Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC
Abstract
:1. Introduction
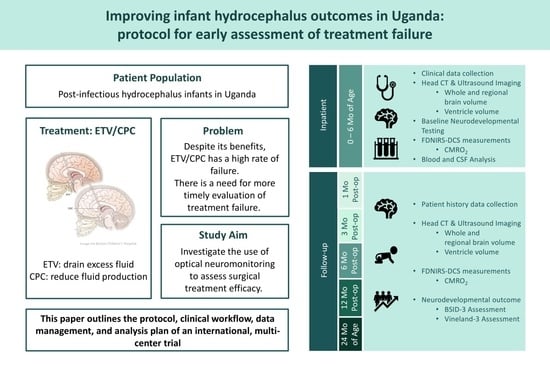
- Aim 1: To establish the first large-scale dataset of the longitudinal evolution of cerebral physiology, structure, treatment outcome, and long-term neurodevelopmental outcome during hydrocephalus progression.
- (a)
- Brain physiology: Perform FDNIRS-DCS measurements during hospital stay and at each follow-up exam to determine SO2, CBF, and CMRO2.
- (b)
- Brain structure: Perform a CT/HUS head scan pre-operatively and post-operatively at day one, six and 12 months, and at 24 months of age to determine brain and CSF volumes as well as extra-axial layer thickness.
- (c)
- Clinical course: Follow response to treatment and occurrence of treatment failure.
- (d)
- Neurodevelopmental outcome: Following the baseline assessment, evaluate the outcomes at six and 12 months post-operatively, and at 24 months of age with the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-3) and the Vineland Adaptive Behavior Scales, Third Edition (Vineland-3).
- Aim 2: To determine the statistical power of optical measures for early prediction of treatment failure, neurodevelopmental outcome, and post-treatment brain growth.
2. Experimental Design
2.1. Study Design
2.2. Study Organization
2.3. Study Period
2.4. Participants
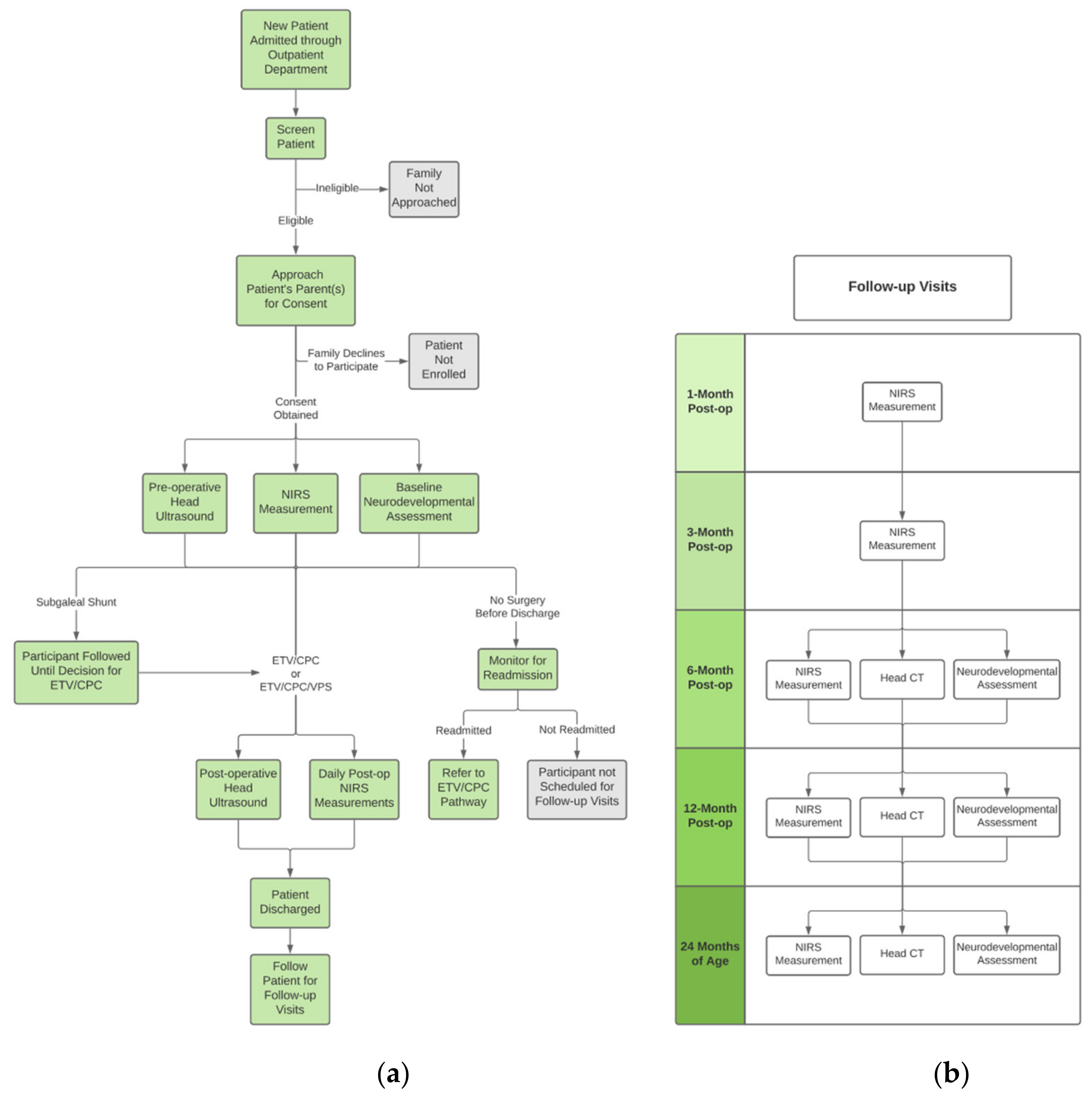
2.5. Patient Screening and Recruitment
2.6. Informed Consent
2.7. Clinical Workflow
2.8. Intervention
2.9. Research Workflow
3. Results
3.1. Data Collection
3.1.1. Patient Demographics and Clinical Data Collection
3.1.2. CT and US Imaging
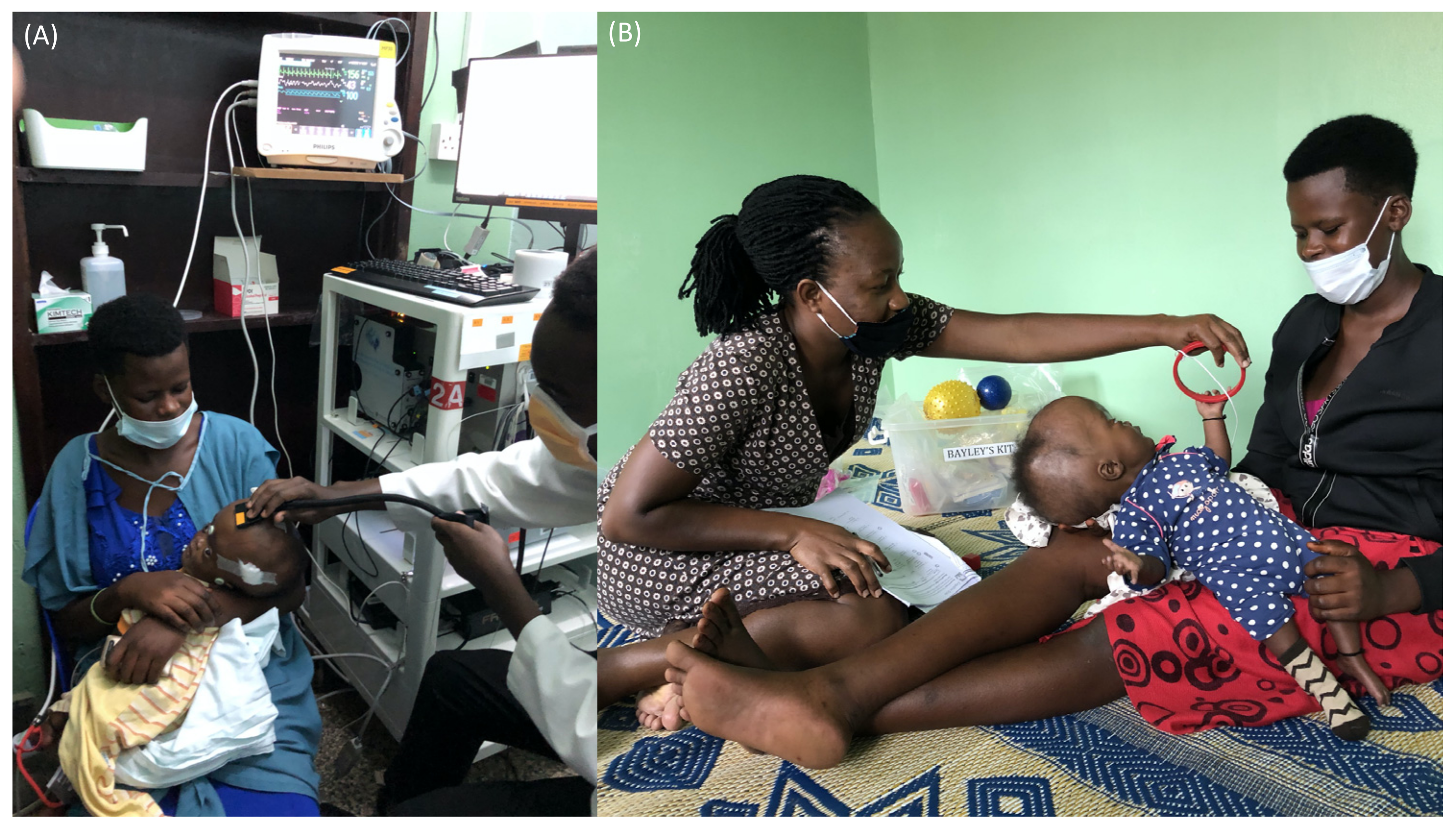
3.1.3. FDNIRS-DCS Measurements
3.1.4. Neurodevelopmental Outcome Assessment
3.2. Data Security, Storage, and Sharing
3.3. Data Analysis
3.3.1. Data Analysis Platform
3.3.2. Cerebral Hemoglobin Concentrations, Calculations, and Oxygen Metabolism Estimation
3.3.3. CT and US Imaging Analysis
3.4. Power Analysis and Statistical Plan
3.5. Staff Training
3.6. Data Quality Assurance
3.7. Reporting
3.8. Ethics Approval, Registration, and Dissemination
3.9. Study Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warf, B.C. Educate One to Save a Few. Educate a Few to Save Many. World Neurosurg. 2013, 79, S15.e15–S15.e18. [Google Scholar] [CrossRef]
- Warf, B.C.; Collaboration, E.A.N.R. Pediatric Hydrocephalus in East Africa: Prevalence, Causes, Treatments, and Strategies for the Future. World Neurosurg. 2010, 73, 296–300. [Google Scholar] [CrossRef]
