Yeast Protein as an Easily Accessible Food Source
Abstract
1. Introduction
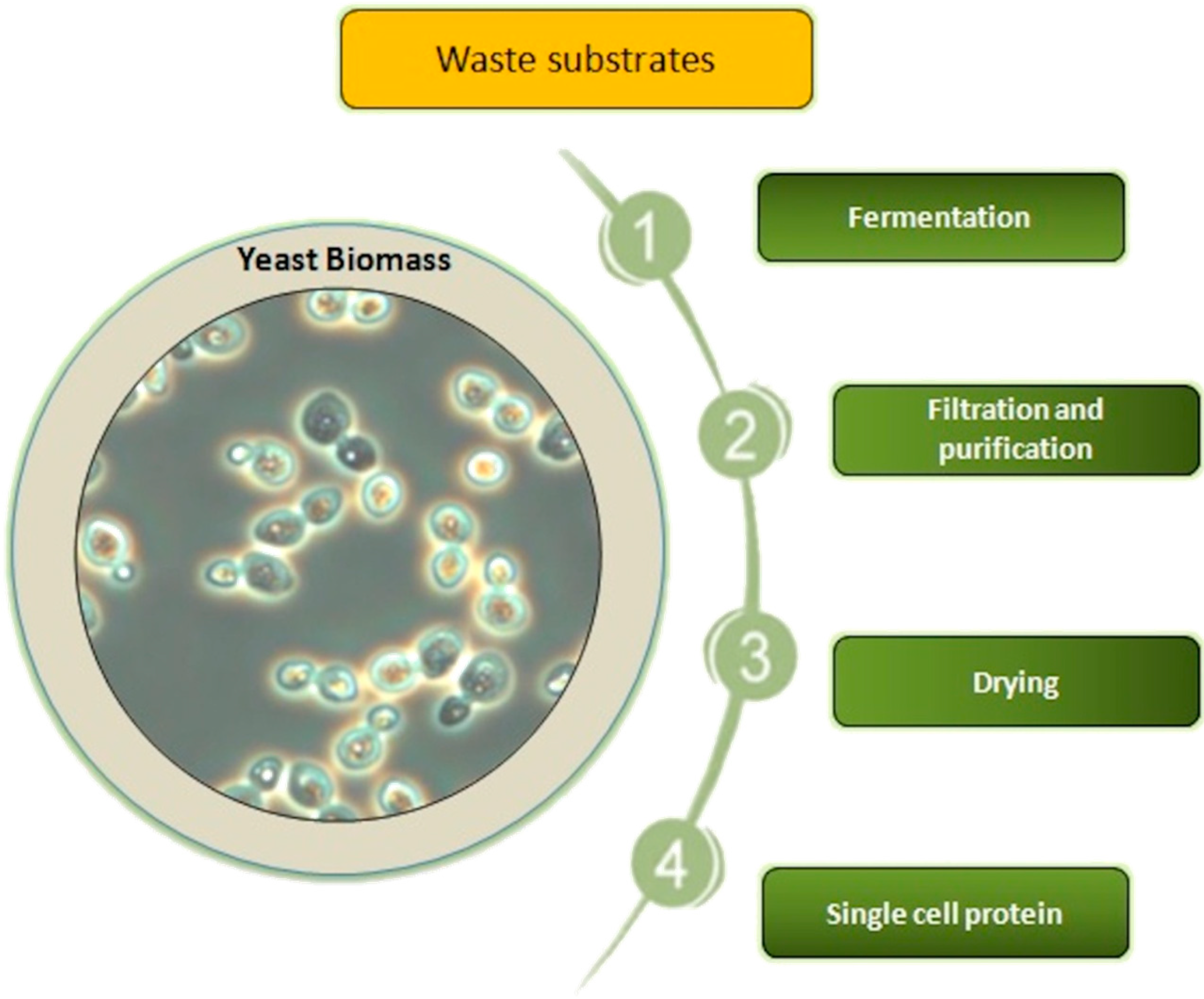
2. Production of Yeast SCP from Specific Waste Substrates
3. Yeast Species as Protein Biomass Producers
3.1. Saccharomyces cerevisiae
3.2. Yarrowia lipolytica
3.3. Candida spp.
3.4. Other Species of Yeasts
4. Nutritional Benefits of Yeast Protein
5. Safety of Yeast Protein Used as Food
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. World Population Prospects. 2019. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 10 November 2021).
- Ritchie, H.; Roser, M. Meat and Dairy Production. Available online: https://ourworldindata.org/meat-production (accessed on 10 November 2021).
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 114. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon (FR): International Agency for Research on Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507972/ (accessed on 10 November 2021).
