Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Physical Activity Questionnaire and Other Lifestyle Factors
2.3. Clinical and Laboratory Measurements
2.4. Measurement of Metabolites
2.5. Calculations
2.6. Statistical Analyses
3. Results
3.1. Baseline Clinical and Laboratory Characteristics of the Participants in the Categories of Physical Activity
3.2. Physical Activity and Incident Type 2 Diabetes
3.3. PA Changes during the Follow-Up
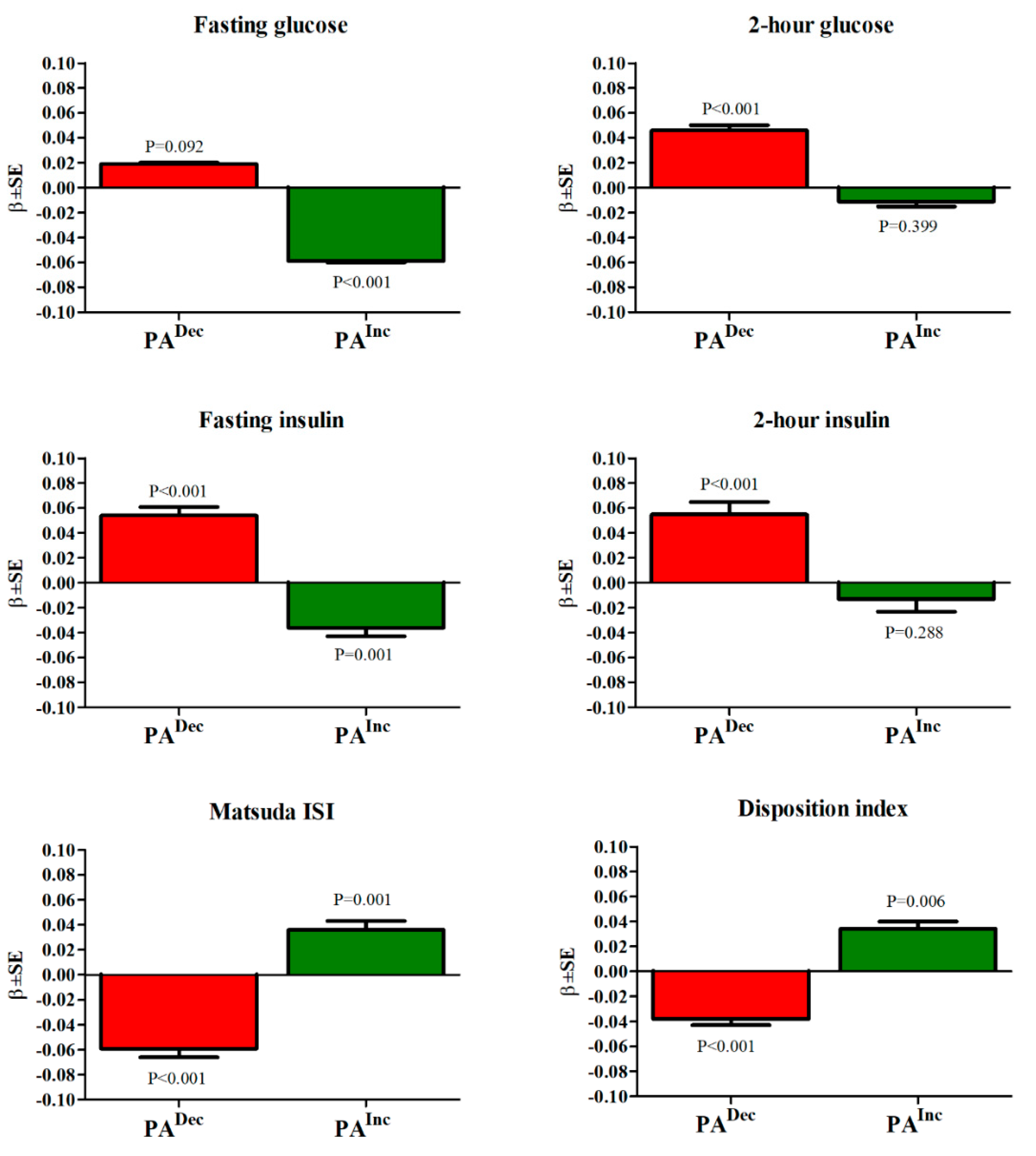
3.4. Association of PA Changes with Glucose and Insulin Concentrations, Insulin Sensitivity, and Insulin Secretion at the Follow-Up
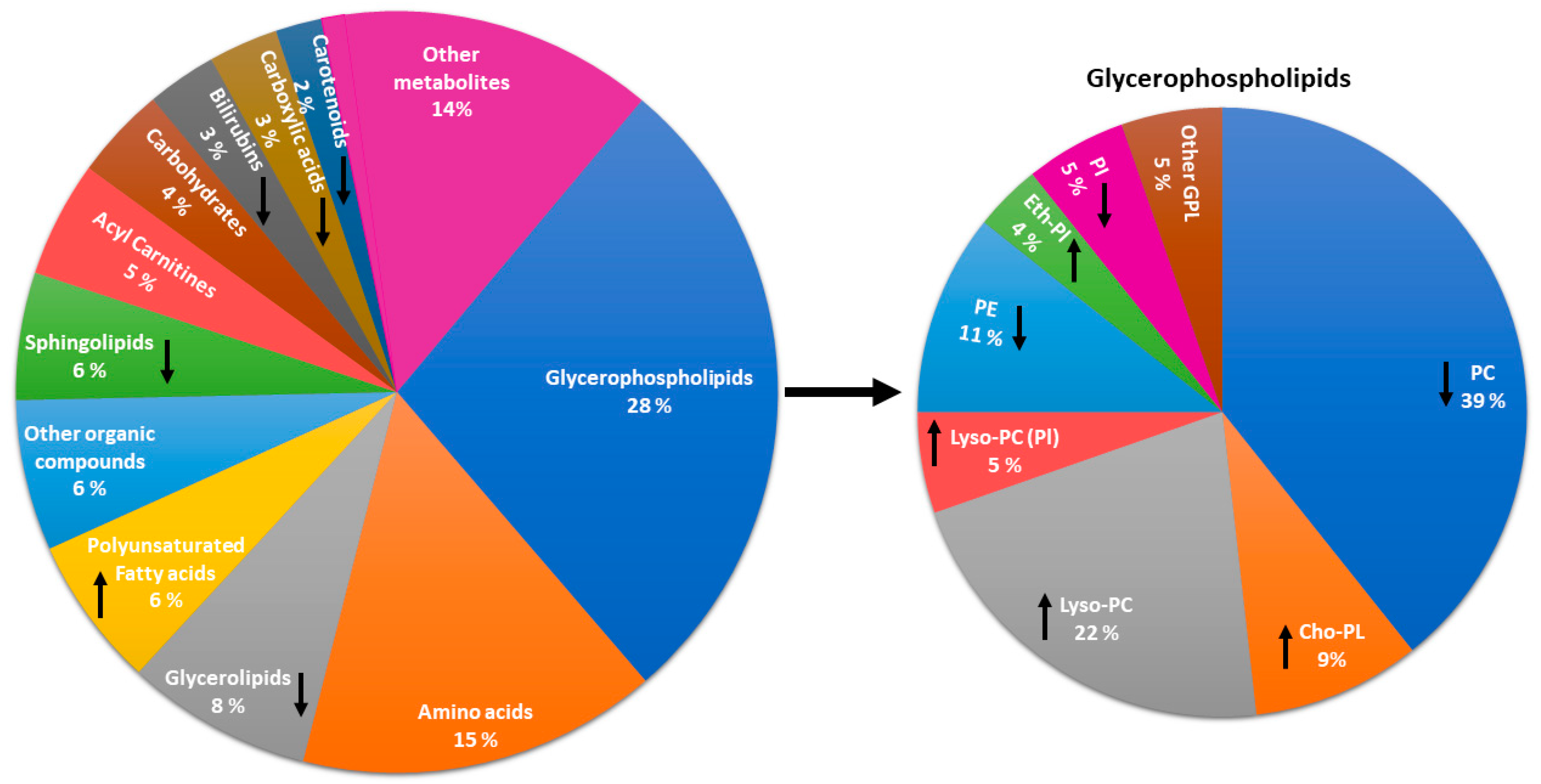
3.5. Metabolites Associated with Physical Activity
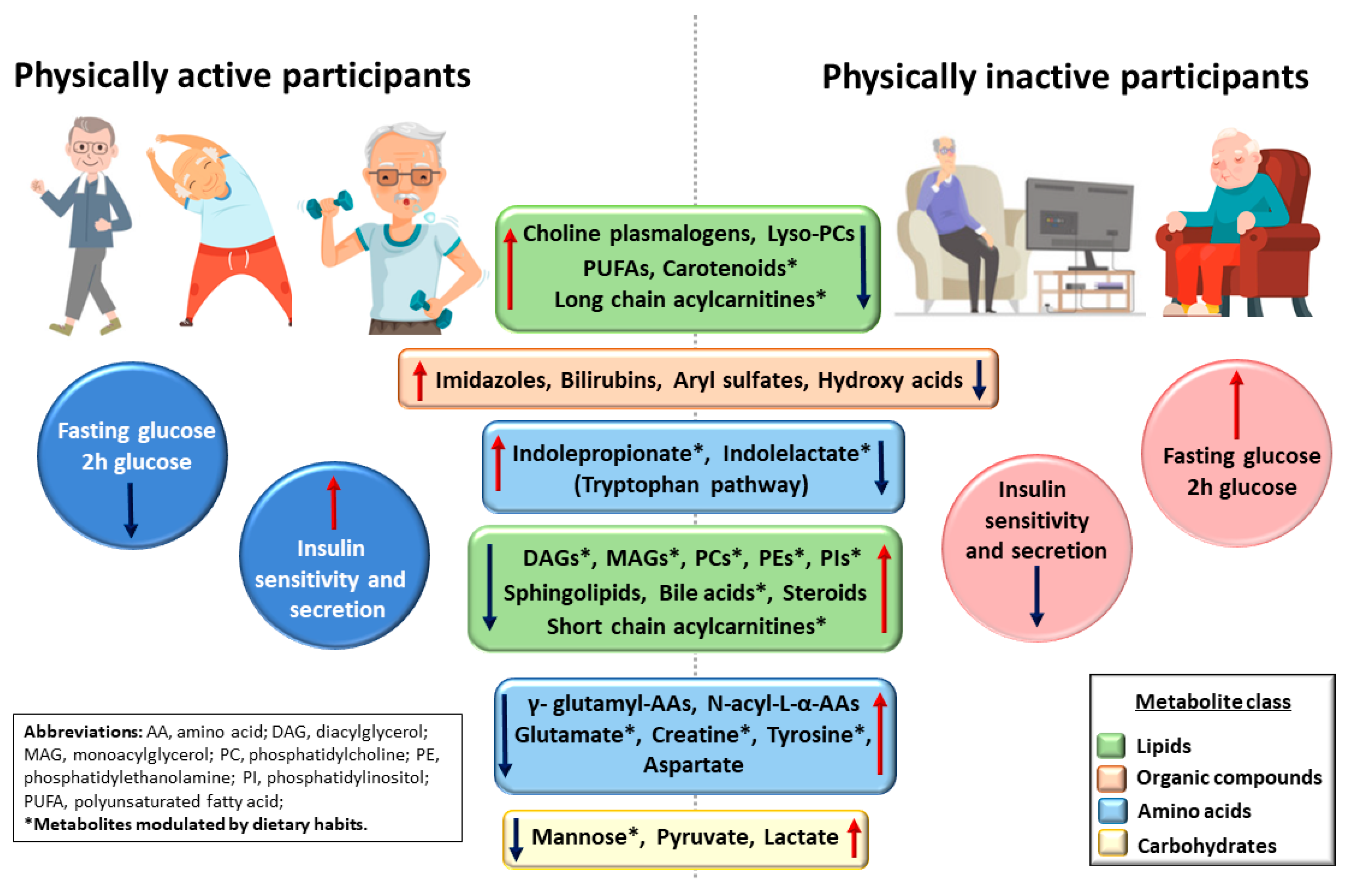
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Knol, M.J.; Corpeleijn, E.; Stolk, R.P. Does physical activity modify the risk of obesity for type 2 diabetes: A review of epidemiological data. Eur. J. Epidemiol. 2010, 25, 5–12. [Google Scholar] [CrossRef]
- Merlotti, C.; Morabito, A.; Pontiroli, A.E. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes. Metab. 2014, 16, 719–727. [Google Scholar] [CrossRef]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chooi, Y.C.; Chan, Z.; Lo, J.; Choo, J.; Ding, B.T.K.; Leow, M.K.S.; Magkos, F. Dose-Dependent Effects of Exercise and Diet on Insulin Sensitivity and Secretion. Med. Sci. Sports Exerc. 2019, 51, 2109–2116. [Google Scholar] [CrossRef]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods. Mol. Biol. 2014, 1198, 3–12. [Google Scholar] [CrossRef]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef]
- Ding, M.; Zeleznik, O.A.; Guasch-Ferre, M.; Hu, J.; Lasky-Su, J.; Lee, I.M.; Jackson, R.D.; Shadyab, A.H.; Lamonte, M.J.; Clish, C.; et al. Metabolome-wide association study of the relationship between habitual physical activity and plasma metabolite levels. Am. J. Epidemiol. 2019, 188, 1932–1943. [Google Scholar] [CrossRef]
