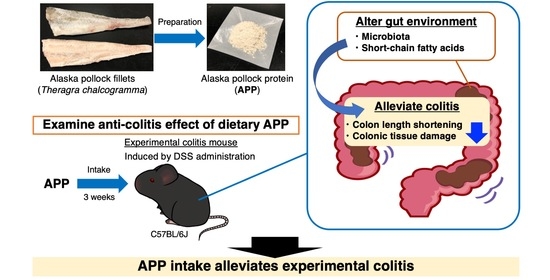
Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice
Abstract
1. Introduction
2. Results
2.1. Nutritional Compositions of APP
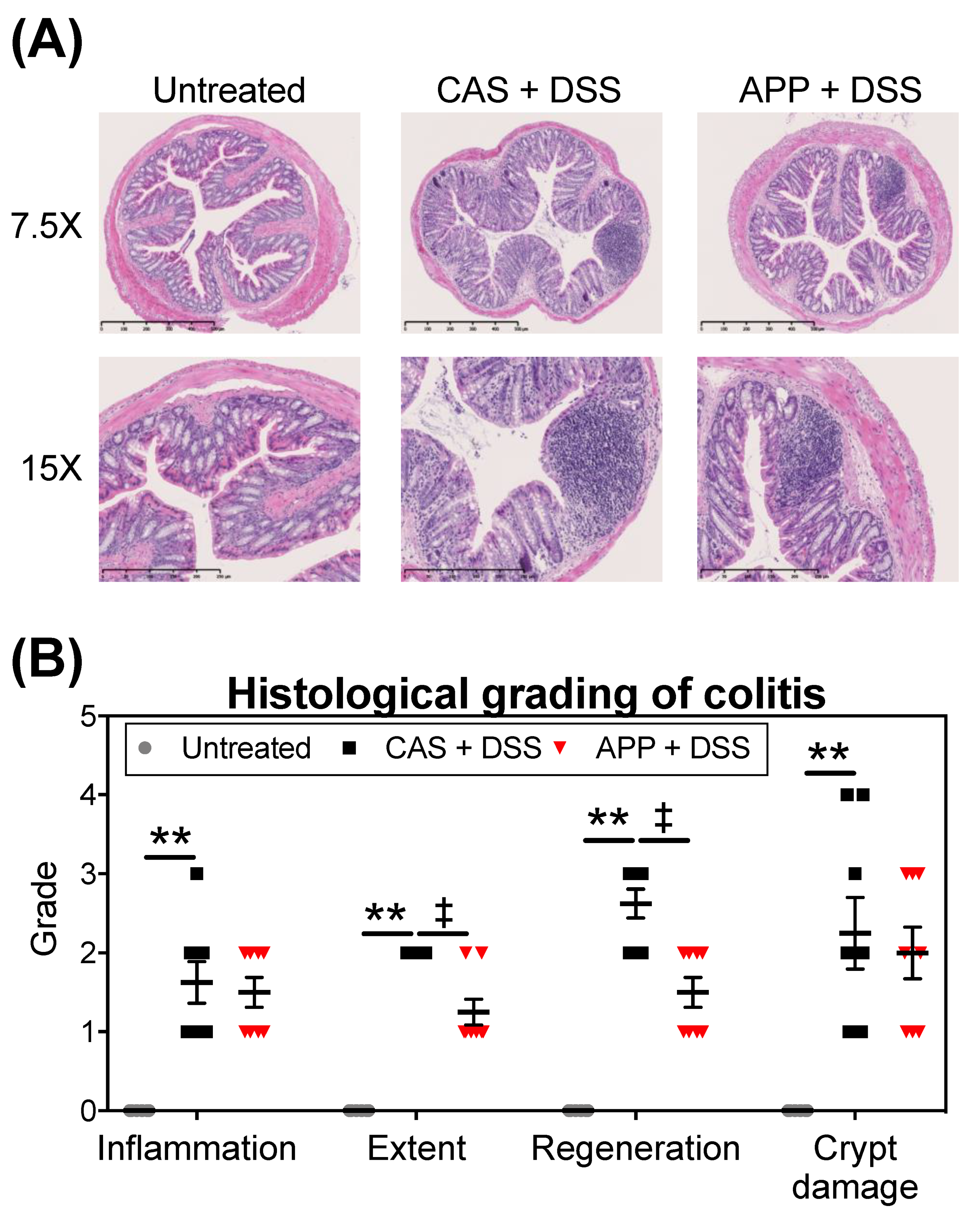
2.2. APP Intake Alleviates Experimental Colitis Symptoms Induced by DSS in Mice
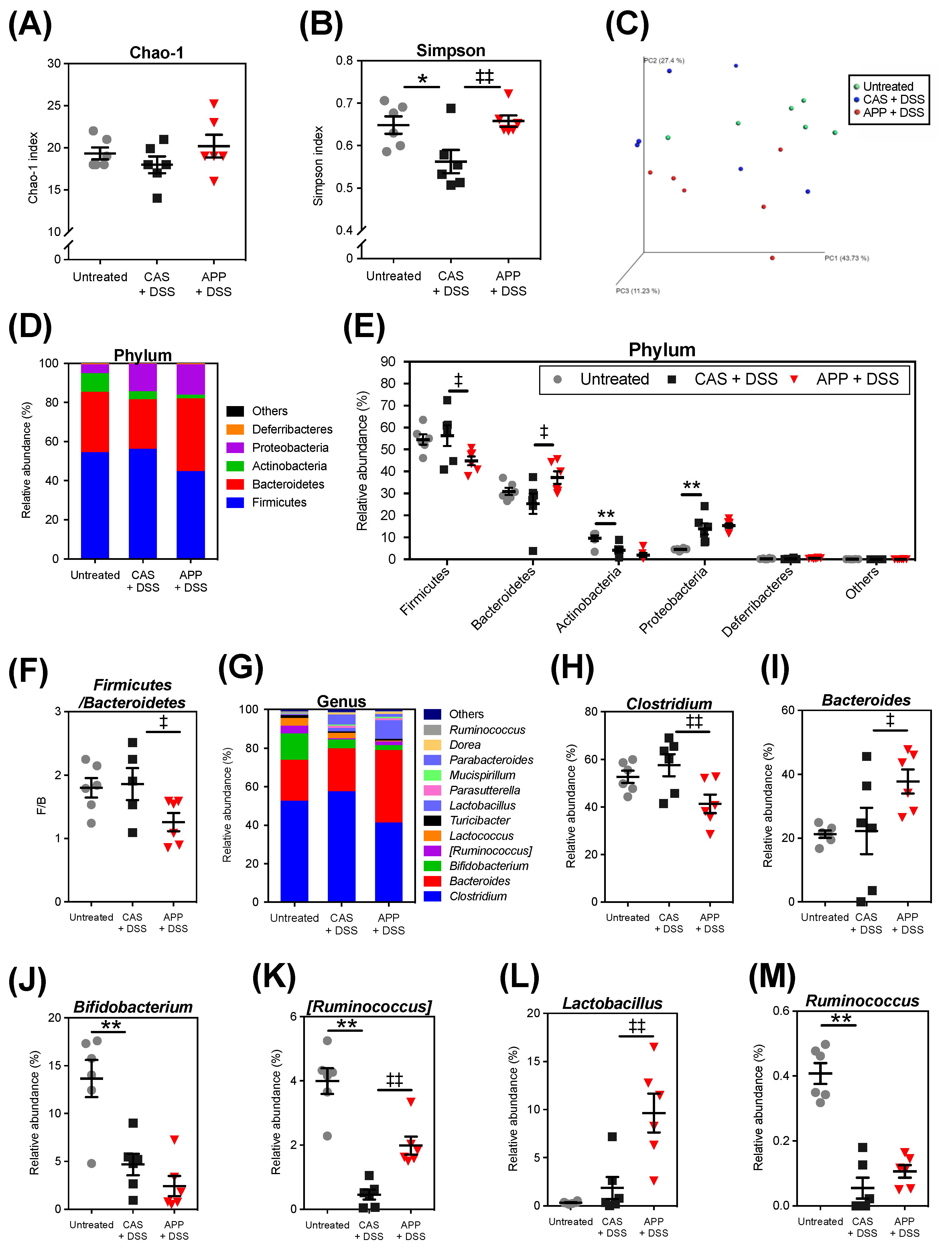
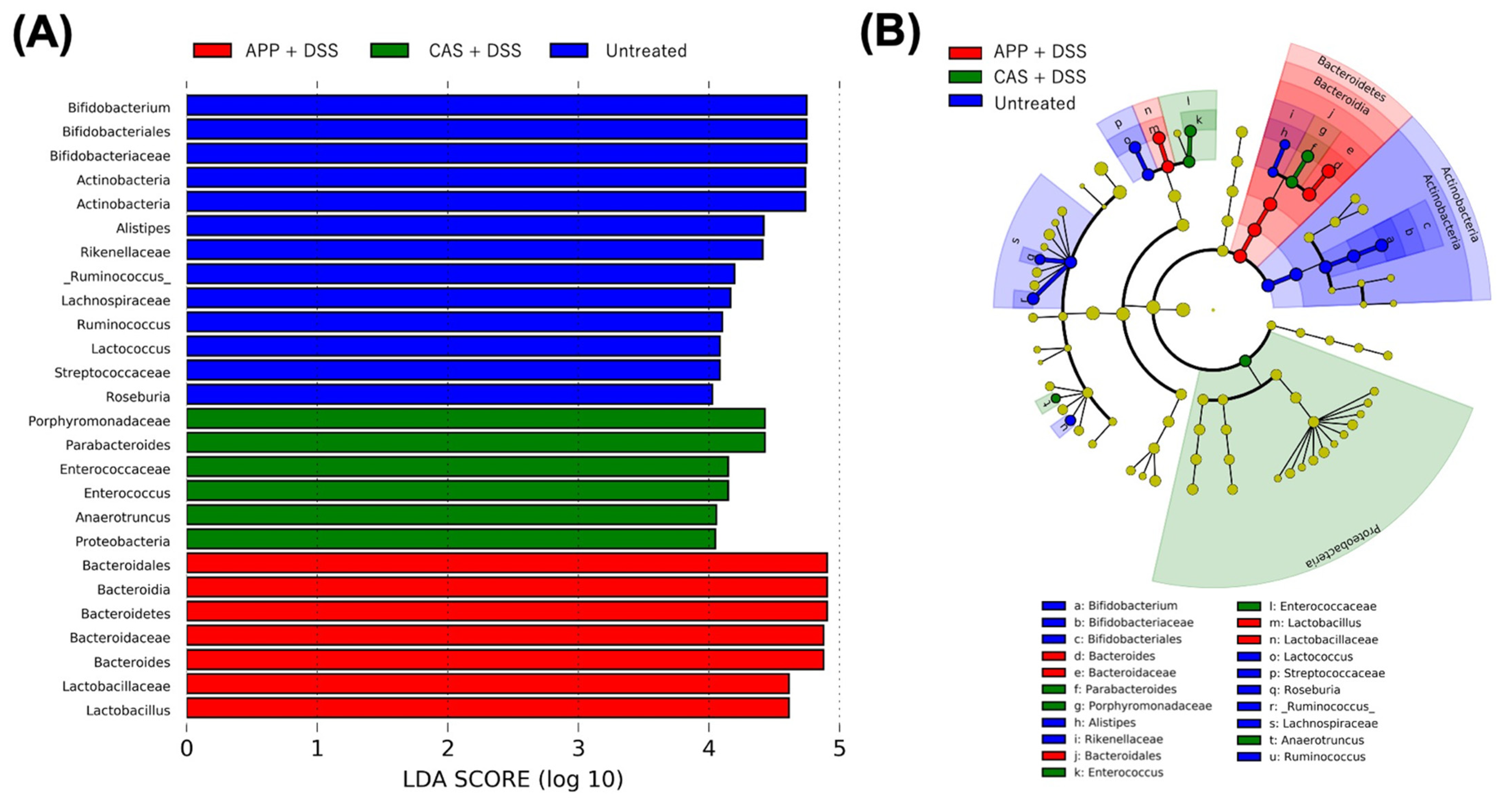
2.3. APP Intake Altered the Composition of the Gut Microbiota of Mice with DSS-Induced Colitis
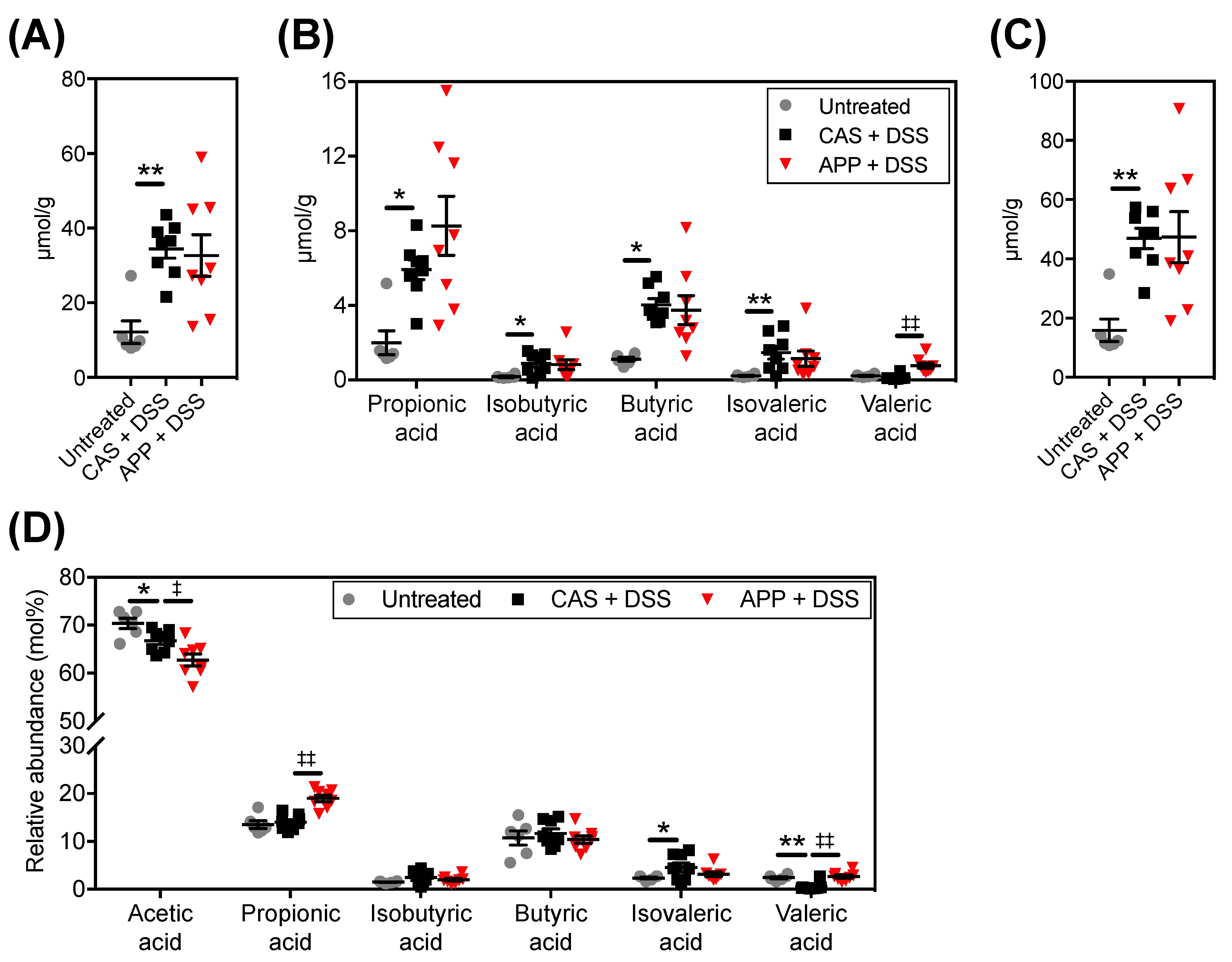
2.4. APP Intake Altered Fecal Short-Chain Fatty Acid (SCFA) Contents/Compositions in Mice with DSS-Induced Colitis
3. Discussion
4. Materials and Methods
4.1. Materials and Experimental Diets
4.2. Nutritional Compositions of the Experimental Proteins (Casein and APP)
4.3. Animal Experiments
4.4. Histopathological Analysis
4.5. mRNA Expression Analysis
4.6. Fecal Short-Chain Fatty Acid (SCFA) Compositions
4.7. The 16S rRNA Amplicon Sequence and Bioinformatics
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarner, V.; Rubio-Ruiz, M.E. Low-Grade Systemic Inflammation Connects Aging, Metabolic Syndrome and Cardiovascular Disease. In Aging and Health-A Systems Biology Perspective; Yashin, A.I., Jazwinski, S.M., Eds.; Karger: Basel, Switzerland, 2015; Volume 40, pp. 99–106. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol 2020, 11, 571731. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Mehta, N.N.; McGillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Furfaro, F.; Sciurti, R.; Bezzio, C.; Ardizzone, S.; de Franchis, R. Glucose intolerance and diabetes mellitus in ulcerative colitis: Pathogenetic and therapeutic implications. World J. Gastroenterol. 