Metabolic Changes by Wine Flor-Yeasts with Gluconic Acid as the Sole Carbon Source
Abstract
1. Introduction
2. Results
2.1. Gluconic Acid Consumption and Cell Viability
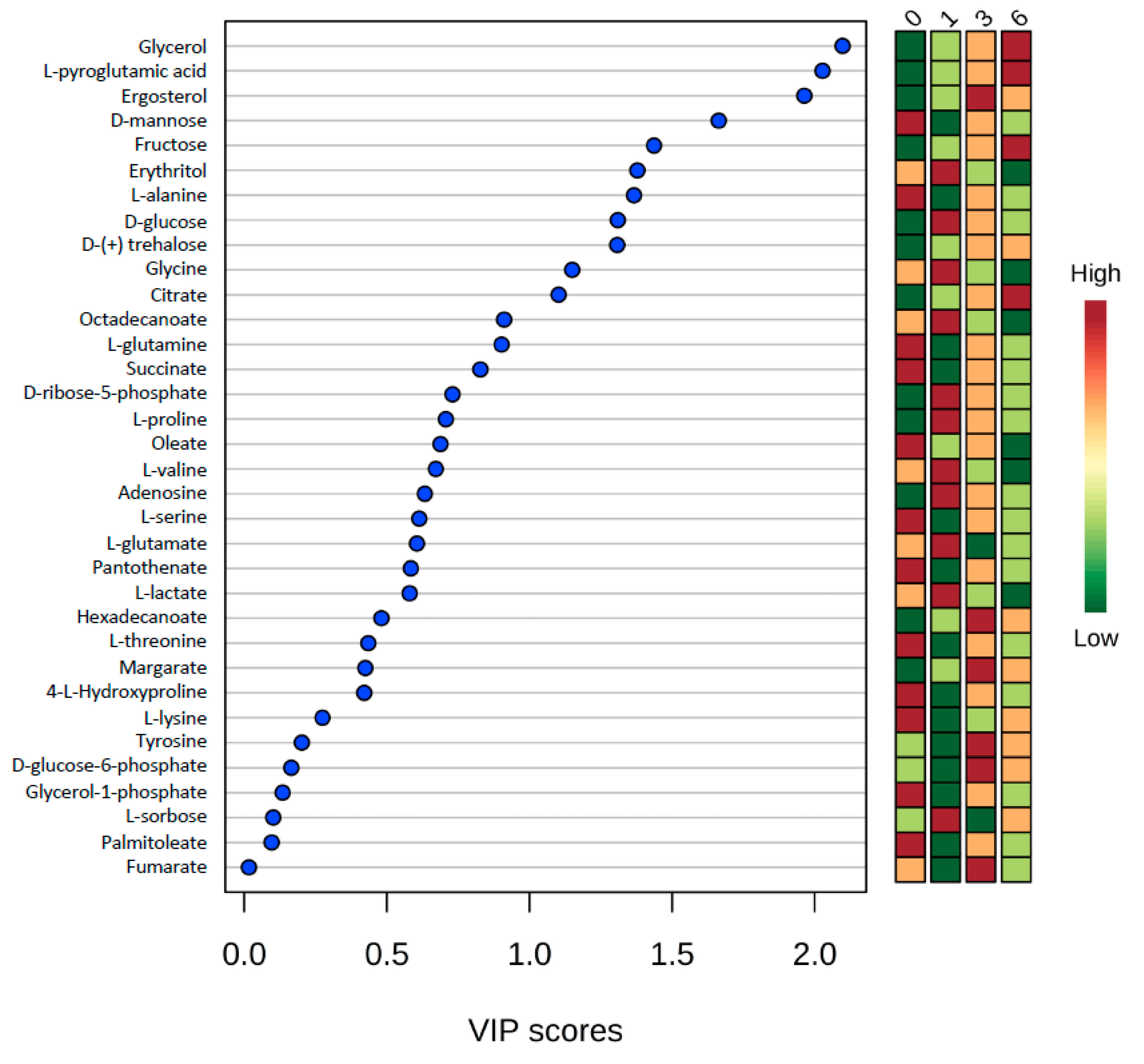
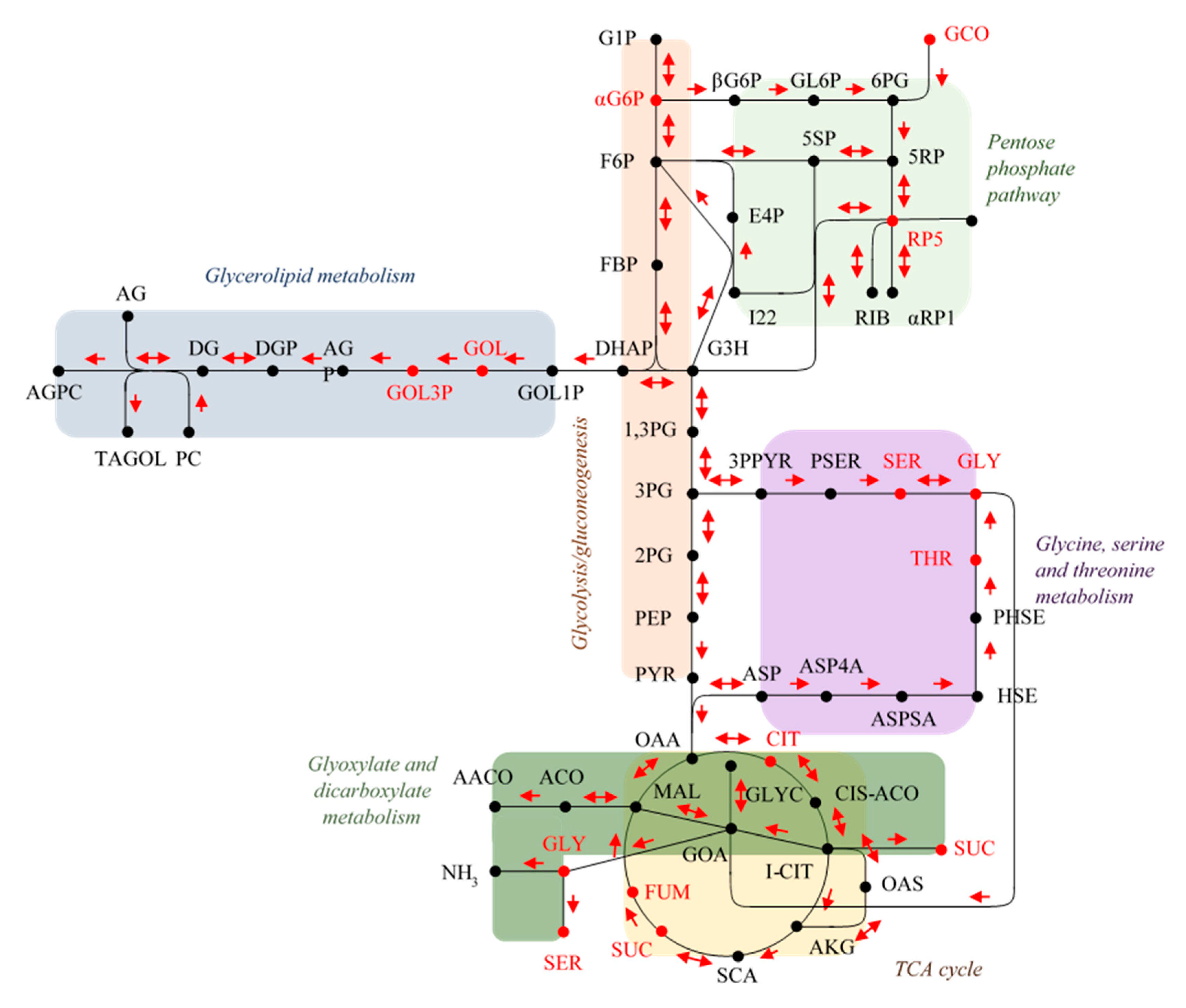
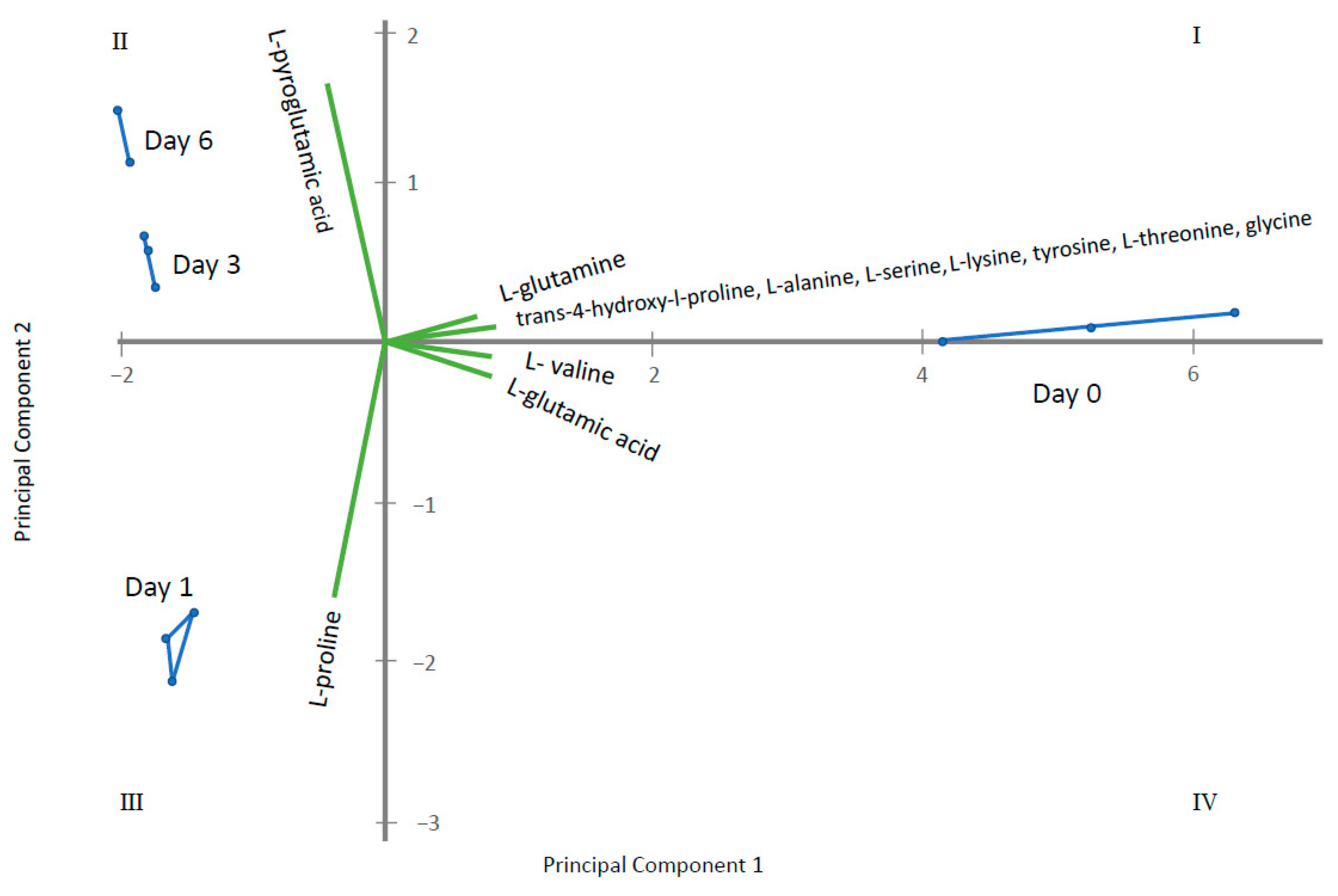
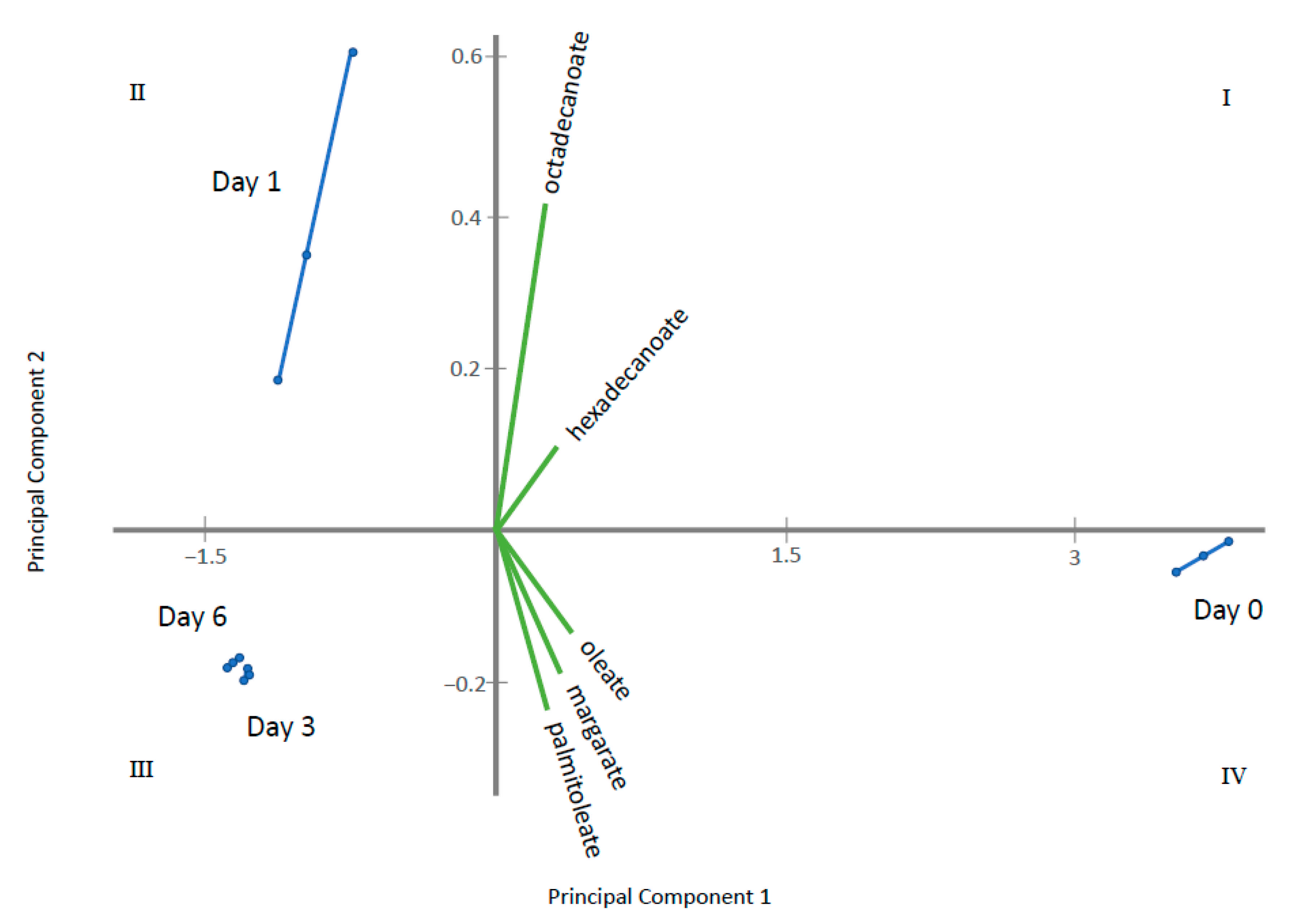
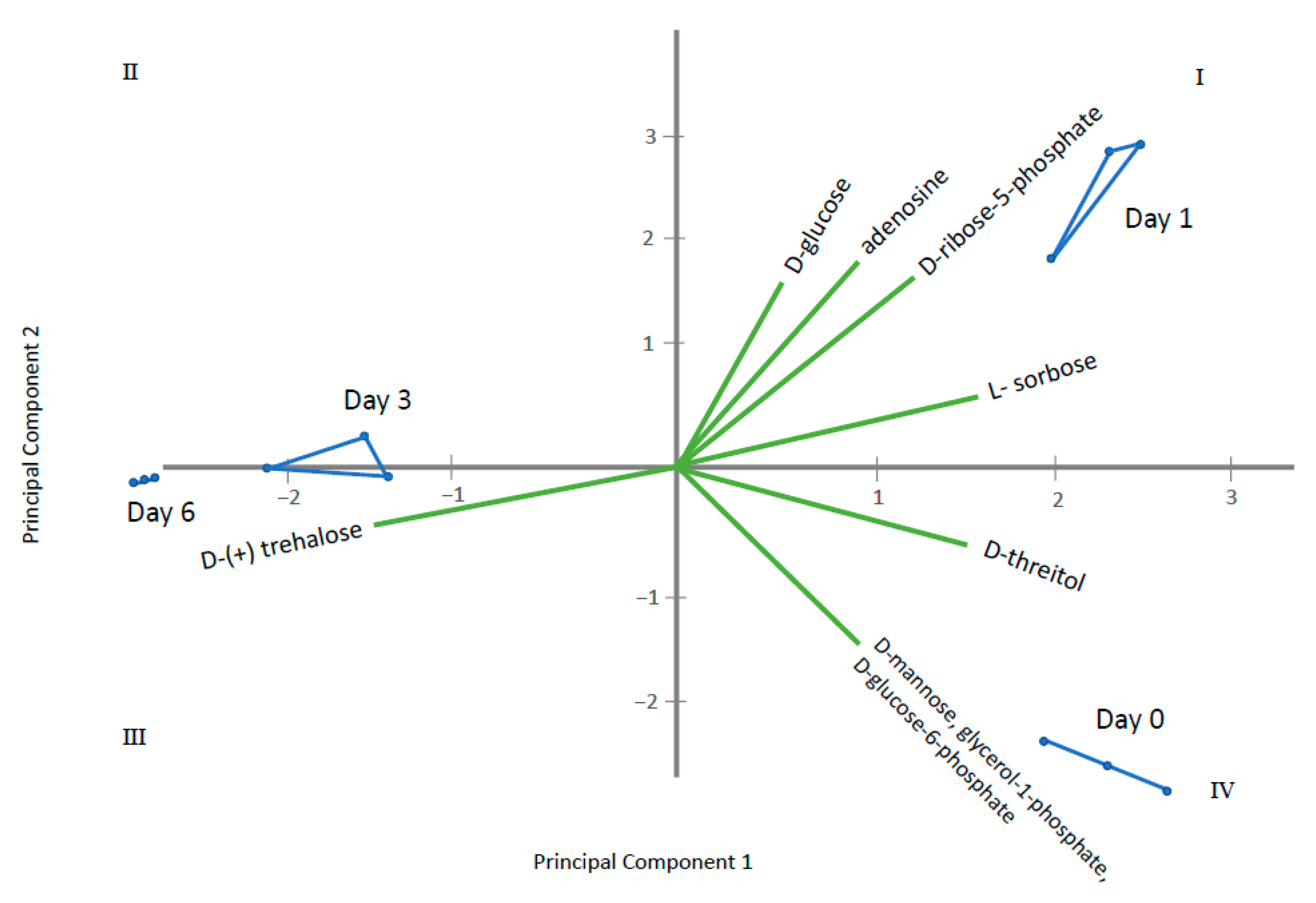
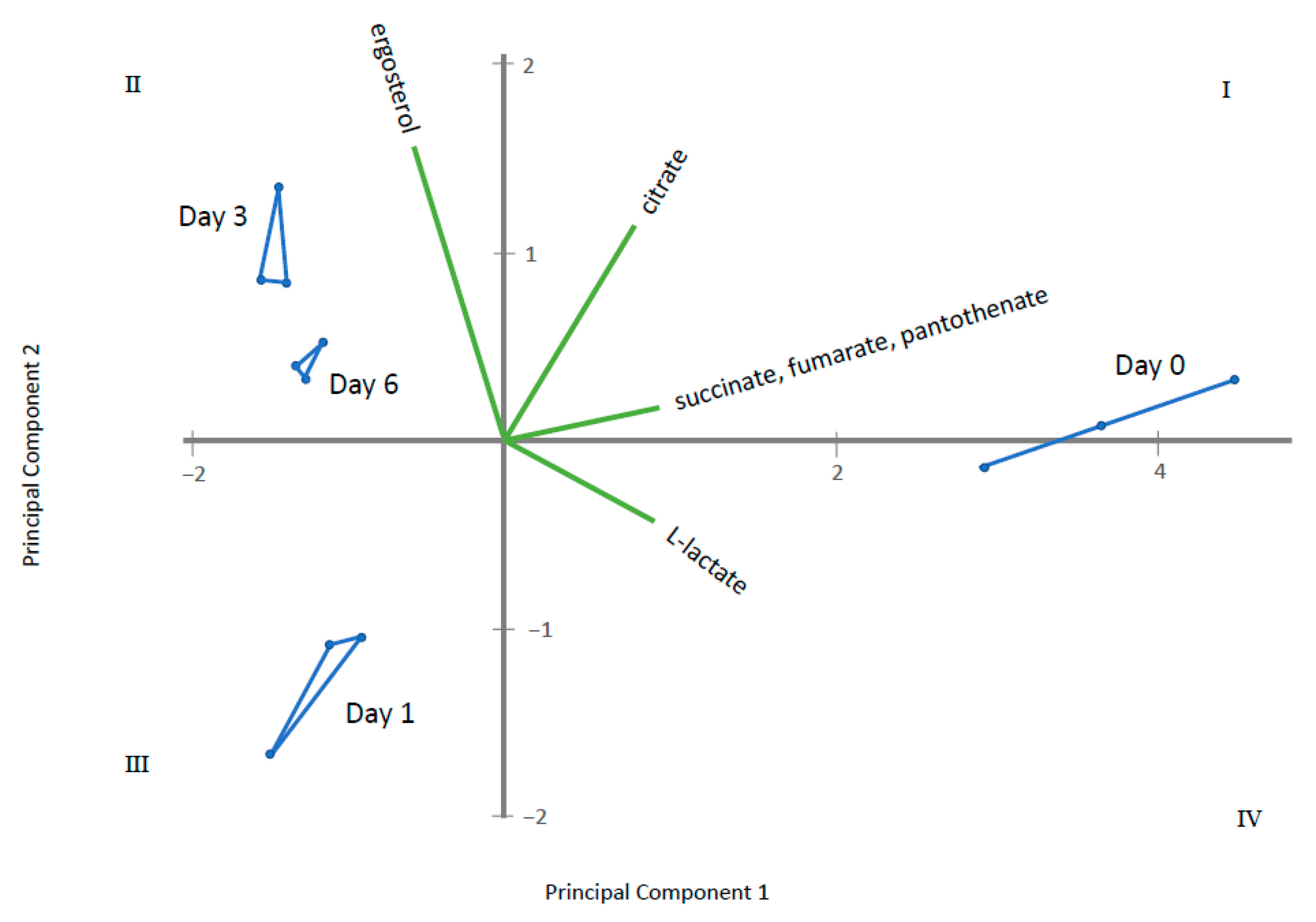
2.2. Endo-Metabolomic Analysis
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
4.2. Gluconic Acid Consumption
4.3. Yeast Cell Viability
4.4. Endo-Metabolite Identification and Quantification
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legras, J.L.; Moreno-Garcia, J.; Zara, S.; Zara, G.; Garcia-Martinez, T.; Mauricio, J.C.; Mannazzu, I.; Coi, A.L.; Bou Zeidan, M.; Dequin, S.; et al. Flor yeast: New perspectives beyond wine aging. Front. Microbiol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae—their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef]
- Bakker, J. SHERRY|The Product and Its Manufacture, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5252–5257. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Millán, M.C.; Mauricio, J.C.; Moreno, J. Proteins involved in wine aroma compounds metabolism by a Saccharomyces cerevisiae flor-velum yeast strain grown in two conditions. Food Microbiol. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- Moreno, J.; Moreno-García, J.; López-Muñoz, B.; Mauricio, J.C.; García-Martínez, T. Use of a flor velum yeast for modulating colour, ethanol and major aroma compound contents in red wine. Food Chem. 2016, 213, 90–97. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.J.; Ortega, J.M.; Mauricio, J.C. Effect of gluconic acid consumption during simulation of biological aging of sherry wines by a flor yeast strain on the final volatile compounds. J. Agric. Food Chem. 2003, 51, 6198–6203. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C.; Medina, M.; Moreno, J.J. Effect of Schizosaccharomyces pombe on aromatic compounds in dry sherry wines containing high levels of gluconic acid. J. Agric. Food Chem. 2004, 52, 4529–4534. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Ortega, J.M.; Medina, M.; Mauricio, J.C. Gluconic acid consumption in wines by Schizosaccharomyces pombe and its effect on the concentrations of major volatile compounds and polyols. J. Agric. Food Chem. 2004, 52, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Medina, M.; Mauricio, J.C. Potential application of a glucose-transport-deficient mutant of Schizosaccharomyces pombe for removing gluconic acid from grape must. J. Agric. Food Chem. 2005, 53, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Removing gluconic acid by using different treatments with a Schizosaccharomyces pombe mutant: Effect on fermentation byproducts. Food Chem. 2007, 104, 457–465. [Google Scholar] [CrossRef]