- Stancáková, A.; Javorský, M.; Kuulasmaa, T.; Haffner, S.M.; Kuusisto, J.; Laakso, M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 finnish men. Diabetes 2009, 58, 1212–1221. [Google Scholar] [CrossRef]
- Genuth, S.; Alberti, K.G.M.M.; Bennett, P.; Buse, J.; DeFronzo, R.; Kahn, R.; Kitzmiller, J.; Knowler, W.C.; Lebovitz, H.; Lernmark, A.; et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar] [CrossRef]
- Borodulin, K.; Mäkinen, T.E.; Leino-Arjas, P.; Tammelin, T.H.; Heliövaara, M.; Martelin, T.; Kestilä, L.; Prättälä, R. Leisure time physical activity in a 22-year follow-up among Finnish adults. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Knowler, W.; Barrett-Connor, E.; Fowler, S.; Hamman, R.; Lachin, J.; Walker, E.; Nathan, D. Reduction of the incidence of type 2 diabetes with lifestyle intervention or metformin. Int. Urol. Nephrol. 2002, 34, 162–163. [Google Scholar]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and diabetes study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Samith Shetty, A.; Nanditha, A. Primary prevention of Type 2 diabetes in South Asians-challenges and the way forward. Diabet. Med. 2013, 30, 26–34. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Lindström, J.; Lakka, T.A.; Eriksson, J.G.; Niskanen, L.; Wikström, K.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Valle, T.T.; et al. Physical activity in the prevention of type 2 diabetes: The Finnish diabetes prevention study. Diabetes 2005, 54, 158–165. [Google Scholar] [CrossRef]
- Short, K.R.; Pratt, L.V.; Teague, A.M.; Man, C.D.; Cobelli, C. Postprandial improvement in insulin sensitivity after a single exercise session in adolescents with low aerobic fitness and physical activity. Pediatr. Diabetes 2013, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bloem, C.J.; Chang, A.M. Short-term exercise improves β-cell function and insulin resistance in older people with impaired glucose tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 387–392. [Google Scholar] [CrossRef]