- Barnard, M.; Levin, S.; Trapp, C. Meat consumption as a risk factor for type 2 diabetes. Nutrients 2014, 6, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A.S. Production of single cell protein (SCP) and essentials amino acids from Candida utilis FMJ12 by solid state fermentation using mango waste supplemented with nitrogen sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar]
- Lee, J.Z.; Logan, A.; Terry, S.; Spear, J.R. Microbial response to single-cell protein production and brewery wastewater treatment. Microb. Biotechnol. 2015, 8, 65–76. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Cherdthong, A.; Sumadong, P.; Foiklang, S.; Milintawisamai, N.; Wanapat, M.; Chanjula, P.; Gunun, N.; Gunun, P. Effect of post-fermentative yeast biomass as a substitute for soybean meal on feed utilization and rumen ecology in Thai native beef cattle. J. Anim. Feed Sci. 2019, 28, 238–243. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of single cell protein (SCP) from food and agricultural waste by using Saccharomyces cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A. Nutritional yeast biomass: Characterization and application. In Diet, Microbiome and Health. Handbook of Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Academic Press: London, UK, 2018; Volume 11, pp. 237–270. ISBN 978-0-12-811440-7. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Świder, R.; Baier, A.; Mickowska, B.; Juda, M.; Chudzik-Rząd, B.; Szyszka, R.; Malm, A. Yarrowia lipolytica grown on biofuel waste as a source of single cell protein and essential amino acids for human diet. Saudi J. Med. Pharm. Sci. 2017, 3, 1344–1351. [Google Scholar]
- Hezarjaribi, M.; Ardestani, F.; Ghorbani, R.H. Single cell protein production by Saccharomyces cerevisiae using an optimized culture medium composition in a batch submerged bioprocess. Appl. Biochem. Biotechnol. 2016, 179, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 251–262. [Google Scholar]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef] [PubMed]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; RaviKumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Bel, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Saygün, A.; Sahin-Yesilcubuk, N.; Aran, N. Effects of different oil sources and residues on biomass and metabolite production by Yarrowia lipolytica YB 423-12. J. Am. Oil Chem. Soc. 2014, 91, 1521–1530. [Google Scholar] [CrossRef]
- Tzirita, M.; Papanikolaou, S.; Chatzifragkou, A.; Quilty, B. Waste fat biodegradation and biomodification by Yarrowia lipolytica and a bacterial consortium composed of Bacillus spp. and Pseudomonas putida. Eng. Life Sci. 2018, 18, 932–942. [Google Scholar] [CrossRef]
- Vasiliadou, I.; Bellou, S.; Daskalaki, A.; Tomaszewska-Hetman, L.; Chatzikotoula, C.; Kompoti, B.; Papanikolaou, S.; Vayenas, D.; Pavlou, S.; Aggelis, G. Biomodification of fats and oils and scenarios of adding value on renewable fatty materials through microbial fermentations: Modelling and trials with Yarrowia lipolytica. J. Clean Prod. 2018, 200, 1111–1129. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Valorization of biodiesel production residues in making porous clay brick. Fuel Process Technol. 2012, 103, 166–173. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Sajnaga, E.; Kozak, E.; Poleszak, E.; Malm, A. Dietary supplements based on the yeast biomass. Curr. Top. Nutraceutical Res. 2015, 13, 83–88. [Google Scholar]
- Vuong, M.D.; Thanh, N.T.; Son, C.K.; Yves, W. Protein enrichment of cassava-based dried distiller’s grain by solid state fermentation using Trichoderma Harzianum and Yarrowia Lipolytica for feed ingredients. Waste Biomass Valoriz. 2021, 12, 3875–3888. [Google Scholar] [CrossRef]
- Anbuselvi, S.; Avinash, S.; Manas, J. Optimization of single cell protein from Chinese potato by submerged fermentation. Pharm. Lett. 2015, 7, 281–283. [Google Scholar]
- Dobrowolski, A.; Mikuła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Accumulation of a Cocoa-Butter-Like Lipid by Yarrowia lipolytica Cultivated on Agro-Industrial Residues. Curr. Microbiol. 2003, 46, 124–130. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol-a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Srividya, A.R.; Vishnuvarthan, V.J.; Murugappan, M.; Dahake, P.G. Single cell protein—A review. Int. J. Pharm. Res. Sch. 2013, 2, 472–485. [Google Scholar]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Lee, C.; Yamakwa, T.; Komada, T. Rapid growth of thermotolerant yeast on palm oil. World J. Microbiol. Biotechnol. 1993, 9, 187–190. [Google Scholar] [CrossRef]
- Petkov, K.; Rymowicz, W.; Musiał, I.; Kinal, S.; Biel, W. Nutritive value of protein Yarrowia lipolytica, yeast obtained on various lipid substrates. Folia Univ. Agric. Stetin. Zootech. 2002, 227, 95–100. [Google Scholar]
- Lapeña, D.; Kosa, G.; Hansen, L.D.; Mydland, L.T.; Passoth, V.; Horn, S.J.; Eijsink, V.G.H. Production and characterization of yeasts grown on media composed of spruce-derived sugars and protein hydrolysates from chicken by-products. Microb. Cell Fact. 2020, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, M.R.; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single cell proteins: As nutritional enhncer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Rhishipal, R.; Philip, R. Selection of marine yeasts for the generation of single cell protein from prawn-shell waste. Bioresour. Technol. 1998, 65, 255–256. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Chen, X.L.; Wang, Z. Microbial biomass production from rice straw hydrolysate in airlift bioreactors. J. Biotechnol. 2005, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.J.G.; Kalaw, S.P.; Galvez, C.T.; Reyes, R.G. Mycota of distillery yeast sludge as source of single cell protein. Mycosphere 2015, 6, 241–247. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, M.; Yang, Z.; Yang, Q. Biomass production from glutamate fermentation wastewater by the co-culture of Candida halophila and Rhodotorula glutinis. Bioresour. Technol. 2005, 96, 1522–1524. [Google Scholar] [CrossRef]
- Yadav, J.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed culture of Kluyveromyces marxianus and Candida krusei for single-cell protein production and organic load removal from whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Nigam, J.N. Cultivation of Candida langeronii in sugar cane bagasse hemicellulosic hydrolyzate for the production of single cell protein. World J. Microbiol. Biotechnol. 2000, 16, 367–372. [Google Scholar] [CrossRef]