- Warf, B.C. Hydrocephalus in Uganda: The Predominance of Infectious Origin and Primary Management with Endoscopic Third Ventriculostomy. J. Neurosurg. Pediatr. 2005, 102, 1–15. [Google Scholar] [CrossRef]
- Warf, B.C.; Alkire, B.C.; Bhai, S.; Hughes, C.; Schiff, S.J.; Vincent, J.R.; Meara, J.G. Costs and Benefits of Neurosurgical Intervention for Infant Hydrocephalus in Sub-Saharan Africa. J. Neurosurg. Pediatr. 2011, 8, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warf, B.C. Comparison of 1-Year Outcomes for the Chhabra and Codman-Hakim Micro Precision Shunt Systems in Uganda: A Prospective Study in 195 Children. J. Neurosurg. 2005, 102, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Limbrick, D.D.; Mathur, A.; Johnston, J.M.; Munro, R.; Sagar, J.; Inder, T.; Park, T.S.; Leonard, J.L.; Smyth, M.D. Neurosurgical Treatment of Progressive Posthemorrhagic Ventricular Dilation in Preterm Infants: A 10-Year Single-Institution Study. J. Neurosurg. Pediatr. 2010, 6, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S. Neonatal Posthemorrhagic Hydrocephalus from Prematurity: Pathophysiology and Current Treatment Concepts. J. Neurosurg. Pediatr. 2012, 9, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Riva-Cambrin, J.; Butler, J.; Browd, S.R.; Drake, J.M.; Holubkov, R.; Kestle, J.R.W.; Limbrick, D.D.; Simon, T.D.; Tamber, M.S.; et al. Outcomes of CSF Shunting in Children: Comparison of Hydrocephalus Clinical Research Network Cohort with Historical Controls: Clinical Article. J. Neurosurg. Pediatr. 2013, 12, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Warf, B.C.; Campbell, J.W. Combined Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization as Primary Treatment of Hydrocephalus for Infants with Myelomeningocele: Long-Term Results of a Prospective Intent-to-Treat Study in 115 East African Infants. J. Neurosurg. Pediatr. 2008, 2, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Warf, B.C.; Mugamba, J.; Kulkarni, A.V. Endoscopic Third Ventriculostomy in the Treatment of Childhood Hydrocephalus in Uganda: Report of a Scoring System That Predicts Success. J. Neurosurg. Pediatr. 2010, 5, 143–148. [Google Scholar] [CrossRef]
- Warf, B.C.; Kulkarni, A.V. Intraoperative Assessment of Cerebral Aqueduct Patency and Cisternal Scarring: Impact on Success of Endoscopic Third Ventriculostomy in 403 African Children. J. Neurosurg. Pediatr. 