2014, 20, 3507. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Elli, L.; Matteis, S.D.; Floriani, I.; Torri, V.; Piodi, L. Autoimmune disorders in patients affected by celiac sprue and inflammatory bowel disease. Ann. Med. 2009, 41, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yorulmaz, E.; Adali, G.; Yorulmaz, H.; Ulasoglu, C.; Tasan, G.; Tuncer, I. Metabolic syndrome frequency in inflammatory bowel diseases. Saudi J. Gastroenterol. 2011, 17, 376. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Kim, K.-A.; Thomas, S.S.; Cha, Y.-S. Effect of Omega-3 Fatty Acid Rich Oils; Fish Oil and Perilla Oil on High-Fat Diet Fed DSS-Induced Colitis Mice. Curr. Dev. Nutr. 2020, 4, 1525. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-κB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2020, 59, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Oyen, J.; Jacques, H.; Kristiansen, K.; Madsen, L. Seafood intake and the development of obesity, insulin resistance and type 2 diabetes. Nutr. Res. Rev. 2019, 32, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosomi, R.; Fukuda, M.; Ikeda, Y.; Yoshida, M.; Fukunaga, K. Dietary Tuna Dark Muscle Protein Attenuates Hepatic Steatosis and Increases Serum High-Density Lipoprotein Cholesterol in Obese Type-2 Diabetic/Obese KK-A(y) Mice. J. Food Sci. 2017, 82, 1231–1238. [Google Scholar] [CrossRef]
- Maeda, H.; Hosomi, R.; Yokoyama, T.; Ikeda, Y.; Nishimoto, A.; Tanaka, G.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; et al. Dietary Alaska pollock protein attenuates liver steatosis and alters gut microbiota in leptin-deficient ob/ob mice. J. Funct. Foods 2020, 75, 104266. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011, 76, H116–H121. [Google Scholar] [CrossRef]