- Rages, A.A.; Haider, M.M. Alkaline hydrolys is of olive fruits wastes for the production of single cell protein by Candida lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999. [Google Scholar] [CrossRef]
- Dos Reis, K.C.; Coimbra, J.M.; Duarte, W.F.; Schwan, R.F.; Silva, C.F. Biological treatment of vinasse with yeast and simultaneous production of single-cell protein for feed supplementation. Int. J. Environ. Sci. Technol. 2019, 16, 763–774. [Google Scholar] [CrossRef]
- Arous, F.; Azabou, S.; Jaouani, A.; Zouari-Mechichi, H.; Nasri, M.; Mechichi, T. Biosynthesis of single-cell biomass from olive mill wastewater by newly isolated yeasts. Environ. Sci. Pollut. Res. 2016, 23, 6783–6792. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, A.; Mancilha, I.M.; Sato, S. Cultivation of Candida tropicalis in sugar cane hemicellulosic hydrolyzate for microbial protein production. J. Biotechnol. 1996, 51, 83–88. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Liu, Y. Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 2012, 62, 1165–1172. [Google Scholar] [CrossRef]
- Barbosa Magalhãesa, C.E.; Souza-Netob, M.S.; Astolfi-Filhob, S.; Rocha Matosb, I.T.S. Candida tropicalis able to produce yeast single cell protein using sugarcane bagasse hemicellulosic hydrolysate as carbon source. Biotechnol. Res. Innov. 2018, 2, 19–21. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, M.; Yang, Z. Biomass production of isolate from salad oil manufacturing wastewater. Bioresour. Technol. 2005, 96, 1183–1187. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, W.; Zhang, G. Production of single cell protein using waste capsicum powder produced during capsanthin extraction. Lett. Appl. Microbiol. 2010, 50, 187–191. [Google Scholar] [CrossRef]
- Yunus, F.-u.-N.; Nadeem, M.; Rashid, F. Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed. J. Inst. Brew. 2015, 121, 553–557. [Google Scholar] [CrossRef]
- Liu, B.; Song, J.; Li, Y.; Niu, J.; Wang, Z.; Yang, Q. Towards industrially feasible treatment of potato starch processing waste by mixed cultures. Appl. Biochem. Biotechnol. 2013, 171, 1001–1010. [Google Scholar] [CrossRef]
- Jalasutram, V.; Kataram, S.; Gandu, B.; Anupoju, G.R. Single cell protein production from digested and undigested poultry litter by Candida utilis: Optimization of process parameters using response surface methodology. Clean Technol. Environ. Policy 2013, 15, 265–273. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol. Trace Elem. Res. 2019, 187, 316–327. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.; Bzducha-Wróbel, A.; Kieliszek, M. Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass Valor. 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Ouedraogo, N.; Savadogo, A.; Somda, M.K.; Tapsoba, F.; Zongo, C.; Traore, A.S. Effect of mineral salts and nitrogen source on yeast (Candida utilis NOY1) biomass production using tubers wastes. Afr. J. Biotechnol. 2017, 16, 359–365. [Google Scholar] [CrossRef]
- Nigam, J.N. Single cell protein from pineapple cannery effluent. World J. Microbiol. Biotechnol. 1998, 14, 693–696. [Google Scholar] [CrossRef]
- Gao, L.; Chi, Z.; Sheng, J.; Ni, X.; Wang, L. Single-cell protein production from Jerusalem artichoke extract by a recently isolated marine yeast Cryptococcus aureus G7a and its nutritive analysis. Appl. Microbiol. Biotechnol. 2007, 7, 825–832. [Google Scholar] [CrossRef]
- Duarte, L.C.; Carvalheiro, F.; Lopes, S.; Neves, I.; Gírio, F.M. Yeast Biomass Production in Brewery’s Spent Grains Hemicellulosic Hydrolyzate. Appl. Biochem. Biotechnol. 2008, 148, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Hesham, A.E.L.; Alamri, S.A.; Alrumman, S.A. Production of single-cell protein from wasted date fruits by Hanseniaspora uvarum KKUY-0084 and Zygosaccharomyces rouxii KKUY-0157. Ann. Microbiol. 2014, 64, 1505–1511. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Babu, M.; Raj, S.P.; Nirmala, C.B.; Deccaraman, M.; Sagadevan, E. Production of single cell protein using Kluyveromyces marxianus isolated from paneer whey. Int. J. Biomed. Adv. Res. 2014, 5, 255–257. [Google Scholar]
- Dhanasekaran, D.; Lawanya, S.; Saha, S.; Thajuddin, N.; Panneerselvan, A. Production of Single Cell Protein from Pineapple Waste Using Yeast. Innov. Rom. Food Biotechnol. 2011, 8, 26–32. [Google Scholar]
- Dunuweera, A.S.; Nikagolla, D.N.; Ranganathan, K. Fruit waste substrates to produce single-cell proteins as alternative human food supplements and animal feeds using baker’s yeast (Saccharomyces cerevisiae). J. Food Qual. 2021, 2021, 9932762. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Khalique, A.; Anjum, A.A.; Jabbar, M.A. Comparative assessment of various agro-industrial wastes for Saccharomyces cerevisiae biomass production and its quality evaluation as single cell protein. J. Anim. Plant Sci. 2011, 21, 844–849. [Google Scholar]
- Onofre, S.K.; Bertoldo, I.C.; Abatti, D.; Refosco, D. Chemical composition of the biomass of saccharomyces cerevisiae-(meyen ex E. C. Hansen, 1883) yeast obtained from the beer manufacturing process. Int. J. Environ. Agric. Biotechnol. 2017, 2, 558–562. [Google Scholar] [CrossRef]
- Haddish, K. Production of single cell protein from fruit of beles (Opuntia Ficus-Indica L.) peels using Saccharomyces cerevisiae. J. Microbiol. Exp. 2015, 2, 00073. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Miedzianka, J.; Jama, D.; Kawalec, S.; Liman, K.; Janek, T.; Skaradziński, G.; Rymowicz, W.; Lazar, Z. High value-added products derived from crude glycerol via microbial fermentation using Yarrowia clade yeast Microb. Cell Fact. 2021, 20, 195. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and oat agricultural wastes as substrate candidates for biomass production of the non-conventional yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kira, A.; Rymowicz, W. Biomass production by novel strain of Yarrowia lipolytica using raw glicerol, derived from biodiesel production. Bioresour. Technol. 2013, 137C, 124. [Google Scholar] [CrossRef]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical composition and biological value of proteins of the yeast Yarrowia lipolytica growing on industrial glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef][Green Version]
- Yunus, F.-N.; Nadeem, M.; Rashid, F. Enhancement of protein contents of rice bran for animal feed by solid state fermentation. Biomed. Lett. 2015, 1, 31–36. Available online: https://www.semanticscholar.org/paper/Enhancement-of-protein-contents-of-rice-bran-for-by-Yunus-Nadeem/2210af954b05ad54ed2f19bc7ec0ba3221f7ab7b (accessed on 1 January 2022).