2010, 5, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, R.T.; Wang, S.; Warf, B.C. Global Surgery for Pediatric Hydrocephalus in the Developing World: A Review of the History, Challenges, and Future Directions. Neurosurg. Focus 2016, 41, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warf, B.C. Congenital Idiopathic Hydrocephalus of Infancy: The Results of Treatment by Endoscopic Third Ventriculostomy with or without Choroid Plexus Cauterization and Suggestions for How It Works. Child’s Nerv. Syst. 2013, 29, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Warf, B.C. The Impact of Combined Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization on the Management of Pediatric Hydrocephalus in Developing Countries. World Neurosurg. 2013, 79, S23.e13–S23.e15. [Google Scholar] [CrossRef]
- Warf, B.C. Comparison of Endoscopic Third Ventriculostomy Alone and Combined with Choroid Plexus Cauterization in Infants Younger than 1 Year of Age: A Prospective Study in 550 African Children. J. Neurosurg. 2005, 103, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.V.; Schiff, S.J.; Mbabazi-Kabachelor, E.; Mugamba, J.; Ssenyonga, P.; Donnelly, R.; Levenbach, J.; Monga, V.; Peterson, M.; MacDonald, M.; et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. N. Engl. J. Med. 2017, 377, 2456–2464. [Google Scholar] [CrossRef]
- Schiff, S.J.; Kulkarni, A.V.; Mbabazi-Kabachelor, E.; Mugamba, J.; Ssenyonga, P.; Donnelly, R.; Levenbach, J.; Monga, V.; Peterson, M.; Cherukuri, V.; et al. Brain Growth after Surgical Treatment for Infant Postinfectious Hydrocephalus in Sub-Saharan Africa: 2-Year Results of a Randomized Trial. J. Neurosurg. Pediatr. 2021, 28, 326–334. [Google Scholar] [CrossRef]
- Warf, B.C.; Dagi, A.R.; Kaaya, B.N.; Schiff, S.J. Five-Year Survival and Outcome of Treatment for Postinfectious Hydrocephalus in Ugandan Infants. J. Neurosurg. Pediatr. 2011, 8, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Marano, P.J.; Stone, S.S.D.; Mugamba, J.; Ssenyonga, P.; Warf, E.B.; Warf, B.C. Reopening of an Obstructed Third Ventriculostomy: Long-Term Success and Factors Affecting Outcome in 215 Infants. J. Neurosurg. Pediatr. 2015, 15, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Grant, P.E.; Roche-Labarbe, N.; Surova, A.; Themelis, G.; Selb, J.; Warren, E.K.; Krishnamoorthy, K.S.; Boas, D.A.; Franceschini, M.A. Increased Cerebral Blood Volume and Oxygen Consumption in Neonatal Brain Injury. J. Cereb. Blood Flow Metab. 2009, 29, 1704–1713. [Google Scholar] [CrossRef]
