Pharmacological Interaction of Quercetin Derivatives of Tilia americana and Clinical Drugs in Experimental Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plant Material
2.2.1. Preparation of the Extract and the Bioactive Fraction
2.2.2. HPLC Analysis
2.2.3. Presence of Flavonoid Derivatives of Quercetin by Brain Histological Analysis
2.3. Drugs and Reagents
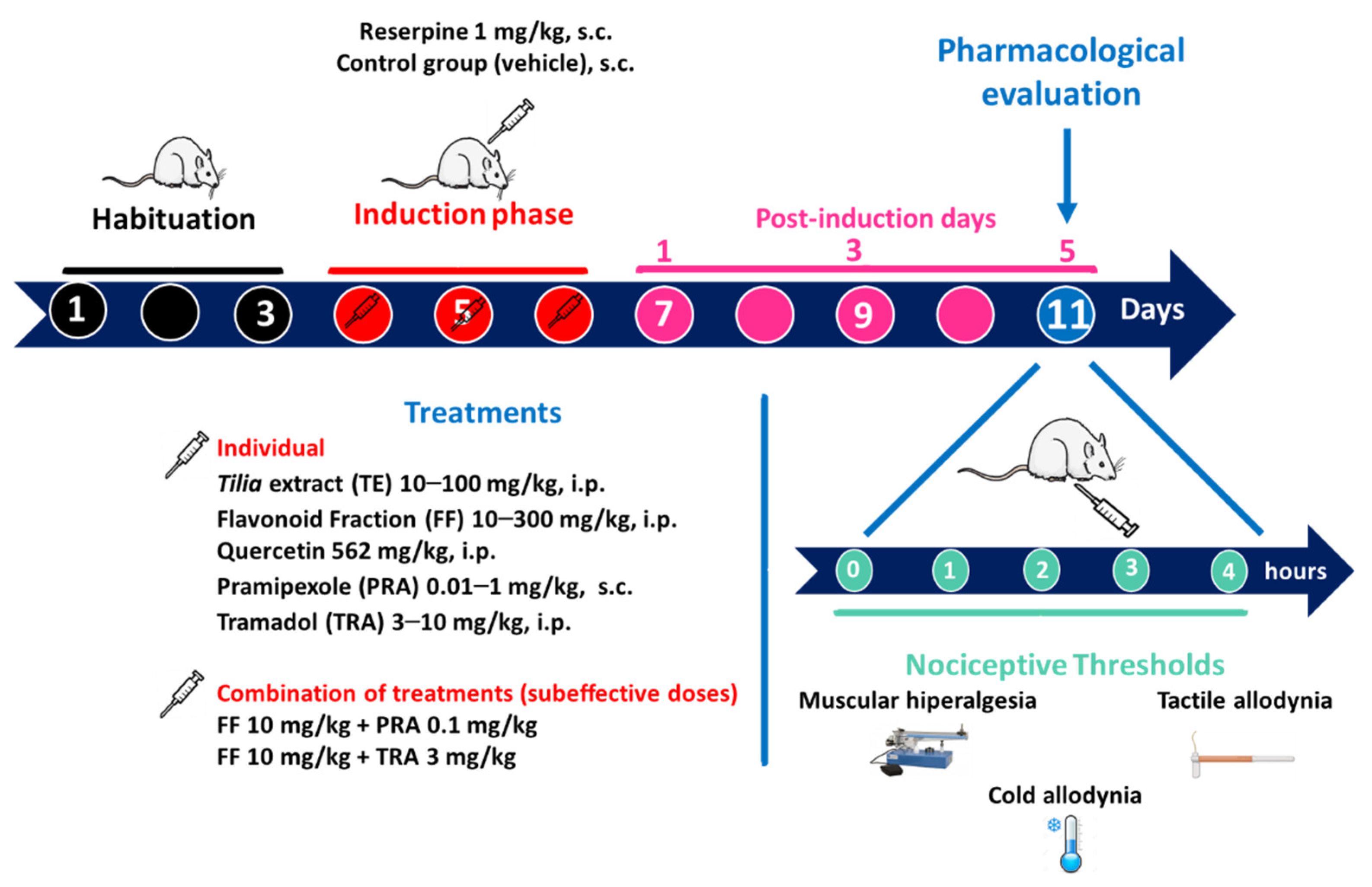
2.4. Reserpine (RES)-Induced FM Model
2.4.1. Experimental Groups
2.4.2. Threshold for Muscle Pressure (Mechanical Hyperalgesia)
2.4.3. Threshold for Tactile Response (Tactile Allodynia)
2.4.4. Threshold for the Cold Stimulus (Cold Allodynia)
2.5. In Silico Analysis
2.6. Statistical Analysis
3. Results
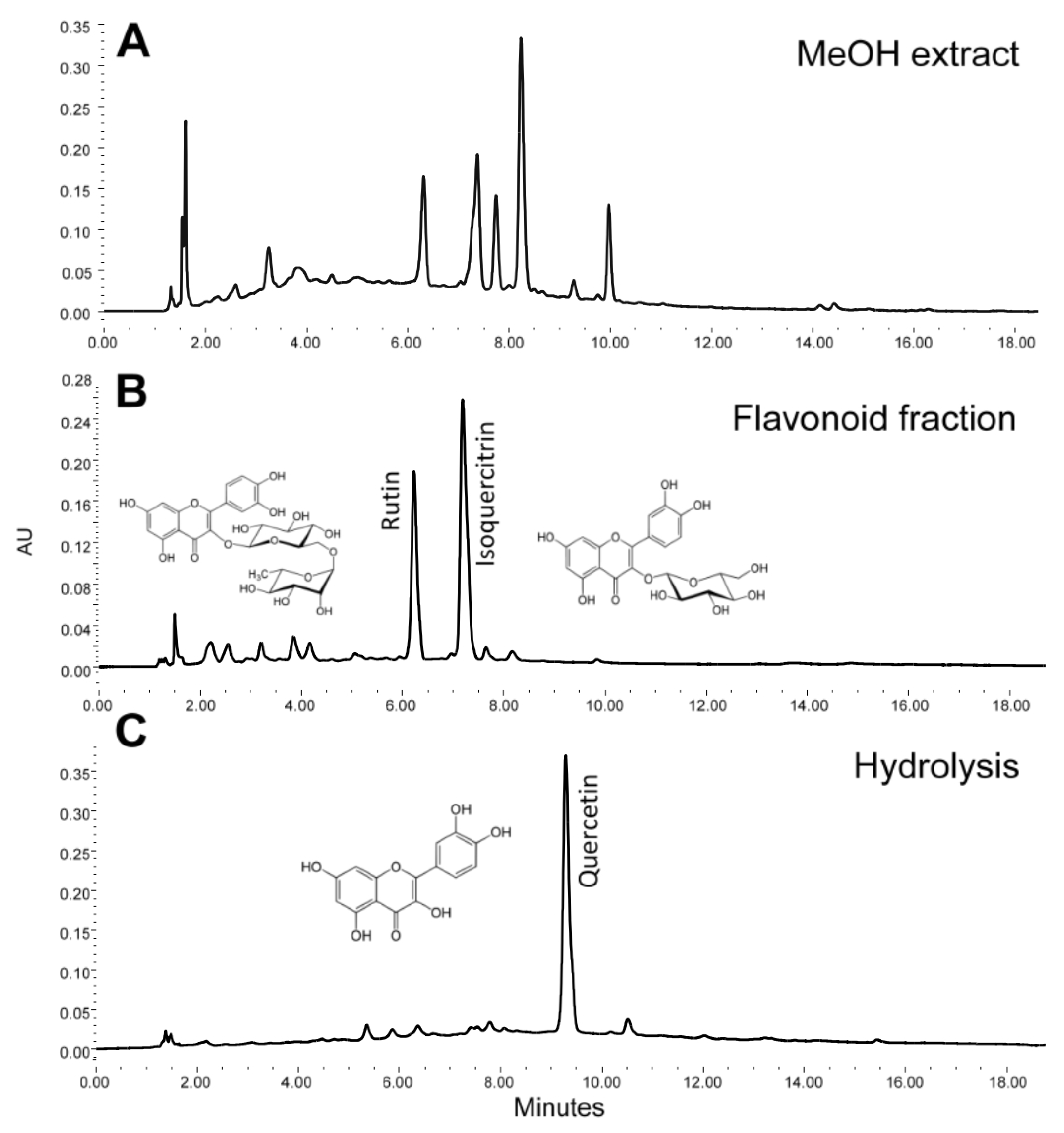
3.1. Phytochemical Analysis
3.1.1. Flavonoid Characterization
3.1.2. Epifluorescence of Flavonoids
3.2. Pharmacological Evaluation
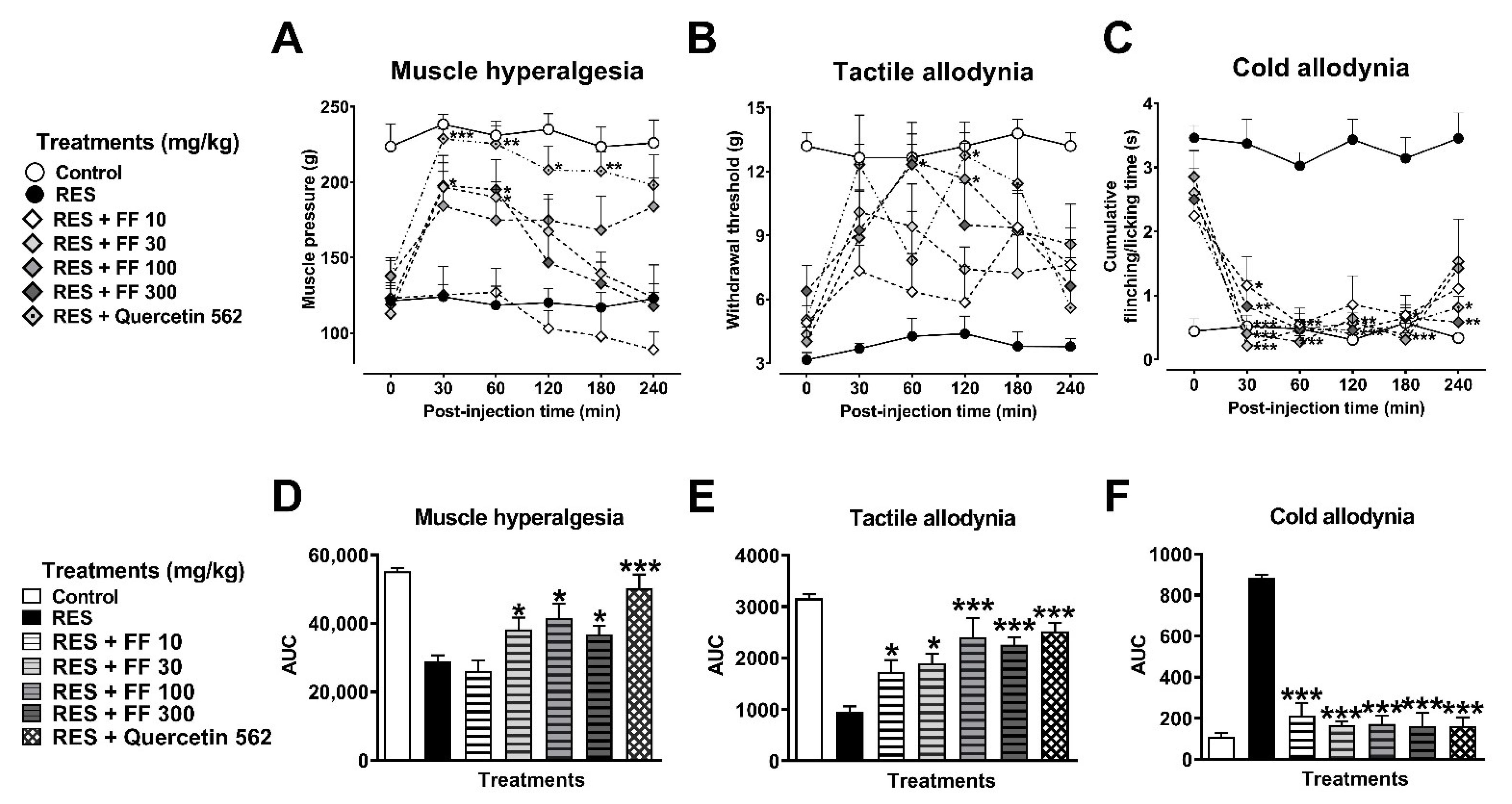
3.2.1. Antinociceptive Effects of Tilia Extract (TE) and Flavonoid Fraction of Tilia (FF)
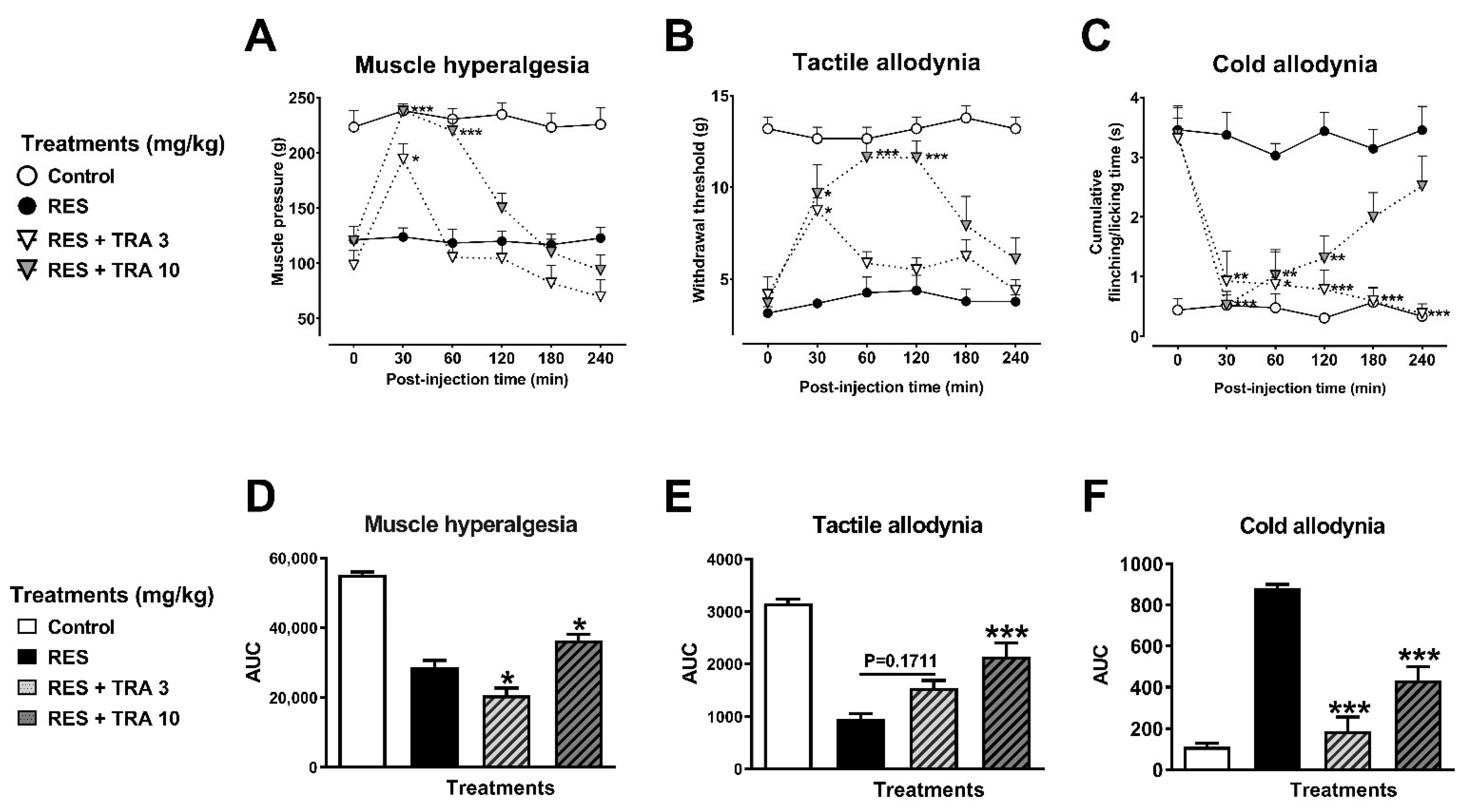
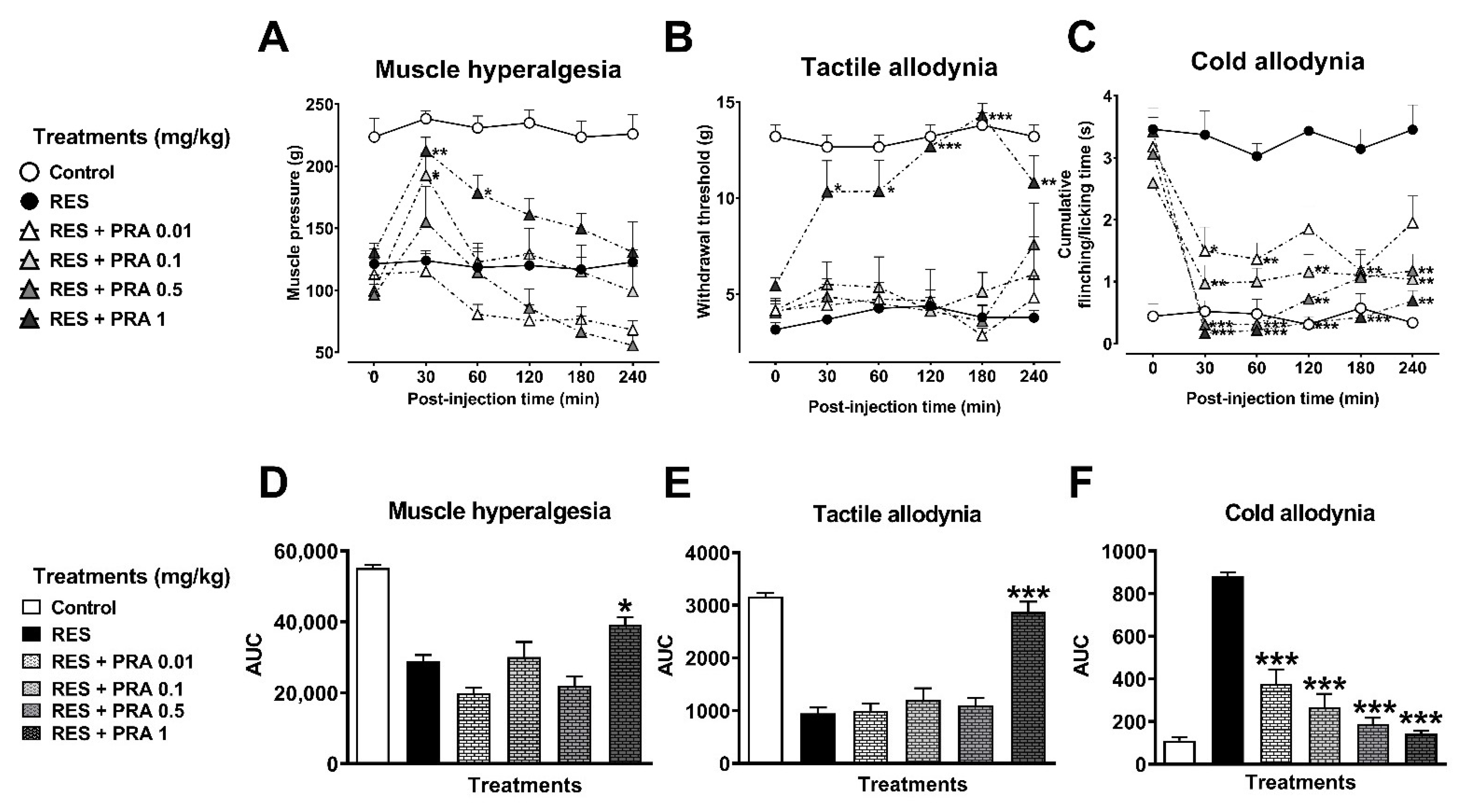
3.2.2. Antihyperalgesic and Antiallodynic Effects of Tramadol (TRA) and Pramipexole (PRA)
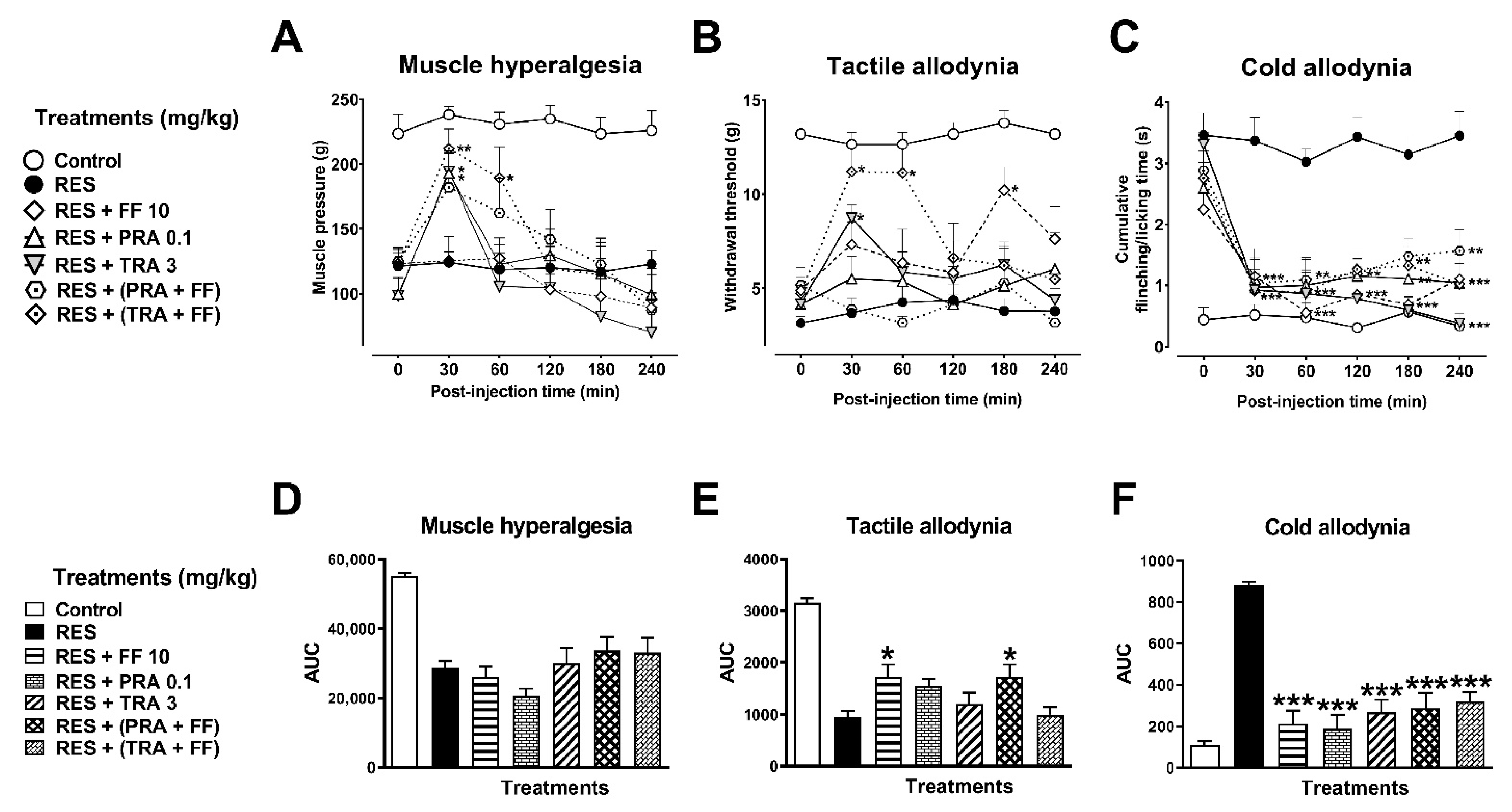
3.2.3. Combination of FF with TRA and PRA
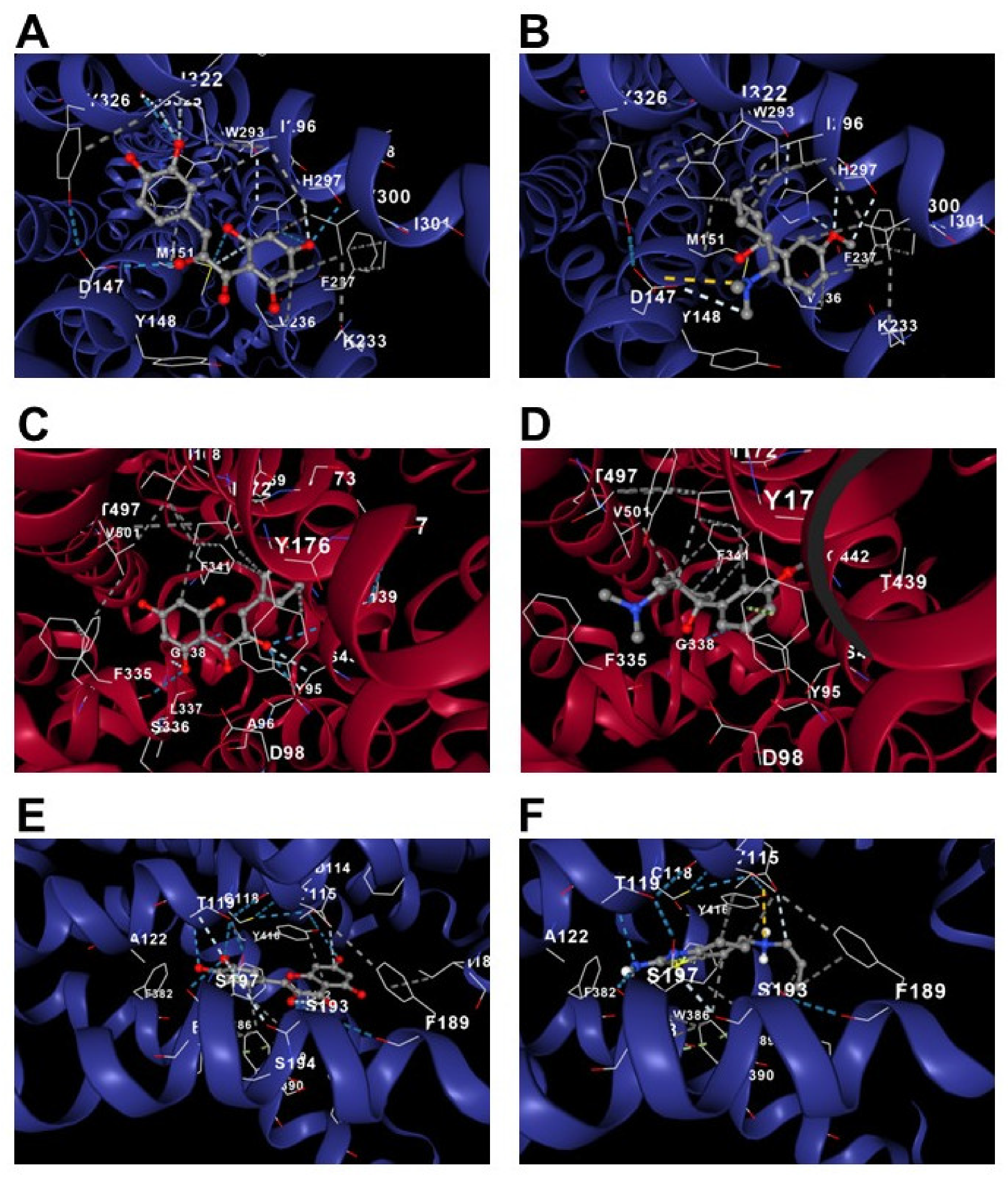
3.2.4. In Silico Analysis of Quercetin, TRA, and PRA on the Serotonin Transporter and µ-opioid and D2 Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giorgi, V.; Sirotti, S.; Romano, M.E.; Marotto, D.; Ablin, J.N.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H. Controversies and challenges in fibromyalgia: A review and a proposal. Ther. Adv. Musculoskelet. Dis. 2017, 9, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med. Clin. 2017, 149, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Franco, L.; Tamayo-Valenzuela, A.C.; Guevara-López, U.; Bautista-Eugenio, V. Tratamiento farmacológico de la fibromialgia: La experiencia de tres años. Rev. Fac. Med. UNAM 2010, 53, 11–18. [Google Scholar]