- Ukaegbu-Obi, K.M. Single cell protein: A resort to global protein challenge and waste management. J. Microbiol. Microb. Technol. 2016, 1, 5. [Google Scholar]
- Avais, M.; Sharif, M.; Ashfaq, K.; Aqib, A.I.; Saeed, M.; Di Cerbo, A.; Alagawany, M. Effect of yeast-fermented citrus pulp as a protein source on nu-2 trient intake, digestibility, nitrogen balance and in situ diges-3 tion kinetics in Nili Ravi buffalo bulls. Animals 2021, 11, 1713. [Google Scholar] [CrossRef]
- Spalvins, K.; Zihare, L.; Blumberga, D. Single cell protein production from waste biomass: Comparison of various industrial by-products. Energy Procedia 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Darvishi, F.; Nahvi, I.; Zarkesh-Esfahani, H.; Momenbeik, F. Effect of plant oils upon lipase and citric acid production in Yarrowia lipolytica yeast. J. Biomed. Biotechnol. 2009, 2019, 562943. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Catoi, A.-F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef]
- Papanicolaou, S.; Chevalot, I.; Galiotou-Panayotou, M.; Marc, I.; Aggelis, G. Industrial derivative of tallow: A promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron. J. Biotechnol. 2007, 10, 426–435. [Google Scholar] [CrossRef]
- Winkelhausen, E.; Velickova, E.; Amartey, S.A.; Kuzmanova, S. Ethanol production by immobilized Saccharomyces cerevisiae in lyophilized cellulose gel. Appl. Biochem. Biotechnol. 2010, 162, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Baj, T.; Juda, M.; Świder, R.; Mickowska, B.; Malm, A. Statistical evaluation of growth parameters in biofuel waste as a culture medium for improved production of single cell protein and amino acids by Yarrowia lipolytica. AMB Express 2020, 10, 35. [Google Scholar] [CrossRef]
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of fruit wastes in producing single cell protein. Int. J. Environ. Sci. 2012, 1, 430–438. [Google Scholar]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kang, B.H.; Lee, H.M.; Lee, S.J.; Kim, H.S.; Choi, H.W.; Park, T.J.; Kong, K.H. Efficient brazzein production in yeast (Kluyveromyces lactis) using a chemically defined medium. Bioprocess Biosyst. Eng. 2021, 44, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Polpass, A.J.; Kunjukrishnan, K.S.; Solomon, R.D.J. Formulation and statistical optimization of culture medium for improved production of antimicrobial compound by Streptomyces sp. JAJ06. Int. J. Microbiol. 2013, 2013, 526260. [Google Scholar] [CrossRef][Green Version]
- Matos, Â.P. The impact of microalgae in food science and technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmon salar). Aquaculture 2013, 402–403, 1–7. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B. Production of single cell protein from ram horn hydrolysate. Turk. J. Biol. 2001, 25, 371–377. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Rasoul-Amini, S.; Moromvat, M.H.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Saeed, M.; Yasmin, I.; Murtaza, M.A.; Fatima, I.; Saeed, S. Single cell proteins a novel value added food product. Pak. J. Food Sci. 2016, 26, 211–217. [Google Scholar]
- Strong, P.J.; Xie, S.; Clarke, W.P. Methane as a resource: Can the methanotrophs add value? Environ. Sci. Technol. 2015, 49, 4001–4018. [Google Scholar] [CrossRef]
- Hames, E.E.; Demir, T. Microbial ribonucleases (RNases): Production and application potential. World J. Microbiol. Biotechnol. 2015, 31, 1853–1862. [Google Scholar] [CrossRef]
- Knight, N.; Roberts, G.; Shelton, D. The thermal stability of QuornTM pieces. Int. J. Food Sci. Technol. 2001, 36, 47–52. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Ajila, C.M.; Bezawada, J.; Tyagi, R.D.; Surampalli, R.Y. Food-grade single-cell protein production, characterization and ultrafiltration recovery of residual fermented whey proteins from whey. Food Bioprod. Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Ibarr, A.; Barbosa-Cánovas, G.V. Introduction to Food Process Engineering; Food Preservation Technology Series; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Baudelaire, E.D. Grinding for food powder production. In Handbook of Food Powders Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 132–149. [Google Scholar]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Amata, I.A. Yeast a single cell protein: Charakteristics and metabolism. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 158–170. [Google Scholar]
- Malav, A.; Meena, S.; Sharma, M.; Sharma, M.; Dub, P. A critical review on single cell protein production using different substrates. Int. J. Dev. Res. 2017, 7, 16682–16687. [Google Scholar]
- Raziq, A.; Lateef, M.; Ullah, A.; Ullah, H.; Khan, M.W. Single cell protein (SCP) production and potential substrates: A comprehensive review. Pure Appl. Biol. 2020, 9, 1743–1754. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Chen, W.N. Engineering the Saccharomyces cerevisiae β-oxidation pathway to increase medium chain fatty acid production as potential biofuel. PLoS ONE 2014, 9, e84853. [Google Scholar] [CrossRef][Green Version]
- Balakumar, S.; Arasaratnam, V. Osmo-, thermo- and ethanol- tolerances of Saccharomyces cerevisiae S1. Braz. J. Microbiol. 2012, 43, 157–166. [Google Scholar] [CrossRef][Green Version]
- Podpora, B.; Świderski, F.; Sadowska, A.; Piotrowska, A.; Rakowska, R. Spent brewer’s yeast autolysates as a new and valuable component of functional food and dietary supplement. J. Food Process. Technol 2015, 6, 12. [Google Scholar]
- Huige, N.J. Brewery by-products and effluents. In Handbook of Brewing; Priest, F.G., Steward, G.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 656–713. [Google Scholar]
- Guo, J.; Qiu, X.; Salze, G.; Davis, D.A. Use of high-protein brewer’s yeast products in practical diets for the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2019, 25, 680–690. [Google Scholar] [CrossRef]