- Whittemore, B.A.; Swift, D.M.; Thomas, J.M.; Chalak, L.F. A Neonatal NeuroNICU Collaborative Approach to Neuromonitoring of Posthemorrhagic Ventricular Dilation in Preterm Infants. Pediatr. Res. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Roche-Labarbe, N.; Dehaes, M.; Carp, S.; Fenoglio, A.; Barbieri, B.; Hagan, K.; Grant, P.E.; Franceschini, M.A. Non-Invasive Optical Measurement of Cerebral Metabolism and Hemodynamics in Infants. J. Vis. Exp. 2013, 73, 4379. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, A.; Yip, L.C.M.; Milej, D.; Suwalski, M.; Kewin, M.; Lo, M.; Carson, J.J.L.; Han, V.; Bhattacharya, S.; Diop, M.; et al. Perfusion and Metabolic Neuromonitoring during Ventricular Taps in Infants with Post-Hemorrhagic Ventricular Dilatation. Brain Sci. 2020, 10, 452. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Hagan, K.; Fenoglio, A.; Grant, P.E.; Franceschini, M.A. Reduced Cerebral Blood Flow and Oxygen Metabolism in Extremely Preterm Neonates with Low-Grade Germinal Matrix- Intraventricular Hemorrhage. Sci. Rep. 2016, 6, 25903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehaes, M.; Aggarwal, A.; Lin, P.-Y.; Fortuno, C.R.; Fenoglio, A.; Roche-Labarbe, N.; Soul, J.S.; Franceschini, M.A.; Grant, P.E. Cerebral Oxygen Metabolism in Neonatal Hypoxic Ischemic Encephalopathy during and after Therapeutic Hypothermia. J. Cereb. Blood Flow Metab. 2013, 34, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Weil, A.G.; Westwick, H.; Wang, S.; Alotaibi, N.M.; Elkaim, L.; Ibrahim, G.M.; Wang, A.C.; Ariani, R.T.; Crevier, L.; Myers, B.; et al. Efficacy and Safety of Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization for Infantile Hydrocephalus: A Systematic Review and Meta-Analysis. Child’s Nerv. Syst. 2016, 32, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Kishimoto, J.; Eagleson, R.; Bhattacharya, S.; De Ribaupierre, S. Does Ventricular Volume Affect the Neurodevelopmental Outcome in Infants with Intraventricular Hemorrhage? Child’s Nerv. Syst. 2020, 36, 569–575. [Google Scholar] [CrossRef]
- Carp, S.A.; Farzam, P.; Redes, N.; Hueber, D.M.; Franceschini, M.A. Combined Multi-Distance Frequency Domain and Diffuse Correlation Spectroscopy System with Simultaneous Data Acquisition and Real-Time Analysis. Biomed. Opt. Express 2017, 8, 3993–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.A.; Walker, A.M.; Horne, R.S.C. The Development of Autonomic Cardiovascular Control Is Altered by Preterm Birth. Early Hum. Dev. 2013, 89, 145–152. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Wallace, E.M.; Whatley, C.; Odoi, A.; Hollis, S.; Weichard, A.J.; Muthusamy, J.S.; Varma, S.; Cameron, J.; Narayan, O.; et al. Sleep: A Window Into Autonomic Control in Children Born Preterm and Growth Restricted. Sleep 2017, 40, zsx048. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Walker, A.M.; Horne, R.S.C. Effects of Sleeping Position on Development of Infant Cardiovascular Control. Arch. Dis. Child. 2008, 93, 868–872. [Google Scholar] [CrossRef]