- Goldenberg, D.L.; Burckhardt, C.; Crofford, L. Management of fibromyalgia syndrome. J. Am. Med. Assoc. 2004, 292, 2388–2395. [Google Scholar] [CrossRef]
- Wright, C.L.; Mist, S.D.; Ross, R.L.; Jones, K.D. Duloxetine for the treatment of fibromyalgia. Expert Rev. Clin. Immunol. 2010, 6, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Flub, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Maffei, M.E. Fibromyalgia: Recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef]
- Ventura-Martinez, R.; Mares-Sánchez, J.J.; Avilés-Herrera, J.; Ángeles-López, G.E.; Déciga-Campos, M.; González-Trujano, M.E.; López-Muñoz, F.J. Antinociceptive synergy between metamizole and hesperidin in a model of visceral pain in mice. Arch. Med. Res. 2021, 52, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Thieme, K.; Turk, D.C. Guidelines on the management of fibromyalgia syndrome—A systematic review. Eur. J. Pain 2010, 14, 5–10. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; Martinez, A.L.; Gonzalez-Trujano, M.E.; Moreno, J.; Vibrans, H.; Soto-Hernandez, M. Pharmacological evaluation of the anxiolytic and sedative effects of Tilia americana L. Var. mexicana in mice. J. Ethnopharmacol. 2007, 109, 140–145. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Martínez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.; Soto-Hernández, M. HPLC/MS Analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97. [Google Scholar] [CrossRef]