- Guo, J.; Reis, J.; Salze, G.; Rhodes, M.; Tilton, S.; Davis, D.A. Using high protein distiller’s dried grain product to replace corn protein concentrate and fishmeal in practical diets for the Pacific white shrimp Litopenaeus vannamei. J. World Aquac. Soc. 2019, 50, 983–992. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Izah, S.C.; Enaregha, E.B.; Epidi, J.O. Vitamin content of Saccharomyces cerevisiae biomass cultured in cassava wastewater. MOJ Toxicol. 2019, 4, 42–45. [Google Scholar]
- Paalme, T.; Kevvai, K.; Vilbaste, A.; Hälvin, K.; Nisamedtinov, I. Uptake and accumulation of B-group vitamers in Saccharomyces cerevisiae in ethanol-stat fed-batch culture. World J. Microbiol. Biotechnol. 2014, 30, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Biermaier, B.; Gross, M.; Schwaiger, K.; Gareis, M. Ochratoxin A in brewer’s yeast used as food supplement. Mycotoxin Res. 2016, 32, 1–5. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of chromium supplementation on glucose metabolism and lipids. A systematic review of randomized controlled trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef]
- EFSA. Selenium-enriched yeast as source for selenium added for nutritional purposes in food for particular nutritional uses and foods (including food supplements) for the general population. EFSA J. 2008, 9, 2137. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Sloth, J.J.; Larsen, E.H.; Bugel, S.H.; Moesgaard, S. Determination of total selenium and Se in isotopically enriched human samples by ICP-dynamic reaction cell-MS. J. Anal. At. Spectrom. 2003, 18, 317–322. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast [β]-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the safety of ‘yeast beta-glucans’ as a novel food ingredient. EFSA Panel on Dietetic Products. Nutrition and Allergies (NDA). EFSA 2011, 9, 2137. [Google Scholar] [CrossRef]
- Pérez-Torrado, R.; Gamero, E.; Gomez-Pastor, R.; Garre, E.; Aranda, A.; Matallana, E. Yeast biomass, an optimised product with myriad applications in the food industry. Trends Food Sci. Technol. 2015, 46, 167–175. [Google Scholar] [CrossRef]
- Wang, W.; Wei, H.; Alahuhta, M.; Chen, X.; Hyman, D.; Johnson, D.K.; Zhang, M.; Himmel, M.E. Heterologous expression of xylanase enzymes in lipogenic yeast Yarrowia lipolytica. PLoS ONE 2014, 9, e111443. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Patsios, S.I.; Dedousi, A.; Sossidou, E.N.; Zdragas, A. Sustainable animal feed protein through the cultivation of Yarrowia lipolytica on agro-industrial wastes and by-products. Sustainability 2020, 12, 1398. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Nicaud, J.-M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef]
- Zhao, L.; Li, B.; Xiong, D.; Zhang, H.; Tang, X.; Zhang, H.; Song, Y.; Yang, S. Cocoa-butter-equivalent production from Yarrowia lipolytica by optimization of fermentation technology. Am. J. Biochem. Biotechnol. 2016, 12, 196–205. [Google Scholar] [CrossRef][Green Version]
- Nicaud, J.-M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Thevenieau, F.; Beopoulos, A.; Desfougeres, T.; Sabirova, J.; Albertin, K.; Zinjarde, S.; Nicaud, J.-M. Uptake and assimilation of hydrophobic substrates by the oleaginous yeast Yarrowia lipolytica. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1513–1527. [Google Scholar]
- Dominguez, A.; Deive, F.J.; Angeles Sanroman, M.; Longo, M.A. Biodegradation and utilization of waste cooking oil by Yarrowia lipolytica CECT 1240. Eur. J. Lipid Sci. Technol. 2010, 112, 1200–1208. [Google Scholar] [CrossRef]
- Czech, A.; Smolczyk, A.; Ognik, K.; Kiesz, M. Nutritional value of Yarrowia lipolytica yeast and its effect on growth performance indicators in piglets. Ann. Anim. Sci. 2016, 16, 1091–1100. [Google Scholar] [CrossRef]
- Jach, M.E.; Masłyk, M.; Juda, M.; Sajnaga, E.; Malm, A. Vitamin B12-enriched Yarrowia lipolytica biomass obtained from biofuel waste. Waste Biomass Valoriz. 2020, 11, 1711–1716. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Janeczko, M.; Juda, M.; Kochanowicz, E.; Baj, T.; Malm, A. Production of enriched in B vitamins biomass of Yarrowia lipolytica grown in biofuel waste. Saudi J. Biol. Sci. 2021, 28, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, B.; Gui, X.; Wang, G.; Xu, L.; Yan, Y.; Madzak, C.; Pan, D.; Wang, Y.; Zha, G.; et al. Engineering Yarrowia lipolytica to simultaneously produce lipase and single cell protein from agro-industrial wastes for feed. Sci. Rep. 2018, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Woźnica, A.; Czech, A. Utilization of glycerol and yeast biomass produced from glycerol in the animal nutrition. Post. Nauk Roln 2010, 62, 136–140. [Google Scholar]
- Esteban, P.F.; Vazquez de Aldana, C.R.; del Rej, F. Cloning and characterization of 1,3-β-glucanase-encoding genes from non-conventional yeasts. Yeast 1999, 15, 1631–1644. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Drzymala, K.; Rzechonek, D.A.; Mitula, P.; Mironczuk, A.M. Lipid production from waste materials in seawater-based medium by the yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 547. [Google Scholar] [CrossRef]
- Nandy, S.K.; Srivastava, R.K. A review on sustainable yeaste biotechnological processes and applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywinska, A. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 2019, 64, 809–820. [Google Scholar] [CrossRef]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijk, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mironczuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.A.Z.; Amaral, P.; Belo, I. Yarrowia lipolytica: An industrial workhorse. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 930–944. [Google Scholar]