- Oishi, Y.; Dohmoto, N. Alaska pollack protein prevents the accumulation of visceral fat in rats fed a high fat diet. J. Nutr. Sci. Vitaminol. 2009, 55, 156–161. [Google Scholar] [CrossRef][Green Version]
- Kawabata, F.; Mizushige, T.; Uozumi, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Kishida, T. Fish protein intake induces fast-muscle hypertrophy and reduces liver lipids and serum glucose levels in rats. Biosci. Biotechnol. Biochem. 2015, 79, 109–116. [Google Scholar] [CrossRef]
- Hosomi, R.; Nishimoto, A.; Kobayashi, T.; Ikeda, Y.; Mitsui, M.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Alaska pollock protein alters insulin sensitivity and gut microbiota composition in rats. J. Food Sci. 2020, 85, 3628–3637. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Ijaz, M.U.; Hussain, M.; Haq, I.U.; Zhao, D.; Li, C. High-Fat Proteins Drive Dynamic Changes in Gut Microbiota, Hepatic Metabolome, and Endotoxemia-TLR-4-NFκB-Mediated Inflammation in Mice. J. Agric. Food Chem. 2020, 68, 11710–11725. [Google Scholar] [CrossRef]
- Harris, K.G.; Chang, E.B. The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: New insights into complex disease. Clin. Sci. 2018, 132, 2013–2028. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.-K. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.G.; Landström, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Salem, F.; Kindt, N.; Marchesi, J.R.; Netter, P.; Lopez, A.; Kokten, T.; Danese, S.; Jouzeau, J.