- Martínez, A.L.; Gonzalez-Trujano, M.E.; Aguirre-Hernandez, E.; Moreno, J.; Soto-Hernandez, M.; Lopez-Munoz, F.J. Antinociceptive activity of Tilia americana var. mexicana inflorescences and quercetin in the formalin test and in an arthritic pain model in rats. Neuropharmacology 2009, 56, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.L.; González-Trujano, M.E.; Chávez, M.; Pellicer, F.; Moreno, J.; López-Muñoz, F.J. Hesperidin produces antinociceptive response and synergistic interaction with ketorolac in an arthritic gout-type pain in rats. Pharmacol. Biochem. Behav. 2011, 97, 683–689. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J. Antihyperalgesic effect of hesperidin improves with diosmin in experimental neuropathic pain. Biomed. Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Roman-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jimenez-Ferrer, J.E. Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J. Ethnopharmacol. 2008, 118, 312–317. [Google Scholar] [CrossRef]
- Quiñonez-Bastidas, G.N.; Navarrete, A. Mexican plants and derivates compounds as alternative for inflammatory and neuropathic pain treatment—A review. Plants 2021, 10, 865. [Google Scholar] [CrossRef]
- Pérez-Ortega, G.; Guevara-Fefer, P.; Chávez, M.; Herrera, J.; Martínez, A.; Martínez, A.L.; González-Trujano, M.E. Sedative and anxiolytic efficacy of Tilia americana var. mexicana inflorescences used traditionally by communities of state of Michoacan, Mexico. J. Ethnopharmacol. 2008, 116, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; De la Luz-Cuellar, Y.E.; Granados-Soto, V.; González-Trujano, M.E.; Fernández-Guasti, A. Sex differences and estradiol involvement in hyperalgesia and allodynia in an experimental model of fibromyalgia. Horm. Behav. 2018, 97, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Leon, A.; Fernández-Guasti, A.; Martínez, A.; Pellicer, F.; González-Trujano, M.E. Sleep architecture is altered in the reserpine-induced fibromyalgia model in ovariectomized rats. Behav. Brain Res. 2019, 364, 383–392. [Google Scholar] [CrossRef]