- Schmitz, K.; Peter, V.; Meinert, S.; Kornfeld, G.; Hardiman, T.; Wiechert, W.; Noack, S. Simultaneous utilization of glucose and gluconate in Penicillium chrysogenum during overflow metabolism. Biotechnol. Bioeng. 2013, 110, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Valcarcel, M.J.; González, P.; Domecq, B. Influence of Botrytis infection of the grapes on the biological aging process of Fino Sherry. Am. J. Enol. Vitic. 1991, 42, 58–62. [Google Scholar]
- Ruiz-Argüeso, T.; Rodriguez-Navarro, A. Gluconic acid-producing bacteria from honey bees and ripening honey. Microbiology 1973, 76, 211–216. [Google Scholar] [CrossRef]
- Couto, J.A.; Graça, A.R.; Soares-Franco, J.M.; Hogg, T. The use of gluconic acid level as an indicator of the activity of acetic acid bacteria in grapes. In Proceedings of the VII Symposium International d’Oenologie, Bordeaux, France, 19–21 June 2003. [Google Scholar] [CrossRef]
- Peinado, J.; Florindo, J.; Lopez-Barea, J. Glutathione reductase from Saccharomyces cerevisiae undergoes redox interconversion in situ and in vivo. Mol. Cell. Biochem. 1992, 110, 135–143. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Ortega, J.M.; Medina, M.; Moreno, J. Changes in gluconic acid, polyols and major volatile compounds in sherry wine during aging with submerged flor yeast cultures. Biotechol. Lett. 2003, 25, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Feuillat, M.; Charpentier, C. Flor yeast metabolism in a model system similar to cellar ageing of the French Vin Jaune: Evolution of some by-products, nitrogen compounds and polysaccharides. Vitis 2000, 39, 129–134. [Google Scholar]
- Martínez, P.; Valcárcel, M.J.; Piérez, L.; Benítez, T. Metabolism of Saccharomyces cerevisiae flor yeasts during fermentation and biological aging of fino sherry: By-products and aroma compounds. Am. J. Enol. Vitic. 1998, 49, 240–250. [Google Scholar]
- Cortes, M.B.; Moreno, J.; Zea, L.; Moyano, L.; Medina, M. Changes in aroma compounds of sherry wines during their biological aging carried out by Saccharomyces cerevisiae races bayanus and capensis. J. Agric. Food Chem. 1998, 46, 2389–2394. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Medina, M.; Mauricio, J.C. Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol Lett. 2004, 26, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.B.; Moreno, J.J.; Zea, L.; Moyano, L.; Medina, M. Response of the aroma fraction in sherry wines subjected to accelerated biological aging. J. Agric. Food Chem. 1999, 47, 3297–3302. [Google Scholar] [CrossRef]