- Lanciotti, R.; Vannini, L.; Lopez, C.C.; Gobbetti, M.; Guerzoni, E. Evaluation of the ability of Yarrowia lipolytica to impart strain-dependent characteristics to cheese when used as a ripening adjunct. Int. J. Dairy Technol. 2005, 58, 89–99. [Google Scholar] [CrossRef]
- Irby, R.F.; Kandula, M.; Zadikany, R.; Sandin, R.; Greene, J. Yarrowia lipolytica as normal human flora: A case series of 24 patients with positive cultures and no attributable disease. Infect. Dis. Clin. Pract. 2014, 22, 207–209. [Google Scholar] [CrossRef]
- Devillers, H.; Brunel, F.; Polomska, X.; Sarilar, V.; Lazar, Z.; Robak, M.; Neuveglise, C. Draft genome sequence of Yarrowia lipolytica strain A-101 isolated from polluted soil in Poland. Genome Announc. 2016, 4, e01094-16. [Google Scholar] [CrossRef] [PubMed]
- Wojtatowicz, M.; Rymowicz, W.; Kautola, H. Comparison of di_erent strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrol. Appl. Biochem. Biotechnol. 1991, 31, 165–174. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 10. [Google Scholar] [CrossRef]
- Stefańska, B.; Komisarek, J.; Stanisławski, D.; Gąsiorek, M.; Kasprowicz-Potocka, M.; Frankiewicz, A.; Nowak, W. The effect of Yarrowia lipolytica culture on growth performance, ruminal fermentation and blood parameters of dairy calves. Anim. Feed Sci. Technol. 2018, 243, 72–79. [Google Scholar] [CrossRef]
- Czech, A.; Smolczyk, A.; Grela, E.R.; Kiesz, M. Effect of dietary supplementation with Yarrowia lipolytica or Saccharomyces cerevisiae yeast and probiotic additives on growth performance, basic nutrients digestibility and biochemical blood profile in piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Smolczyk, A.; Ognik, K.; Wlazło, Ł.; Nowakowicz-Dębek, B.; Kiesz, M. Effect of dietary supplementation with Yarrowia lipolytica or Saccharomyces cerevisiae yeast and probiotic additives on haematological parameters and the gut microbiota in piglets. Res. Vet. Sci. 2018, 119, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Merska, M.; Ognik, K. Blood immunological and biochemical indicators in turkey hens fed diets with a different content of the yeast Yarrowia lipolytica. Ann. Anim. Sci. 2014, 14, 935–946. [Google Scholar] [CrossRef]
- Merska, M.; Czech, A.; Ognik, K. The effect of yeast Yarrowia lipolytica on the antioxidant indices and macro-and microelements in blood plasma of turkey hens. Pol. J. Vet. Sci. 2015, 18, 709–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merska-Kazanowska, M.; Czech, A.; Katarzyna, O. The effect of different doses of dried yeast Yarrowia lipolytica on production effects of turkey hens and hematological indicators of blood. Ann. Univ. Mariae Curie-Skłodowska Sect. Zootech. 2013, 31, 35–41. [Google Scholar]
- Berge, G.M.; Hatlen, B.; Odom, J.M.; Ruyter, B. Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquac. Nutr. 2013, 19, 110–121. [Google Scholar] [CrossRef]
- Alamillo, E.; Reyes-Becerril, M.; Cuesta, A.; Angulo, C. Marine yeast Yarrowia lipolytica improves the immune responses in Pacific red snapper (Lutjanus peru) leukocytes. Fish Shellfish Immunol. 2017, 70, 48–56. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Chiglintseva, M.N.; Yusupova, A.I.; Vinokurova, N.G.; Lysanskaya, V.Y.; Morgunov, I.G. Biotechnological potential of Yarrowia lipolytica grown under thiamine limitation. Food Technol. Biotechnol. 2012, 50, 412–419. [Google Scholar]
- EFSA. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, 5594. [Google Scholar] [CrossRef]
- Munawar, R.A.; Irfan, M.; Nadeem, M.; Syed, Q.A.; Siddique, Z.H. Biosynthesis of single cell biomass of Candida utilis by submerged fermentation. Pak. J. Sci. 2010, 62, 1–5. [Google Scholar]
- Akkani, G.B.; du Preez, J.C.; Steyn, L.; Kilian, S.G. Protein enrichment of an Opuntia ficus-indica cladode hydrolysate by cultivation of Candida utilis and Kluyveromyces marxianus. J. Sci. Food Agric. 2015, 95, 1094–1102. [Google Scholar] [CrossRef]
- Lemmel, S.A.; Heimsch, R.C.; Edwards, L.L. Optimizing the continuous production of Candida utilis and Saccharomycopsis fibuliger on potato processing wastewater. Appl. Environ. Microbiol. 1979, 37, 227–232. [Google Scholar] [CrossRef]
- Hu, Z.; Que, Y.; Gao, Y.; Yin, Y.; Zhao, Y. Using black liquor from the soda pulping process for protein production by Candida utilis. BioResources 2015, 10, 3908–3921. [Google Scholar] [CrossRef]
- Harder, W.; Brooke, A.C. Methylotrophic yeasts. In Yeast Biotechnology and Biocatalysis; Verachtert, H., De Mot, R., Eds.; Marcel Dekker: New York, NY, USA, 1990; pp. 395–428. [Google Scholar]
- Iida, H.; Sakagachi, S.; Yagawa, Y.; Anraku, Y. Cell cycle control by Ca2+ in Saccharomyces cerevisiae. J. Biol. Chem. 1990, 265, 21216–21222. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef]
- Schultz, N.; Chang, L.; Hauck, A.; Reuss, M.; Syldatk, C. Microbial production of single-cell protein from deproteinized whey concentrates. Appl. Microbiol. Biotechnol. 2006, 69, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wróbel, A.; Błażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Rutherfurd, S.M.; Kim, I.Y.; Moughan, P.J. Protein quality as determined by the digestible indispensable amino acid score: Evaluation of factors underlying the calculation. Nutr. Rev. 2016, 74, 584–599. [Google Scholar] [CrossRef]
- Paraskevopouloua, A.; Athanasiadisa, I.; Kanellakib, M.; Bekatoroua, A.; Blekasa, G.; Kiosseogloua, V. Functional properties of single cell protein produced by Kefir Microflora. Food Res. Int. 2003, 36, 431–438. [Google Scholar] [CrossRef]
- Cristiani-Urbina, E.; Netzahuatl-Munoz, A.R.; Manriquez-Rojas, F.J.; Juarez-Ramrez, C.; Ruiz-Ordaz, N.; Galndez-Mayer, J. Batch and fed-batch cultures for the treatment of whey with mixed yeast cultures. Process Biochem. 2000, 35, 649–657. [Google Scholar] [CrossRef]