- Pepperdine, C.R.; McCrimmon, A.W. Test Review: Vineland Adaptive Behavior Scales, Third Edition (Vineland-3) by Sparrow, S.S., Cicchetti, D.V., & Saulnier, C.A. Can. J. Sch. Psychol. 2018, 33, 157–163. [Google Scholar] [CrossRef]
- Warf, B.; Ondoma, S.; Kulkarni, A.; Donnelly, R.; Ampeire, M.; Akona, J.; Kabachelor, C.R.; Mulondo, R.; Nsubuga, B.K. Neurocognitive Outcome and Ventricular Volume in Children with Myelomeningocele Treated for Hydrocephalus in Uganda. J. Neurosurg. Pediatr. 2009, 4, 564–570. [Google Scholar] [CrossRef]
- Drotar, D.; Olness, K.; Wiznitzer, M.; Guay, L.; Marum, L.; Svilar, G.; Hom, D.; Fagan, J.F.; Ndugwa, C.; Kiziri-Mayengo, R. Neurodevelopmental Outcomes of Ugandan Infants With Human Immunodeficiency Virus Type 1 Infection. Pediatrics 1997, 100, e5. [Google Scholar] [CrossRef] [Green Version]
- Wray, S.; Cope, M.; Delpy, D.T.; Wyatt, J.S.; Reynolds, E.O.R. Characterization of the near Infrared Absorption Spectra of Cytochrome Aa3 and Haemoglobin for the Non-Invasive Monitoring of Cerebral Oxygenation. Biochim. Biophys. Acta Bioenerg. 1988, 933, 184–192. [Google Scholar] [CrossRef]
- Boas, D.A.; Campbell, L.E.; Yodh, A.G. Scattering and Imaging with Diffusing Temporal Field Correlations. Phys. Rev. Lett. 1995, 75, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Yodh, A.G. Spatially Varying Dynamical Properties of Turbid Media Probed with Diffusing Temporal Light Correlation. J. Opt. Soc. Am. 1997, 14, 192. [Google Scholar] [CrossRef]
- Shang, Y.; Li, T.; Yu, G. Clinical Applications of Near-Infrared Diffuse Correlation Spectroscopy and Tomography for Tissue Blood Flow Monitoring and Imaging. Physiol. Meas. 2017, 38, R1–R26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diop, M.; Verdecchia, K.; Lee, T.-Y.; Lawrence, K.S. Calibration of Diffuse Correlation Spectroscopy with a Time-Resolved near-Infrared Technique to Yield Absolute Cerebral Blood Flow Measurements: Errata. Biomed. Opt. Express 2012, 3, 1476–1477. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Buckley, E.M.; Licht, D.J.; Lynch, J.M.; Schwab, P.J.; Naim, M.Y.; Lavin, N.A.; Nicolson, S.C.; Montenegro, L.M.; Yodh, A.G.; et al. Cerebral Oxygen Metabolism in Neonates with Congenital Heart Disease Quantified by MRI and Optics. J. Cereb. Blood Flow Metab. 2013, 34, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche-Labarbe, N.; Fenoglio, A.; Aggarwal, A.; Dehaes, M.; Carp, S.A.; Franceschini, M.A.; Grant, P.E. Near-Infrared Spectroscopy Assessment of Cerebral Oxygen Metabolism in the Developing Premature Brain. J. Cereb. Blood Flow Metab. 