biol. Rep. 2014, 41, 2119–2128. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Li, Y.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E. Effects of bovine lactoferrin on the immature porcine intestine. Br. J. Nutr. 2014, 111, 321–331. [Google Scholar] [CrossRef]
- Ray, A.K.; Ghosh, K.; Ringø, E. Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr. 2012, 18, 465–492. [Google Scholar] [CrossRef]
- Liu, C.; Chi, K.; Yang, M.; Guo, N. Staphylococcal enterotoxin a induces intestinal barrier dysfunction and activates NLRP3 inflammasome via NF-κB/MAPK signaling pathways in mice. Toxins 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocoll. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Peng, P.; Chen, J.; Yao, K.; Yin, Y.; Long, L.; Fang, R. The effects of dietary supplementation with porous zinc oxide on growth performance, intestinal microbiota, morphology, and permeability in weaned piglets. Anim. Sci. J. 2019, 90, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Ruan, P.; Gong, Z.J.; Zhang, Q.R. Changes of plasma D(-)-lactate, diamine oxidase and endotoxin in patients with liver cirrhosis. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 58–61. [Google Scholar]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Zhang, J.; Wang, L.; Sun, Y.; Zhang, C. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed. Aquaculture 2021, 531, 735975. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Dong, Y.; Yan, C.; Zhang, B. The effects of flavomycin and colistin sulfate pre-treatment on ileal bacterial community composition, the response to salmonella typhimurium and host gene expression in broiler chickens. Microorganisms 2019, 7, 574. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, W.; Ye, B.; Wu, X.; Li, X.; Dong, Y. Effects of replacing fishmeal protein with poultry by-product meal protein and soybean meal protein on growth, feed intake, feed utilization, gut and liver histology of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2020, 516, 734503. [Google Scholar] [CrossRef]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tomé, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. Biometals 2014, 27, 857–874. [Google Scholar] [CrossRef]
- Shimizu, H. Development of an enteric-coated lactoferrin tablet and its application. Biometals 2004, 17, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, T.; Xu, Z.; Liu, J.; Feng, J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J. Anim. Sci. 2006, 84, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, Z.; Zhou, R.; Li, C.; Shao, S.; Li, J. The mechanism of intestinal flora dysregulation mediated by intestinal bacterial biofilm to induce constipation. Bioengineered 2021, 12, 6484–6498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yang, Z.; Li, C.; Liang, H.; Wu, Z.; Pu, W. Yeast probiotics shape the gut microbiome and improve the health of early-weaned piglets. Front. Microbiol. 2018, 9, 2011. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Estruch, G.; Collado, M.C.; Penaranda, D.S.; Tomas, V.A.; Jover, C.M.; Perez, M.G.; Martinez-Llorens, S. Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA Gene. PLoS ONE 2015, 10, e0136389. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Feng, J.; He, J.; Lou, Y.; Zhu, J. Effect of dietary fermented soybean meal on growth, intestinal morphology and microbiota in juvenile large yellow croaker, Larimichthys crocea. Aquac. Res. 2019, 50, 748–757. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Lee, C.; Zhu, H.; Zheng, S.; Pierro, A. Protective effects of lactoferrin on injured intestinal epithelial cells. J. Pediatr. Surg. 2019, 54, 2509–2513. [Google Scholar] [CrossRef]
- Prgomet, C.; Prenner, M.L.; Schwarz, F.J.; Pfaffl, M.W. Effect of lactoferrin on selected immune system parameters and the gastrointestinal morphology in growing calves. J. Anim. Physiol. Anim. Nutr. 2007, 91, 109–119. [Google Scholar] [CrossRef]
- Valenti, P.; Berlutti, F.; Conte, M.P.; Longhi, C.; Seganti, L. Lactoferrin functions: Current status and perspectives. J. Clin. Gastroenterol. 2004, 38, S127–S129. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Takakura, N.; Yamauchi, K.; Tamura, Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin. Vaccine Immunol. 2006, 13, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhou, X.; Zhou, Y.; Kuang, W.; Chen, Y.; Luo, L.; Dai, F. Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish Shellfish Immunol. 2020, 103, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Lu, Y.; Li, Q. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2010, 19, 405–419. [Google Scholar] [CrossRef]
- Gu, M.; Bai, N.; Zhang, Y.; Krogdahl, Å. Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 2016, 464, 286–295. [Google Scholar] [CrossRef]
- Urán, P.A.; Gonçalves, A.A.; Taverne-Thiele, J.J.; Schrama, J.W.; Verreth, J.A.J.; Rombout, J.H.W.M. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Tusi, S.K.; Manesh, T.E.; Fathollahi, M.S.; Bagherian, A. Can tert-butylhydroquinone improve the healing of extracted tooth socket in rats? J. Dent. Res. 2017, 14, 8–12. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 2018, 8, 13482–13492. [Google Scholar] [CrossRef]
- Aguiló, A.; Tauler, P.; Fuentespina, E.; Tur, J.A.; Córdova, A.; Pons, A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol. Behav. 2005, 84, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.H.; Mui, J.J. Comparison of dietary inclusion of commercial and fermented soybean meal on oxidative status and non-specific immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 63, 208–212. [Google Scholar] [CrossRef]
- Baeverfjord, G.T.; Krogdahl, A. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Torstensen, B.E.; Hemre, G.I.; Sanden, M.; Waagbø, R. Hepatic oxidative stress in Atlantic salmon (Salmo salar L.) transferred from a diet based on marine feed ingredients to a diet based on plant ingredients. Aquac. Nutr. 2011, 17, e424–e436. [Google Scholar] [CrossRef]