- Kahn, S.E.; Larson, V.G.; Beard, J.C.; Cain, K.C.; Fellingham, G.W.; Schwartz, R.S.; Veith, R.C.; Stratton, J.R.; Cerqueira, M.D.; Abrass, I.B. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am. J. Physiol. Endocrinol. Metab. 1990, 258, E937–E943. [Google Scholar] [CrossRef]
- DiMenna, F.J.; Arad, A.D. The acute vs. chronic effect of exercise on insulin sensitivity: Nothing lasts forever. Cardiovasc. Endocrinol. Metab. 2021, 10, 149–161. [Google Scholar] [CrossRef]
- Kitabchi, A.E. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes 2005, 54, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeon, J.Y. Role of exercise on insulin sensitivity and beta-cell function: Is exercise sufficient for the prevention of youth-onset type 2 diabetes? Ann. Pediatr. Endocrinol. Metab. 2020, 25, 208–216. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferré, M.; et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef]
- Yea, K.; Kim, J.; Yoon, J.H.; Kwon, T.; Kim, J.H.; Lee, B.D.; Lee, H.J.; Lee, S.J.; Kim, J.I.; Lee, T.G.; et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J. Biol. Chem. 2009, 284, 33833–33840. [Google Scholar] [CrossRef]
- Qian, F.; Ardisson Korat, A.V.; Imamura, F.; Marklund, M.; Tintle, N.; Virtanen, J.K.; Zhou, X.; Bassett, J.K.; Lai, H.; Hirakawa, Y.; et al. N-3 fatty acid biomarkers and incident type 2 diabetes: An individual participant-level pooling project of 20 prospective cohort studies. Diabetes Care 2021, 44, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39,740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; van der A, D.L.; Spijkerman, A.M.W.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Levy, D.; Cupples, L.A.; Evans, J.C.; D’Agostino, R.B.; Ellison, R.C. Total serum bilirubin and risk of cardiovascular disease in the Framingham Offspring Study. Am. J. Cardiol. 2001, 87, 1196–1200. [Google Scholar] [CrossRef]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Holland, W.L.; Adams, A.C.; Brozinick, J.T.; Bui, H.H.; Miyauchi, Y.; Kusminski, C.M.; Bauer, S.M.; Wade, M.; Singhal, E.; Cheng, C.C.; et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013, 17, 790–797. [Google Scholar] [CrossRef]