- Rajoka, M.; Ahmed, S.; Hashmi, A.; Athar, M. Production of microbial biomass protein from mixed substrates by sequential culture fermentation of Candida utilis and Brevibacterium lactofermentum. Ann. Microbiol. 2012, 62, 1173–1179. [Google Scholar] [CrossRef]
- Manilal, V.B.; Narayanan, C.S.; Balagopalan, C. Cassava starch effluent treatment with concomitant SCP production. World J. Microbiol. Biotechnol. 1991, 7, 185–190. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I. Ethanol from Lignocellulosic Materials by a Simultaneous Saccharification and Fermentation Process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Supplementation requirements of brewery’s spent grain hydrolysate for biomass and xylitol production by Debaryomyces hansenii CCMI 941. J. Ind. Microbiol. Biotechnol. 2006, 33, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 2006, 17, 320–326. [Google Scholar] [CrossRef]
- Terentiev, Y.; Pico, A.H.; Böer, E.; Wartmann, T.; Klabunde, J.; Breuer, U.; Babel, W.; Suckow, M.; Gellissen, G.; Kunze, G. A wide-range integrative yeast expression vector system based on Arxula adeninivorans-derived elements. J. Ind. Microbiol. Biotechnol. 2004, 31, 223–228. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). QPS: Qualified Presumption of Safety of Micro-Organisms in Food and Feed; EFSA: Parma, Italy, 2005. [Google Scholar]
- Paul, D.; Mukhopadhyay, R.; Chatterjee, B.P.; Guha, A.K. Nutritional profile of food yeast Kluyveromyces fragilis biomass grown on whey. Appl. Biochem. Biotechnol. 2002, 97, 209–218. [Google Scholar] [CrossRef]
- Meyer, P.S.; du Preez, J.C.; Kilian, S.G. Chemostat cultivation of Candida blankii on sugar cane bagasse hemicellulose hydrolysate. Biotechnol. Bioeng. 1992, 40, 353–358. [Google Scholar] [CrossRef]
- Tavares, J.M.; Duarte, L.C.; Amaral-Collaço, M.T.; Gírio, F.M. Phosphate limitation stress induces xylitol overproduction by Debaryomyces hansenii. FEMS Microbiol. Lett. 1999, 171, 115–120. [Google Scholar] [CrossRef][Green Version]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Bekatorou, A.; Pandey, A.; Kanellaki, M.; Koutinas, A.A. Discarded oranges and brewer’s spent grains as promoting ingredients for microbial growth by submerged and solid state fermentation of agro-industrial waste mixtures. Appl. Biochem. Biotechnol. 2013, 170, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, P.; Lu, D.; Shen, A.; Jiang, N. Screening of pyruvate-producing yeast and effect of nutritional conditions on pyruvate production. Lett. Appl. Microbiol. 2002, 35, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Kamal, M.A. Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res. 2004, 38, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.S.; Bezawada, J.; Elharche, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Simultaneous single-cell protein production and COD removal with characterization of residual protein. Bioprocess Biosyst. Eng. 2014, 37, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-López, F.N.; Guillamon, J.M. Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol. 2012, 31, 25–32. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Vagelas, I.; Kalorizou, H.; Papachatzis, A.; Botu, M. Bioactivity of olive oil mill wasterwater against plant pathogens and post harvest diseases. Biotechnol. Biotechnol. Equip. 2009, 23, 1217–1219. [Google Scholar] [CrossRef]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018, 63, 129–140. [Google Scholar] [CrossRef]
- Han, Y.W.; Cheeke, P.R.; Anderson, A.W.; Lekprayoon, C. Growth of Aureobasidium pullulans on straw hydrolysate. Appl. Environ. Microbiol. 1976, 32, 799–802. [Google Scholar] [CrossRef]
- Malak, A.; Baronian, K.; Kunze, G. Blastobotrys (Arxula) adeninivorans: A promising alternative yeast for biotechnology and basic research. Yeast 2016, 33, 535–547. [Google Scholar] [CrossRef]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Betaglucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Di Maro, A. Amino Acid Composition of Milk from Cow, Sheep and Goat Raised in Ailano and Valle Agricola, Two Localities of ‘Alto Casertano’ (Campania Region). Foods 2021, 10, 2431. [Google Scholar] [CrossRef]
- Biel, W.; Maciorowski, R. Assessing nutritional value of grains of selected wheat cultivars. Zywnosc-Nauka Technol. Jakosc 2012, 19, 45–55. [Google Scholar] [CrossRef]
- AAFCO. Association of American Feed Control Official Incorporated; AAFCO: Champaign, IL, USA, 2010; ISBN 1-878341-22-7. [Google Scholar]
- Regulation (EU) No 1169/2011; Provision of Food Information to Costumers; European Parliament and Council: Strasbourg, France, 2011.
- Millward, D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012, 108, S31–S43. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Sadiq, K. Protein Deficiency. In Encyclopedia of Human Nutrition, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 111–115. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.S.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in grain, flour, amino acid composition, protein profiling, and proportion of total flour proteins of different wheat cultivars of north India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Overland, M.; Skrede, A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 27, 68. [Google Scholar] [CrossRef]
- Huyben, D.; Boqvist, S.; Passoth, V.; Renstrom, L.; Allard Bengtsson, U.; Andreoletti, O.; Kiessling, A.; Lundh, T.; Vagsholm, I. Screening of intact yeasts and cell extracts to reduce Scrapie prions during biotransformation of food waste. Acta Vet. Scand. 2018, 60, 9. [Google Scholar] [CrossRef]
- Food for Thought: The Protein Transformation. Available online: https://www.bcg.com/publications/2021/the-benefits-of-plant-based-meats (accessed on 1 January 2022).