- Hung, G.C.; Brown, C.R.; Wolfe, A.B.; Liu, J.; Chiang, H.L. Degradation of the gluconeogenic enzymes fructose-1, 6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J. Biol. Chem. 2004, 279, 49138–49150. [Google Scholar] [CrossRef]
- Przybyla-Zawislak, B.; Gadde, D.M.; Ducharme, K.; McCammon, M.T. Genetic and biochemical interactions involving tricarboxylic acid cycle (TCA) function using a collection of mutants defective in all TCA cycle genes. Genetics 1999, 152, 153–166. [Google Scholar] [PubMed]
- Hartig, A.; Simon, M.M.; Schuster, T.; Daugherty, J.R.; Yoo, H.S.; Cooper, T.G. Differentially regulated malate synthase genes participate in carbon and nitrogen metabolism of S. cerevisiae. Nucleic Acids Res. 1992, 20, 5677–5686. [Google Scholar] [CrossRef][Green Version]
- Fernandez, E.; Moreno, F.; Rodicio, R. The ICL1 gene from Saccharomyces cerevisiae. Eur. J. Biochem. 1992, 204, 983–990. [Google Scholar] [CrossRef]
- Strijbis, K.; Distel, B. Intracellular acetyl unit transport in fungal carbon metabolism. Eukaryot. Cell 2010, 9, 1809–1815. [Google Scholar] [CrossRef]
- Kunze, M.; Hartig, A. Permeability of the peroxisomal membrane: Lessons from the glyoxylate cycle. Front. Physiol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Petersen, J.G.; Kielland-Brandt, M.C.; Nilsson-Tillgren, T.; Bornaes, C.; Holmberg, S. Molecular genetics of serine and threonine catabolism in Saccharomyces cerevisiae. Genetics 1988, 119, 527–534. [Google Scholar] [CrossRef]