- Ramos-Roman, M.A.; Sweetman, L.; Valdez, M.J.; Parks, E.J. Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism. 2012, 61, 202–212. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A.; Peserico, D.; Cattazzo, F.; Bevilacqua, M.; Salvagno, G.L.; Lippi, G.; Targher, G.; Danese, E.; Fava, C. Plasma bile acid profile in patients with and without type 2 diabetes. Metabolites 2021, 11, 453. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Stančáková, A.; Lotta, L.A.; Kuusisto, J.; Boren, J.; Blüher, M.; Wareham, N.J.; Ferrannini, E.; Groop, P.H.; Laakso, M.; et al. Plasma mannose levels are associated with incident type 2 diabetes and cardiovascular disease. Cell Metab. 2017, 26, 281–283. [Google Scholar] [CrossRef]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. The metabolic signature associated with the Western dietary pattern: A cross-sectional study. Nutr. J. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Lankinen, M.A.; Pedret, A.; Schwab, U.; Kolehmainen, M.; Paananen, J.; de Mello, V.; Sola, R.; Lehtonen, M.; Poutanen, K.; et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J. Nutr. 2015, 145, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary intake and circulating concentrations of carotenoids and risk of type 2 diabetes: A dose-response meta-analysis of prospective observational studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Sonne, D.P.; Van Nierop, F.S.; Kulik, W.; Soeters, M.R.; Vilsbøll, T.; Knop, F.K. Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Barlow, C.K.; Mellett, N.A.; Mundra, P.A.; Bonham, M.P.; Larsen, A.; Cameron-Smith, D.; Sinclair, A.; Nestel, P.J.; Wong, G. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J. Nutr. 2015, 145, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyçi, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Rexhaj, Z.; Zhubi, E.; Banach, M.; International Lipid Expert Panel (ILEP). The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2021, 40, 255–276. [Google Scholar] [CrossRef]
- Zeisel, S.H. A brief history of choline. Ann. Nutr. Metab. 2012, 61, 254–258. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Cӑtoi, A.F.; Vodnar, D.C.; Corina, A.; Nikolic, D.; Citarrella, R.; Pérez-Martínez, P.; Rizzo, M. Gut microbiota, obesity and bariatric surgery: Current knowledge and future perspectives. Curr. Pharm. Des. 2019, 25, 2038–2050. [Google Scholar] [CrossRef]
- Giglio, R.V.; Stoian, A.P.; Patti, A.M.; Rizvi, A.A.; Sukhorukov, V.; Ciaccio, M.; Orekhov, A.; Rizzo, M. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of metabolic syndrome. Curr. Pharm. Des. 2021, 27, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Aadahl, M.; Kjær, M.; Kristensen, J.H.; Mollerup, B.; Jørgensen, T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur. J. Prev. Cardiol. 2007, 14, 422–428. [Google Scholar] [CrossRef] [PubMed]
| Leisure-Time Physical Activity Categories | ||||||
|---|---|---|---|---|---|---|
| PA1 (n = 537) | PA2 (n = 2517) | PA3 (n = 1498) | PA4 (n = 4197) | All (n = 8749) | ||
| Variables | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-Value |
| Age, years | 57.1 ± 6.9 | 57.4 ± 7.0 | 56.0 ± 6.7 | 57.5 ± 7.2 | 57.2 ± 7.1 | <0.001 |
| BMI, kg/m2 | 29.1 ± 5.6 | 27.4 ± 4.0 | 27.1 ± 3.8 | 26.1 ± 3.2 | 26.8 ± 3.8 | <0.001 |
| Waist, cm | 104.5 ± 14.0 | 100.0 ± 10.7 | 98.1 ± 10.3 | 94.8 ± 9.2 | 97.4 ± 10.6 | <0.001 |
| Total alcohol consumption, g/wk | 132.0 ± 187.0 | 103.0 ± 139.0 | 98.0 ± 114.0 | 89.0 ± 116.0 | 97.0 ± 129.0 | <0.001 |
| Current smokers, % | 36.5 | 24.1 | 16.8 | 12.9 | 18.2 | <0.001 |
| Physical Activity | PA1 | PA2 | PA3 | PA4 | Unadjusted p | Adjusted p * |