-Y.; Peyrin-Biroulet, L.; Moulin, D. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United Eur. Gastroenterol. J. 2019, 7, 1008–1032. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Tonkonogy, S.L.; Albright, C.A.; Tsang, J.; Balish, E.J.; Braun, J.; Huycke, M.M.; Sartor, R.B. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 2005, 128, 891–906. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, K.A.; Kang, J.L.; Kim, K.H.; Woo, S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, W.; Xue, F.; Ma, Y.; Dong, L.; Wang, T.; Zheng, Y.; Feng, D.; Chang, M.; Yuan, G.; et al. Schistosoma japonicum peptide SJMHE1 inhibits acute and chronic colitis induced by dextran sulfate sodium in mice. Parasit Vectors 2021, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Zhang, F.; Cao, R.; Ni, X.; Xin, Z.; Deng, J.; Wu, G.; Ren, W.; Yin, Y.; Deng, B. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice With DSS-Induced IBD. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015, 21, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: A pilot study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The Administration of Escherichia coli Nissle 1917 Ameliorates Development of DSS-Induced Colitis in Mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef]
- Wang, C.-S.-E.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef]
- Long, T.; Yu, Z.-J.; Wang, J.; Liu, J.; He, B.-S. Orally administered chitooligosaccharides modulate colon microbiota in normal and colitis mice. Int. J. Pharmacol. 2018, 14, 291–300. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef]