- Pascual, M.E.; Carretero, M.E.; Slowing, K.V.; Villar, A. Simplified screening by TLC of plant drugs. Pharm. Biol. 2002, 40, 139–143. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier Science: Sidney, Australia, 2007; Volume 7, ISBN 9780080475134. [Google Scholar]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, Y.W.; Na, H.S.; Kim, S.H.; Chung, J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar]
- Oe, T.; Tsukamoto, M.; Nagakura, Y. Reserpine causes biphasic nociceptive sensitivity alteration in conjunction with brain biogenic amine tones in rats. Neuroscience 2010, 169, 1860–1871. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.-T.; Hou, M.-C.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2019, 41, 138–144. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Wahner-Roedler, D.L.; Elkin, P.L.; Vincent, A.; Thompson, J.M.; Oh, T.H.; Loehrer, L.L.; Mandrekar, J.N.; Bauer, B.A. Use of complementary and alternative medical therapies by patients referred to a fibromyalgia treatment program at a tertiary care center. Mayo Clin. Proc. 2005, 80, 55–60. [Google Scholar] [CrossRef]
- Mogil, J.S.; Davis, K.D.; Derbyshire, S.W. The necessity of animal models in pain research. Pain 2010, 151, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Vaeroy, H.; Javors, M.; Nyberg, F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992, 35, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Legangneux, E.; Mora, J.J.; Spreux-Varoquaux, O.; Thorin, I.; Herrou, M.; Alvado, G.; Gomeni, C. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H] imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology 2001, 40, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Dadabhoy, D.; Crofford, L.J.; Spaeth, M.; Russell, I.J.; Clauw, D.J. Biology and therapy of fibromyalgia. evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res. Ther. 2008, 10, 211. [Google Scholar] [CrossRef]