- Hashem, N.M.A.; El Son, M.A.M.; Ateya, A.I.; Saleh, R.M. Impact of lactoferrin supplementation on oxidative stress, gene expression and immunity dysfunction induced by Aeromonas veronii in Nile tilapia (Oreochromis niloticus). Aquac. Res. 2022, 53, 2392–2407. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huang, H.; Liu, X.; Wang, N.; Zhong, G. Effect of bovine lactoferricin on the growth performance, digestive capacity, immune responses and disease resistance in Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2022, 123, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wen, H.; Gou, G.W.; Liu, T.L.; Lu, X.; Deng, D.F. Preliminary study to evaluate the effects of dietary bile acids on growth performance and lipid metabolism of juvenile genetically improved farmed tilapia (Oreochromis niloticus) fed plant ingredient-based diets. Aquac. Nutr. 2018, 24, 1175–1183. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; El-Hais, A.M.; Olivier, A. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 2018, 490, 228–235. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwashita, Y.; Matsunari, H.; Sugita, T.; Furuita, H.; Akimoto, A.; Okamatsu, K.; Suzuki, N. Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus mykiss. Aquaculture 2010, 309, 173–180. [Google Scholar] [CrossRef]
- Ye, H.; Xu, M.; Chen, L.; Tan, X.; Chen, S.; Zou, C.; Sun, Z.; Liu, Q.; Ye, C.; Wang, A. Effects of dietary plant protein sources influencing hepatic lipid metabolism and hepatocyte apoptosis in hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquaculture 2019, 506, 437–444. [Google Scholar] [CrossRef]
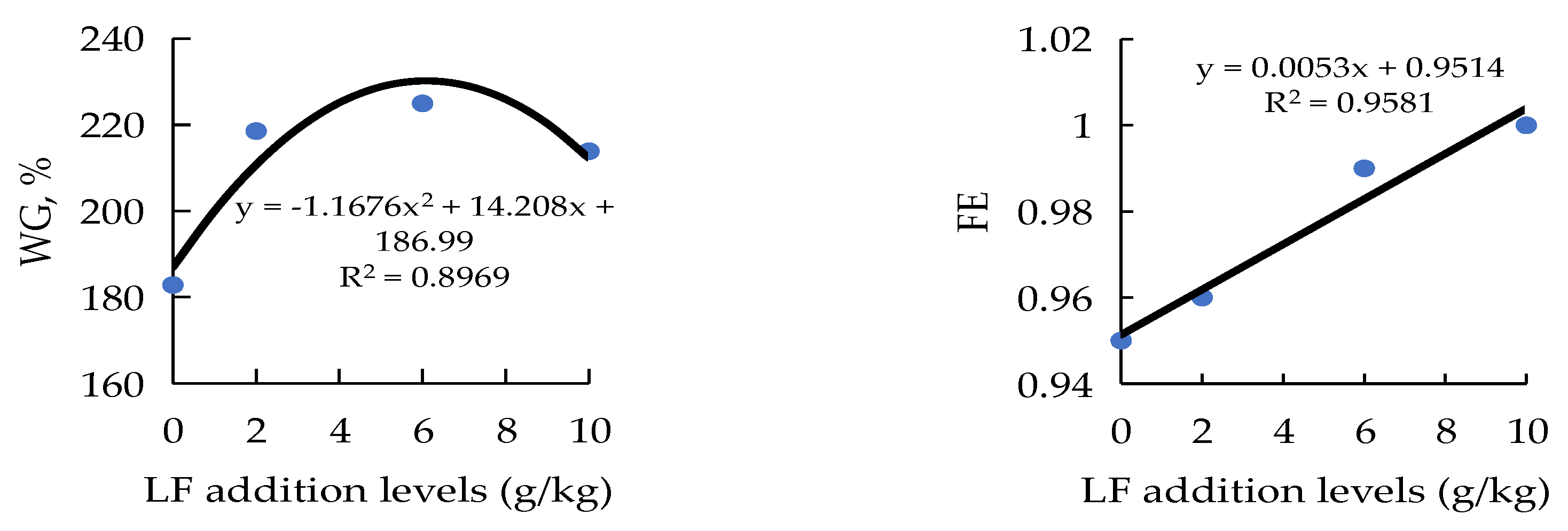

| Items | Diets 1 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| Ingredients | |||||
| Fish meal 2 | 520 | 220 | 220 | 220 | 220 |
| Casein | 119.8 | 112.7 | 112.7 | 112.7 | 112.7 |
| Gelatin | 30 | 28.2 | 28.2 | 28.2 | 28.2 |
| Soybean meal 3 | — | 470 | 470 | 470 | 470 |
| Soybean oil | 35 | 35 | 35 | 35 | 35 |
| Fish oil | 8.2 | 35.2 | 35.2 | 35.2 | 35.2 |
| Soybean lecithin | 20 | 20 | 20 | 20 | 20 |
| Lactoferrin 4 | — | — | 2 | 6 | 10 |
| Corn starch | 177.2 | 32.6 | 30.6 | 26.6 | 22.6 |
| Sodium alginate | 10 | 10 | 10 | 10 | 10 |
| Ca(H2PO4)2 | 15 | 15 | 15 | 15 | 15 |
| Choline chloride | 4 | 4 | 4 | 4 | 4 |
| Stay-C (350 g/kg) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin premix 5 | 4 | 4 | 4 | 4 | 4 |
| Mineral premix 5 | 5 | 5 | 5 | 5 | 5 |
| Taurine | 5 | 8 | 8 | 8 | 8 |
| Microcrystalline cellulose | 46.5 | — | — | — | — |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutrient level (analyzed values) | |||||
| Dry matter | 950.6 | 957.8 | 955.6 | 952.7 | 954.7 |
| Crude protein | 480.5 | 503.4 | 517.2 | 513.3 | 515 |
| Crude lipid | 120.6 | 114.2 | 116.3 | 116.4 | 116.3 |
| Ash | 91.7 | 84.2 | 84.8 | 84 | 82.9 |
| Genes 1 | Primer Sequence (5′ to 3′) 2 | E-Value (%) | Accession Number |
|---|---|---|---|
| IL-8 | F: AAGTTTGCCTTGACCCCGAA | 94.0 | FJ913064.1 |
| R: TGAAGCAGATCTCTCCCGGT | |||
| IL-1β | F: GCAACTCCACCGACTGATGA | 116.0 | EF582837.1 |