- Chen, R.C.; Xu, L.M.; Du, S.J.; Huang, S.S.; Wu, H.; Dong, J.J.; Huang, J.R.; Wang, X.D.; Feng, W.K.; Chen, Y.P. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol. Lett. 2016, 241, 103–110. [Google Scholar] [CrossRef]
- Yang, K.M.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Yang, X.F. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, M.-F.; Wang, Y.-D.; Chen, L.-P.; Xu, W.-F.; Wang, Y.-D.; Deng, F.; Liu, S.-D. Protein kinase d2 protects against acute colitis induced by dextran sulfate sodium in mice. Sci. Rep. 2016, 6, 34079. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. Msystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Medina, D.A.; Ortúzar, V.; Gotteland, M.; Garrido, D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res. Int. 2018, 109, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 2, 712–715. [Google Scholar] [CrossRef]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. Short-chain fatty acids and thyroid hormone interact in regulating enterocyte gene transcription. Surgery 1999, 126, 293–298. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef]
- McDonald, J.A.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R. Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology 2018, 155, 1495–1507.e1415. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Zhao, F.; Wang, C.; Zamaratskaia, G.; Li, C. Chicken-eaters and pork-eaters have different gut microbiota and tryptophan metabolites. Sci. Rep. 2021, 11, 11934. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Sieber, J.; Schmidt, A.; Waldron, C.; Theis, K.; Schmidt, T. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, A.; Yu, Y.; Guo, S.; Wang, M.; Wang, H. L-arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced apoptosis through alleviating oxidative stress. J. Agric. Food Chem. 2019, 67, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Kawadu, D.; Ohshima, N.; Okuzumi, M.; Fujii, T. Comparisons of Microflora and Metabolic Products between Cecum and Feces of Rats. Jpn. J. Food Microbiol. 1994, 11, 125–128. [Google Scholar] [CrossRef][Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Aoac International: Rockville, MD, USA, 2019. [Google Scholar]
- Fukunaga, K.; Hosomi, R.; Fukao, M.; Miyauchi, K.; Kanda, S.; Nishiyama, T.; Yoshida, M. Hypolipidemic Effects of Phospholipids (PL) Containing n-3 Polyunsaturated Fatty Acids (PUFA) Are Not Dependent on Esterification of n-3 PUFA to PL. Lipids 2016, 51, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Kita, M.; Ueda, Y.; Iwakura, Y.; et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 2006, 146, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Fat Influences the Expression of Genes Related to Sterol Metabolism and the Composition of Cecal Microbiota and Its Metabolites in Rats. J. Oleo Sci. 2019, 68, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Eicosapentaenoic Acid and Docosahexaenoic Acid Ethyl Esters Influence the Gut Microbiota and Bacterial Metabolites in Rats. J. Oleo Sci. 2021, 70, 1469–1480. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]


| Nutritional Composition | Dietary Proteins | |||
|---|---|---|---|---|
| Casein 1 | APP 2 | |||
| Water (g/100 g) | 2.6 | 1.0 | ||
| Ash (g/100 g) | 1.8 | 6.3 | ||