- Nagakura, Y.; Miwa, M.; Yoshida, M.; Miura, R.; Tanei, S.; Tsuji, M.; Takeda, H. Spontaneous pain-associated facial expression and efficacy of clinically used drugs in the reserpine-induced rat model of fibromyalgia. Eur. J. Pharmacol. 2019, 864, 172716. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Katanosaka, K.; Yasui, M.; Hayashi, K.; Yamashita, M.; Wakatsuki, K.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain 2015, 156, 415–427. [Google Scholar] [CrossRef]
- De la Luz-Cuellar, Y.E.; Rodríguez-Palma, E.J.; Franco-Enzástiga, Ú.; Salinas-Abarca, A.B.; Delgado-Lezama, R.; Granados-Soto, V. Blockade of spinal A5-GABAA receptors differentially reduces reserpine-induced fibromyalgia-type pain in female rats. Eur. J. Pharmacol. 2019, 858, 172443. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Serra, A.; Alfosea-Cuadrado, G.; Cervera-Ferri, A.; González-Soler, E.M.; Lloret, A.; Martínez-Ricós, J.; Teruel-Martí, V.; Valverde-Navarro, A.A. Hippocampal oscillatory dynamics and sleep atonia are altered in an animal model of fibromyalgia: Implications in the search for biomarkers. J. Comp. Neurol. 2020, 528, 1367–1391. [Google Scholar] [CrossRef]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; González-Soler, E.M.; Martínez-Expósito, F.; Blasco-Ausina, M.C.; Martínez-Bellver, S.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Biasi, G.; Manca, S.; Manganelli, S.; Marcolongo, R. Tramadol in the fibromyalgia syndrome: A controlled clinical trial versus placebo. Int. J. Clin. Pharmacol. Res. 1998, 18, 13–19. [Google Scholar]
- da Rocha, A.P.; Mizzaci, C.C.; Pinto, A.C.P.N.; da Silva Vieira, A.G.; Civile, V.; Trevisani, V.F.M. Tramadol for management of fibromyalgia pain and symptoms: Systematic review. Int. J. Clin. Pract. 2020, 74, e13455. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B.; Holman, A.J. An elephant among us: The role of dopamine in the pathophysiology of fibromyalgia. J. Rheumatol. 