| R: ACCAGGCTGTTATTGACCCG | |||
| IL-10 | F: GTCCACCAGCATGACTCCTC | 99.0 | KJ741852.1 |
| R: AGGGAAACCCTCCACGAATC | |||
| TGF-β1 | F: GCTTACGTGGGTGCAAACAG | 102.0 | GQ503351.1 |
| R: ACCATCTCTAGGTCCAGCGT | |||
| IL-12 | F: CCAGATTGCACAGCTCAGGA | 115.0 | KC662465.1 |
| R: CCGGACACAGATGGCCTTAG | |||
| TNF-α | F: GGATCTGGCGCTACTCAGAC | 91.0 | FJ009449.1 |
| R: CGCCCAGATAAATGGCGTTG | |||
| β-actin | F: TGCTGTCCCTGTATGCCTCT | 104.0 | AY510710.2 |
| R: CCTTGATGTCACGCACGAT |
| Items | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| Growth performance | |||||
| IBW (g/fish) 3 | 33.82 ± 0.10 | 33.76 ± 0.06 | 33.74 ± 0.05 | 33.80 ± 0.07 | 33.98 ± 0.01 |
| FBW (g/fish) 3 | 113.24 ± 0.66 b | 95.47 ± 2.59 a | 107.49 ± 4.28 b | 109.85 ± 3.46 b | 106.65 ± 2.96 b |
| WG (%) 3 | 234.81 ± 2.54 b | 182.81 ± 7.24 a | 218.56 ± 12.96 b | 224.98 ± 9.89 b | 208.68 ± 5.73 ab |
| SGR (%/d) 3 | 2.16 ± 0.01 b | 1.86 ± 0.05 a | 2.07 ± 0.07 b | 2.10 ± 0.05 b | 2.04 ± 0.05 b |
| FE 3 | 0.98 ± 0.00 | 0.95 ± 0.00 | 0.96 ± 0.12 | 0.99 ± 0.07 | 1.00 ± 0.12 |
| Survival (%) 3 | 100.00 ± 0.00 | 97.78 ± 1.11 | 100.00 ± 0.00 | 100.00 ± 0.00 | 98.89 ± 1.11 |
| HSI (%) 4 | 1.31 ± 0.09 | 1.24 ± 0.04 | 1.28 ± 0.07 | 1.24 ± 0.05 | 1.17 ± 0.02 |
| CF (g/cm3) 4 | 3.16 ± 0.07 | 3.05 ± 0.11 | 3.19 ± 0.02 | 2.93 ± 0.12 | 2.94 ± 0.03 |
| Proximate composition (%) | |||||
| Moisture | 67.05 ± 0.21 | 67.27 ± 0.22 | 67.56 ± 0.37 | 67.42 ± 0.34 | 68.26 ± 0.39 |
| Crude protein | 18.01 ± 0.49 | 17.95 ± 0.27 | 17.93 ± 0.90 | 19.20 ± 0.33 | 17.75 ± 0.42 |
| Crude lipid | 8.25 ± 0.17 | 7.90 ± 0.29 | 8.18 ± 0.40 | 7.85 ± 0.32 | 7.49 ± 0.11 |
| Ash | 5.00 ± 0.15 | 4.96 ± 0.07 | 4.90 ± 0.06 | 4.87 ± 0.22 | 4.93 ± 0.09 |
| Items 3 | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| SOD (U/mg protein) | 71.67 ± 5.50 | 68.92 ± 1.76 | 62.16 ± 5.85 | 64.26 ± 3.23 | 60.70 ± 2.15 |
| GSH-Px (U/mg protein) | 79.58 ± 3.31 bc | 65.29 ± 2.97 a | 82.52 ± 1.76 bc | 72.99 ± 1.35 b | 86.76 ± 4.00 c |
| T-AOC (U/mg protein) | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.02 | 0.18 ± 0.01 |
| MDA (nmol/mg protein) | 3.00 ± 0.28 a | 4.56 ± 0.88 b | 1.97 ± 0.21 a | 1.86 ± 0.12 a | 2.55 ± 0.05 a |
| YMDA = 0.0783X2 − 0.9351X + 4.2006, R2 = 0.8297, X = LF supplementation levels (g/kg) | |||||
| Items 3 | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| HDL-C (mmol/L) | 1.06 ± 0.05 b | 1.00 ± 0.03 b | 0.81 ± 0.05 a | 1.03 ± 0.03 b | 0.83 ± 0.09 a |
| LDL-C (mmol/L) | 0.28 ± 0.01 c | 0.19 ± 0.01 b | 0.18 ± 0.01 b | 0.12 ± 0.01 a | 0.12 ± 0.01 a |
| TC (mmol/L) | 3.77 ± 0.21 b | 3.49 ± 0.23 ab | 3.35 ± 0.19 ab | 3.09 ± 0.09 ab | 2.90 ± 0.09 a |
| TG (mmol/L) | 1.61 ± 0.17 | 1.36 ± 0.08 | 1.25 ± 0.06 | 1.57 ± 0.10 | 1.55 ± 0.07 |
| YLDL-C = −0.0079X + 0.188, R2 = 0.8573, X = LF supplementation levels (g/kg) YTC = −0.0592x + 3.4741, R2 = 0.9931, X = LF supplementation levels (g/kg) | |||||
| Items | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| Lipase (U/mg protein) | 0.68 ± 0.00 a | 0.61 ± 0.00 a | 0.84 ± 0.05 b | 0.86 ± 0.02 b | 0.95 ± 0.05 bc |