- Bornaes, C.; Petersen, J.G.; Holmberg, S. Serine and threonine catabolism in Saccharomyces cerevisiae: The CHA1 polypeptide is homologous with other serine and threonine dehydratases. Genetics 1992, 131, 531–539. [Google Scholar] [CrossRef]
- Kastanos, E.K.; Woldman, Y.Y.; Appling, D.R. Role of mitochondrial and cytoplasmic serine hydroxymethyltransferase isozymes in de novo purine synthesis in Saccharomyces cerevisiae. Biochemistry 1997, 36, 14956–14964. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, T.; Gätgens, C.; Weber, U.; Stahmann, K.P. Alanine: Glyoxylate aminotransferase of Saccharomyces cerevisiae–encoding gene AGX1 and metabolic significance. Yeast 2004, 21, 63–73. [Google Scholar] [CrossRef]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef]
- Reynolds, T.B.; Fink, G.R. Bakers’ yeast, a model for fungal biofilm formation. Science 2001, 291, 878–881. [Google Scholar] [CrossRef]
- Kumar, A.; Bachhawat, A.K. OXP1/YKL215c encodes an ATP-dependent 5-oxoprolinase in Saccharomyces cerevisiae: Functional characterization, domain structure and identification of actin-like ATP-binding motifs in eukaryotic 5-oxoprolinases. FEMS Yeast Res. 2010, 10, 394–401. [Google Scholar] [CrossRef]
- Bagnat, M.; Simons, K. Lipid rafts in protein sorting and cell polarity in budding yeast Saccharomyces cerevisiae. Biol. Chem. 2002, 383, 1475–1480. [Google Scholar] [CrossRef]