|---|---|---|---|---|---|---|
| Fasting glucose | 5.79 ± 0.53 | 5.75 ± 0.48 | 5.73 ± 0.48 | 5.68 ± 0.47 | <0.001 | 0.030 |
| 2-h glucose | 6.54 ± 1.90 | 6.20 ± 1.71 | 6.08 ± 1.69 | 5.88 ± 1.62 | <0.001 | <0.001 |
| Fasting insulin | 11.50 ± 8.99 | 9.08 ± 6.50 | 8.49 ± 5.94 | 7.22 ± 4.58 | <0.001 | <0.001 |
| 2-h insulin | 75.29 ± 68.44 | 57.99 ± 56.28 | 54.57 ± 54.53 | 44.42 ± 44.97 | <0.001 | <0.001 |
| Matsuda ISI | 5.32 ± 3.97 | 6.26 ± 3.92 | 6.70 ± 4.08 | 7.58 ± 4.21 | <0.001 | <0.001 |
| DI | 151.6 ± 74.2 | 157.8 ± 68.6 | 161.5 ± 69.1 | 169.2 ± 74.0 | <0.001 | <0.001 |
| Unadjusted | Adjusted * | |||||||
|---|---|---|---|---|---|---|---|---|
| Physical Activity Category | Total (n = 8749) | Incident Diabetes, (n = 1151) | HR | 95% CI | p | HR | 95% CI | p * |
| PA1 | 537 | 102 (19.0%) | 1.00 | 1.00 | ||||
| PA2 | 2517 | 401 (15.9%) | 0.87 | 0.70, 1.08 | 0.213 | 1.15 | 0.92, 1.44 | 0.22 |
| PA3 | 1498 | 204 (13.6%) | 0.70 | 0.55, 0.89 | 0.004 | 1.03 | 0.81, 1.31 | 0.82 |
| PA4 | 4195 | 444 (10.6%) | 0.61 | 0.49, 0.75 | <0.001 | 0.99 | 0.79, 1.24 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemppainen, S.M.; Fernandes Silva, L.; Lankinen, M.A.; Schwab, U.; Laakso, M. Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites 2022, 12, 69. https://doi.org/10.3390/metabo12010069
Kemppainen SM, Fernandes Silva L, Lankinen MA, Schwab U, Laakso M. Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites. 2022; 12(1):69. https://doi.org/10.3390/metabo12010069
Chicago/Turabian StyleKemppainen, Susanna Maria, Lilian Fernandes Silva, Maria Anneli Lankinen, Ursula Schwab, and Markku Laakso. 2022. "Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men" Metabolites 12, no. 1: 69. https://doi.org/10.3390/metabo12010069
APA StyleKemppainen, S. M., Fernandes Silva, L., Lankinen, M. A., Schwab, U., & Laakso, M. (2022). Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites, 12(1), 69. https://doi.org/10.3390/metabo12010069