2009, 36, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-Y.; Liu, C.-T.; Su, Y.-L.; Chen, S.-Y.; Chen, Y.-H.; Tsai, M.-Y. A review of complementary therapies with medicinal plants for chemotherapy-induced peripheral neuropathy. Complement. Ther. Med. 2019, 42, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Rodríguez, N.; González-Trujano, M.E.; Aguirre-Hernández, E.; Ruíz-García, M.; Sampieri, A.; Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid. Med. Cell. Longev. 2014, 2014, 329172. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Santi, D.; Tabach, R. Flavonol glycosides found in hydroethanolic extracts from Tilia cordata, a species utilized as anxiolytics. Rev. Bras. Plantas Med. 2013, 15, 217–224. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Lapa, F.D.R.; Gadotti, V.M.; Missau, F.C.; Pizzolatti, M.G.; Marques, M.C.A.; Dafré, A.L.; Farina, M.; Rodrigues, A.L.S.; Santos, A.R.S. Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin. Pharmacol. Toxicol. 2009, 104, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Leon, A.; Fernández-Guasti, A.; González-Trujano, M.E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats. Eur. J. Pain 2016, 20, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-Inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.C.; Wong, D.V.T.; Lima-Júnior, R.C.P.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In Vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-ΚB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, V.M.; Santos, A.R.S.; Meyre-Silva, C.; Schmeling, L.O.; Machado, C.; Liz, F.H.; Filho, V.C. Antinociceptive action of the extract and the flavonoid quercitrin isolated from Bauhinia microstachya leaves. J. Pharm. Pharmacol. 2010, 57, 1345–1351. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Jiang, G.; Dong, X.; Zhu, Z.; Huang, G. Regulation effects of isoquercitrin on inflammatory in LPS-induced RAW264.7 cell. Her. Med. 2017, 36, 601–605. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; Da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Maj, J.; Rogóz, Z.; Skuza, G.; Kołodziejczyk, K. Antidepressant effects of pramipexole, a novel dopamine receptor agonist. J. Neural Transm. 1997, 104, 525–533. [Google Scholar] [CrossRef]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. J. Pharmacol. Exp. Ther. 1992, 260, 275–285. [Google Scholar] [PubMed]
- Kaneko, K.; Umehara, M.; Homan, T.; Okamoto, K.; Oka, M.; Oyama, T. The analgesic effect of tramadol in animal models of neuropathic pain and fibromyalgia. Neurosci. Lett. 2014, 562, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Villafán-Gutiérrez, R.; Espinosa-Juárez, J.V.; Jaramillo-Morales, O.A.; López-Muñoz, F.J. Synergistic interaction between haloperidol and gabapentin in a model of neuropathic nociception in rat. Eur. J. Pharmacol. 2021, 891, 1–9. [Google Scholar] [CrossRef]
- Déciga-Campos, M.; Beltrán-Villalobos, K.L.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Ángeles-López, G.E.; Ventura-Martínez, R. Synergistic herb-herb interaction of the antinociceptive and anti-inflammatory effects of Syzygium aromaticum and Rosmarinus officinalis combination. Evid.-Based Complement. Altern. Med. 2021, 2021, 8916618. [Google Scholar] [CrossRef]
- Greco, W.R.; Faessel, H.; Levasseur, L. The search for cytotoxic synergy between anticancer agents: A case of Dorothy and the ruby slippers? J. Natl. Cancer Inst. 1996, 88, 699–700. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front. Pharmacol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]
- Machado, D.G.; Bettio, L.E.B.; Cunha, M.P.; Santos, A.R.S.; Pizzolatti, M.G.; Brighente, I.M.C.; Rodrigues, A.L.S. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004, 75, 1659–1699. [Google Scholar] [CrossRef]