2012, 32, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Mandell, J.G.; Langelaan, J.W.; Webb, A.G.; Schiff, S.J. Volumetric Brain Analysis in Neurosurgery: Part 1. Particle Filter Segmentation of Brain and Cerebrospinal Fluid Growth Dynamics from MRI and CT Images. J. Neurosurg. Pediatr. 2015, 15, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.R.; Cherukuri, V.; Paulson, J.N.; Ssentongo, P.; Kulkarni, A.V.; Warf, B.C.; Monga, V.; Schiff, S.J. Normal Childhood Brain Growth and a Universal Sex and Anthropomorphic Relationship to Cerebrospinal Fluid. J. Neurosurg. Pediatr. 2021, 28, 458–468. [Google Scholar] [CrossRef]
- Fenster, A.; Downey, D.B.; Cardinal, H.N. Three-Dimensional Ultrasound Imaging. Phys. Med. Biol. 2001, 46, R67. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Lo, M.; Bhattacharya, S.; Eagleson, R.; Fenster, A.; de Ribaupierre, S. Does the Head Position Affect Neonatal Lateral Ventricular Volume? Am. J. Perinatol. 2020, 29. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Mallucci, C.L.; Sgouros, S.; Roth, J.; Constantini, S.; Group, C.P.N.S. Endoscopic Third Ventriculostomy in the Treatment of Childhood Hydrocephalus. J. Pediatr. 2009, 155, 254–259.e1. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadset, T.A.; Rajaram, A.; Hsiao, C.-H.; Kemigisha Katungi, M.; Magombe, J.; Seruwu, M.; Kaaya Nsubuga, B.; Vyas, R.; Tatz, J.; Playter, K.; et al. Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC. Metabolites 2022, 12, 78. https://doi.org/10.3390/metabo12010078
Vadset TA, Rajaram A, Hsiao C-H, Kemigisha Katungi M, Magombe J, Seruwu M, Kaaya Nsubuga B, Vyas R, Tatz J, Playter K, et al. Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC. Metabolites. 2022; 12(1):78. https://doi.org/10.3390/metabo12010078
Chicago/Turabian StyleVadset, Taylor A., Ajay Rajaram, Chuan-Heng Hsiao, Miriah Kemigisha Katungi, Joshua Magombe, Marvin Seruwu, Brian Kaaya Nsubuga, Rutvi Vyas, Julia Tatz, Katharine Playter, and et al. 2022. "Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC" Metabolites 12, no. 1: 78. https://doi.org/10.3390/metabo12010078
APA StyleVadset, T. A., Rajaram, A., Hsiao, C.-H., Kemigisha Katungi, M., Magombe, J., Seruwu, M., Kaaya Nsubuga, B., Vyas, R., Tatz, J., Playter, K., Nalule, E., Natukwatsa, D., Wabukoma, M., Neri Perez, L. E., Mulondo, R., Queally, J. T., Fenster, A., Kulkarni, A. V., Schiff, S. J., ... Lin, P.-Y. (2022). Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC. Metabolites, 12(1), 78. https://doi.org/10.3390/metabo12010078