- Bratosin, B.C.; Darjan, S.; Vodnar, C.D. Single cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Pedersen, L.L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Allred, L.K.; Nye-Wood, M.G.; Colgrave, M.L. Analysis of gluten in dried yeast and yeast-containing products. Foods 2020, 9, 1790. [Google Scholar] [CrossRef] [PubMed]
| Yeast Species | Waste Substrate | Protein Content | References |
|---|---|---|---|
| Blastobotrys adenininvorans (syn. Arxula adeninivorans) | Spruce-derived sugars and protein hydrolysates from chicken by-products | 50% | [35] |
| Candida sp. | n-alkanes | 65% | [36] |
| Prawn-shell waste | 60.6–70.4% | [37] | |
| Candida arborea | Rice straw hydrolysate | 58.5% | [38] |
| Candida guilliermondii | Treated distillery sludge | 32% | [39] |
| Candida halophila + Rhodotorula glutinis | Glutamate fermentation wastewater | 55% | [40] |
| Candida krusei | Cheese whey | 48% | [41] |
| Candida langeronii | Bagasse hemicelloses hydrolysate | 48% | [42] |
| Candida lipolytica | Alkaline hydrolysis of olive fruits wastes | 59% | [43] |
| Candida parapsilosis | Treated distillery sludge | 31% | [44] |
| Vinasse | 55% | [44] | |
| Candida pararugosa | Olive mill wastewater | 35.9% | [45] |
| Candida tropicalis | Sugar cane hemicellulosic hydrolysate (bagasse) | 31% | [46] |
| Soy molasses | 56% | [47] | |
| Sugarcane bagasse hemicellulosic hydrolysate | 60% | [48] | |
| Candida utilis | Salad oil wastewater | 26% | [49] |
| Waste capsicum powder | 29–48% | [50] | |
| Fermented rice bran | 33% | [51] | |
| Potato starch industry waste | 46% | [52] | |
| Poultry litter, waste capsicum powder | 48% | [53] | |
| Potato wastewater | 42% | [54,55] | |
| Ethanol, sulfite waste liquor | 50–54% | [36] | |
| Tubers wastes | 54% | [56] | |
| Pineapple cannery | 55% | [57] | |
| Mango waste | 56% | [5] | |
| Cyberlindnera jadinii (anamorph name Candida utilis) | Spruce-derived sugars and protein hydrolysates from chicken by-products | 57% | [35] |
| Cryptococcus aureus | Jerusalem artichoke extract | 53% | [58] |
| Debaryomyces hansenii | Brewery’ spent grains hemicellulosic hydrolysate | 32% | [59] |
| Hanseniaspora uvarum | Spoiled date palm fruit | 49% | [60] |
| Kluyveromyces fragilis | Cheese whey (lactose) | 45–54% | [36] |
| Kluyveromyces marxianus | Food waste mixture of orange pulp, whey, brewer’s spent grain | 34% | [61] |
| Cheese whey | 43% | [41] | |
| Paneer whey | 48% | [62] | |
| Saccharomyces cerevisiae | Treated distillery sludge | 33% | [39] |
| Food waste mixture of orange pulp, molasses, brewer’s spent grain | 39% | [61] | |
| Fruit processing residues (pineapple waste) | 45% | [63] | |
| Fruit wastes (peels/mesocarps): mango (Mangifera indica), prickly custard apple (Annona muricata), pineapple (Ananas comosus), papaya (Carica papaya), banana (Musa accuminara Colla), mangosteen (Garcinia mangostana), cashew apple (Anacardium occidentale), cacao (Theobroma cacao), jackfruit (Artocarpus heterophyllus), and pomegranate (Punica granatum) | 48% | [64] | |
| Vegetable processing residues (potato waste:peels) | 49% | [65] | |
| From the beer manufacturing process | 49% | [66] | |
| Molasses | 53% | [36] | |
| Fruit of Beles (Opuntia Ficus-Indica L.) peels hydrolysate | 53% | [67] | |
| Spruce-derived sugars and protein hydrolysates from chicken by-products | 54% | [35] | |
| Wickerhamomyces anomalus | Spruce-derived sugars and protein hydrolysates from chicken by-products | 50% | [35] |
| Zygosaccharomyces rouxii | Spoiled date palm fruit | 49% | [60] |
| Yarrowia lipolytica | Crude glycerol | 30% | [68] |
| Rye and oat agricultural wastes | 30–44.5% | [69] | |
| Biofuel waste | 40–50% | [12] | |
| Pure glycerol, raw glycerol | 45% | [70] | |
| Industrial glycerol obtained in the production of biofuel from rapeseed | 46% | [71] |
| Organisms | Average Amounts of Protein (% Dry Weight) |
|---|---|
| Bacteria | 50–65% |
| Yeast | 29–65% |
| Algae | 40–60% |
| Fungi | 30–45% |
| Meat | 45% |
| Soybean | 35% |
| Milk | 25% |
| Amino Acids | Saccharomyces cerevisiae | Yarrowia lipolytica | Candida utilis | Wheat | Egg | Cow Milk | FAO Amino Acid Requirements for Adults |
|---|---|---|---|---|---|---|---|
| mg/g Protein (Mean) | |||||||
| Arginine | 46.5 | 48 | 32 | 48 | 11.5 | 33 | - |
| Histidine | 23.5 | 26 | 16 | 16 | 4 | 37 | 15 |
| Isoleucine | 37 | 44 | 48 | 33 | 68 | 40 | 30 |
| Leucine | 63 | 68 | 71 | 67 | 90 | 88 | 59 |
| Lysine | 65 | 70 | 51 | 28 | 63 | 78 | 45 |
| Cysteine | 9 | 11 | 24 | 25 | 24 | 9 | |
| Methionine | 14 | 12 | 15.5 | 15 | 32 | 29 | |
| SAA | 23 | 23 | 39.5 | 40 | 56 | 38 | 22 |
| Phenylalanine | 33 | 40 | 41 | 45 | 63 | 47 | |
| Tryptophan | 9 | 47 | 39 | 11 | 16 | Nd | |
| Tyrosine | 26 | 66 | 20 | 36 | 19,5 | 16 | |
| AAA | 68 | 153 | 100 | 92 | 98.5 | 63 | 38 |
| Threonine | 48 | 48 | 41 | 29 | 50 | 48.7 | 23 |
| Valine | 53 | 53 | 55 | 44 | 74 | 47.9 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. https://doi.org/10.3390/metabo12010063
Jach ME, Serefko A, Ziaja M, Kieliszek M. Yeast Protein as an Easily Accessible Food Source. Metabolites. 2022; 12(1):63. https://doi.org/10.3390/metabo12010063
Chicago/Turabian StyleJach, Monika Elżbieta, Anna Serefko, Maria Ziaja, and Marek Kieliszek. 2022. "Yeast Protein as an Easily Accessible Food Source" Metabolites 12, no. 1: 63. https://doi.org/10.3390/metabo12010063
APA StyleJach, M. E., Serefko, A., Ziaja, M., & Kieliszek, M. (2022). Yeast Protein as an Easily Accessible Food Source. Metabolites, 12(1), 63. https://doi.org/10.3390/metabo12010063