- Naidu, P.S.; Singh, A.; Kulkarni, S.K. D2-Dopamine receptor and alpha2-adrenoreceptor-mediated analgesic response of quercetin. Indian J. Exp. Biol. 2003, 41, 1400–1404. [Google Scholar]
- Chen, Q.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2015, 33, 840–848. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinto-Ortiz, Y.E.; González-Trujano, M.E.; Sánchez-Jaramillo, E.; Moreno-Pérez, G.F.; Jacinto-Gutiérrez, S.; Pellicer, F.; Fernández-Guasti, A.; Hernandez-Leon, A. Pharmacological Interaction of Quercetin Derivatives of Tilia americana and Clinical Drugs in Experimental Fibromyalgia. Metabolites 2022, 12, 916. https://doi.org/10.3390/metabo12100916
Quinto-Ortiz YE, González-Trujano ME, Sánchez-Jaramillo E, Moreno-Pérez GF, Jacinto-Gutiérrez S, Pellicer F, Fernández-Guasti A, Hernandez-Leon A. Pharmacological Interaction of Quercetin Derivatives of Tilia americana and Clinical Drugs in Experimental Fibromyalgia. Metabolites. 2022; 12(10):916. https://doi.org/10.3390/metabo12100916
Chicago/Turabian StyleQuinto-Ortiz, Yara Elena, María Eva González-Trujano, Edith Sánchez-Jaramillo, Gabriel Fernando Moreno-Pérez, Salomón Jacinto-Gutiérrez, Francisco Pellicer, Alonso Fernández-Guasti, and Alberto Hernandez-Leon. 2022. "Pharmacological Interaction of Quercetin Derivatives of Tilia americana and Clinical Drugs in Experimental Fibromyalgia" Metabolites 12, no. 10: 916. https://doi.org/10.3390/metabo12100916
APA StyleQuinto-Ortiz, Y. E., González-Trujano, M. E., Sánchez-Jaramillo, E., Moreno-Pérez, G. F., Jacinto-Gutiérrez, S., Pellicer, F., Fernández-Guasti, A., & Hernandez-Leon, A. (2022). Pharmacological Interaction of Quercetin Derivatives of Tilia americana and Clinical Drugs in Experimental Fibromyalgia. Metabolites, 12(10), 916. https://doi.org/10.3390/metabo12100916