| Crude protein (g/100 g) | 93.7 | 92.0 | ||
| Amino acid composition (wt% of total amino acid) | ||||
| Arginine | 3.56 | 6.66 | ||
| Lysine | 8.04 | 10.16 | ||
| Histidine | 2.68 | 2.04 | ||
| Phenylalanine | 4.88 | 4.17 | ||
| Tyrosine | 5.32 | 3.72 | ||
| Leucine | 9.03 | 8.67 | ||
| Isoleucine | 5.23 | 5.03 | ||
| Methionine | 2.53 | 3.29 | ||
| Valine | 6.23 | 5.35 | ||
| Alanine | 3.06 | 6.41 | ||
| Glycine | 1.81 | 4.65 | ||
| Proline | 10.6 | 3.36 | ||
| Glutamic acid 3 | 21.08 | 16.20 | ||
| Serine | 4.92 | 4.16 | ||
| Threonine | 3.85 | 4.36 | ||
| Aspartic acid 4 | 6.81 | 10.65 | ||
| Cysteine | 0.37 | 1.12 | ||
| Crude fat (g/100 g) | 1.3 | 0.7 | ||
| EPA + DHA (g/100 g) | ND | 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, G.; Hagihara, N.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice. Metabolites 2022, 12, 44. https://doi.org/10.3390/metabo12010044
Tanaka G, Hagihara N, Hosomi R, Shimono T, Kanda S, Nishiyama T, Yoshida M, Fukunaga K. Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice. Metabolites. 2022; 12(1):44. https://doi.org/10.3390/metabo12010044
Chicago/Turabian StyleTanaka, Genki, Nozomi Hagihara, Ryota Hosomi, Takaki Shimono, Seiji Kanda, Toshimasa Nishiyama, Munehiro Yoshida, and Kenji Fukunaga. 2022. "Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice" Metabolites 12, no. 1: 44. https://doi.org/10.3390/metabo12010044
APA StyleTanaka, G., Hagihara, N., Hosomi, R., Shimono, T., Kanda, S., Nishiyama, T., Yoshida, M., & Fukunaga, K. (2022). Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice. Metabolites, 12(1), 44. https://doi.org/10.3390/metabo12010044