- Alexandre, H.; Nguyen Van Long, T.; Feuillat, M.; Charpentier, C. Contribution à l’étude des bourbes: Influence sur la fermentescibilité des moûts. Rev. Française D’oenologie 1994, 34, 11–20. [Google Scholar]
- Aguilera, F.; Peinado, R.A.; Millan, C.; Ortega, J.M.; Mauricio, J.C. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int. J. Food Microbiol. 2006, 110, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, D.G. Carbohydrate Metabolism. The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1982; pp. 1–37. [Google Scholar]
- Herrero, P.; Galindez, J.; Ruiz, N.; Martínez-Campa, C.; Moreno, F. Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes. Yeast 1995, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Boles, E.; Hollenberg, C.P. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 1997, 21, 85–111. [Google Scholar] [CrossRef]
- Milewski, S.; Gabriel, I.; Olchowy, J. Enzymes of UDP-GlcNAc biosynthesis in yeast. Yeast 2006, 23, 1–14. [Google Scholar] [CrossRef]
- Mio, T.; Yabe, T.; Arisawa, M.; Yamada-Okabe, H. The eukaryotic UDP-N-acetylglucosamine pyrophosphorylases. Gene cloning, protein expression, and catalytic mechanism. J. Biol. Chem. 1998, 273, 14392–14397. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef]