| Amylase (U/mg protein) | 0.76 ± 0.06 | 0.73 ± 0.11 | 0.90 ± 0.04 | 0.73 ± 0.07 | 0.82 ± 0.06 |
| Trypsin (U/g protein) | 256.07 ± 17.23 b | 175.55 ± 17.55 a | 238.95 ± 17.46 b | 235.03 ± 9.36 b | 283.57 ± 8.83 bc |
| Protease (U/mg protein) | 20.54 ± 0.87 | 15.91 ± 2.04 | 17.37 ± 2.91 | 23.54 ± 2.56 | 26.54 ± 2.53 |
| YLipase = 0.0283X + 0.6876, R2 = 0.7515, X = LF supplementation levels (g/kg) YTrypsin = 8.8954X + 193.25, R2 = 0.7917, X = LF supplementation levels (g/kg) YProtease = 1.1231X + 15.786, R2 = 0.9775, X = LF supplementation levels (g/kg) | |||||
| Items 3 | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| DAO (U/L) | 19.75 ± 1.39 | 20.59 ± 1.05 | 16.09 ± 1.22 | 17.77 ± 2.40 | 17.72 ± 1.47 |
| D-Lac (nmol/mL) | 2.03 ± 0.20 a | 4.05 ± 0.23 b | 1.41 ± 0.18 a | 1.59 ± 0.21 a | 1.41 ± 0.07 a |
| ET-1 (ng/L) | 1.91 ± 0.07 | 2.12 ± 0.09 | 2.27 ± 0.10 | 1.92 ± 0.13 | 2.36 ± 0.20 |
| ET (EU/L) | 1.51 ± 0.03 b | 1.70 ± 0.10 c | 1.23 ± 0.01 a | 1.25 ± 0.01 a | 1.25 ± 0.04 a |
| Items 3 | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM60 | LF2 | LF6 | LF10 | |
| PI | |||||
| lMF (μm) | 577.30 ± 87.68 | 489.10 ± 54.31 | 574.92 ± 35.62 | 513.26 ± 50.67 | 737.53 ± 95.20 |
| tML (μm) | 63.24 ± 6.74 | 64.56 ± 8.11 | 79.05 ± 2.27 | 86.60 ± 9.31 | 86.00 ± 2.51 |
| nMF (unit) | 42.50 ± 4.25 | 45.83 ± 3.09 | 51.67 ± 1.36 | 50.33 ± 8.62 | 48.00 ± 3.55 |
| MI | |||||
| lMF (μm) | 465.12 ± 50.20 ab | 381.90 ± 42.42 a | 580.47 ± 9.06 b | 356.66 ± 9.37 a | 540.48 ± 47.06 b |
| tML (μm) | 53.53 ± 2.44 ab | 44.96 ± 4.06 a | 76.61 ± 7.02 b | 63.13 ± 4.61 ab | 69.62 ± 7.81 b |
| nMF (unit) | 34.33 ± 2.20 | 31.67 ± 1.01 | 43.00 ± 4.36 | 34.83 ± 3.49 | 39.17 ± 0.33 |
| DI | |||||
| lMF (μm) | 417.87 ± 63.72 | 337.13 ± 44.48 | 437.82 ± 22.32 | 397.03 ± 4.38 | 466.67 ± 53.64 |
| tML (μm) | 87.58 ± 7.61 b | 51.53 ± 1.48 a | 74.80 ± 3.34 b | 86.49 ± 1.35 b | 69.78 ± 7.20 b |
| nMF (unit) | 32.00 ± 5.20 | 37.00 ± 4.00 | 40.83 ± 5.33 | 35.00 ± 1.04 | 34.00 ± 3.21 |
| DI: YtML = −1.0492X2 + 12.167X + 52.619, R2 = 0.9884, X = LF supplementation levels (g/kg) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Qin, Y.; Ke, L.; Wang, X.; Wang, K.; Sun, Y.; Ye, J. Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides). Metabolites 2022, 12, 915. https://doi.org/10.3390/metabo12100915
Song T, Qin Y, Ke L, Wang X, Wang K, Sun Y, Ye J. Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides). Metabolites. 2022; 12(10):915. https://doi.org/10.3390/metabo12100915
Chicago/Turabian StyleSong, Tao, Yingmei Qin, Liner Ke, Xuexi Wang, Kun Wang, Yunzhang Sun, and Jidan Ye. 2022. "Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides)" Metabolites 12, no. 10: 915. https://doi.org/10.3390/metabo12100915
APA StyleSong, T., Qin, Y., Ke, L., Wang, X., Wang, K., Sun, Y., & Ye, J. (2022). Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides). Metabolites, 12(10), 915. https://doi.org/10.3390/metabo12100915