- Moreno-García, J.; Ogawa, M.; Joseph, C.L.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Comparative analysis of intracellular metabolites, proteins and their molecular functions in a flor yeast strain under two enological conditions. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, R.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electronica 2001, 4, 1–9. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, M.; Moreno-García, J.; Joseph, L.C.M.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Metabolic Changes by Wine Flor-Yeasts with Gluconic Acid as the Sole Carbon Source. Metabolites 2021, 11, 150. https://doi.org/10.3390/metabo11030150
Ogawa M, Moreno-García J, Joseph LCM, Mauricio JC, Moreno J, García-Martínez T. Metabolic Changes by Wine Flor-Yeasts with Gluconic Acid as the Sole Carbon Source. Metabolites. 2021; 11(3):150. https://doi.org/10.3390/metabo11030150
Chicago/Turabian StyleOgawa, Minami, Jaime Moreno-García, Lucy C. M. Joseph, Juan C. Mauricio, Juan Moreno, and Teresa García-Martínez. 2021. "Metabolic Changes by Wine Flor-Yeasts with Gluconic Acid as the Sole Carbon Source" Metabolites 11, no. 3: 150. https://doi.org/10.3390/metabo11030150
APA StyleOgawa, M., Moreno-García, J., Joseph, L. C. M., Mauricio, J. C., Moreno, J., & García-Martínez, T. (2021). Metabolic Changes by Wine Flor-Yeasts with Gluconic Acid as the Sole Carbon Source. Metabolites, 11(3), 150. https://doi.org/10.3390/metabo11030150








