Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target
Abstract
1. Focusing on Lipids in Obesity and Metabolic Disorders
2. Trefoil Factor 2 (Tff2) as a High-Fat-Induced Gene
3. TFF2 Metabolic Properties and Implications
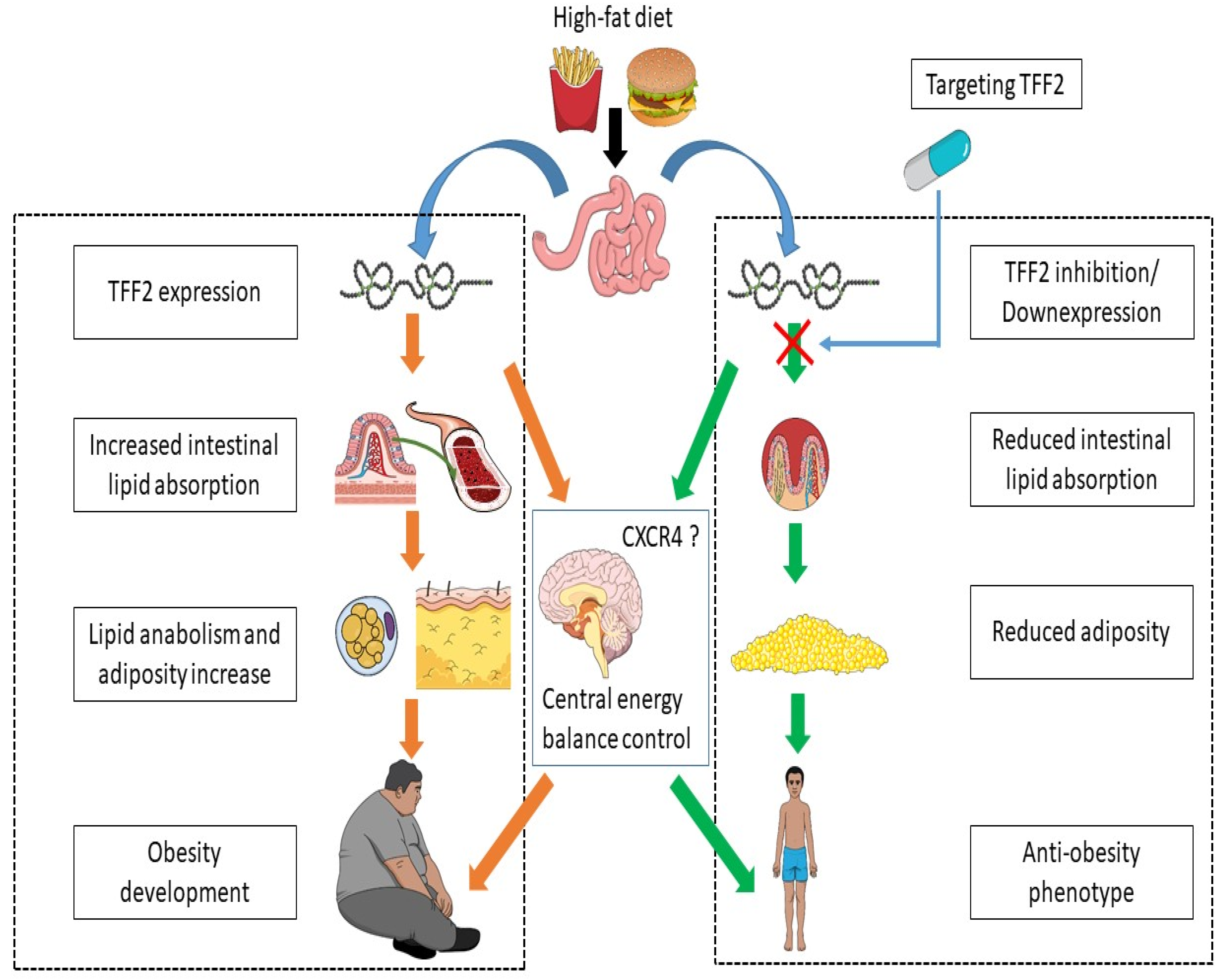
4. Experimental Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effect of red pepper on feeding behavior and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [CrossRef]
- Lusis, A.J. Genetic factors affecting blood lipoproteins: The candidate gene approach. J. Lipid Res. 1988, 29, 397–429. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Julien, P.; Perusse, L.; Vohl, M.-C.; Rudkowska, I. Natural Rumen-Derived trans Fatty Acids Are Associated with Metabolic Markers of Cardiac Health. Lipids 2015, 50, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Perusse, L.; Tremblay, A.; Leblanc, C.; Cloninger, C.R.; Reich, T.; Rice, J.; Bouchard, C. Familial resemblance in energy intake: Contribution of genetic and environmental factors. Am. J. Clin. Nutr. 1988, 47, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.; Day, F.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Rudkowska, I.; Pérusse, L.; Bellis, C.; Blangero, J.; Després, J.-P.; Bouchard, C.; Vohl, M.-C. Interaction between Common Genetic Variants and Total Fat Intake on Low-Density Lipoprotein Peak Particle Diameter: A Genome-Wide Association Study. J. Nutr. Nutr. 2015, 8, 44–53. [Google Scholar] [CrossRef]
- Perusse, L.; Rankinen, T.; Zuberi, A.; Chagnon, Y.C.; Weisnagel, S.J.; Argyropoulos, G.; Walts, B.; Snyder, E.E.; Bouchard, C. The Human Obesity Gene Map: The 2004 Update. Obes. Res. 2005, 13, 381–490. [Google Scholar] [CrossRef]
- Bierman, E.L.; Bagdade, J.D.; Porte, D. Obesity and Diabetes: The Odd Couple. Am. J. Clin. Nutr. 1968, 21, 1434–1437. [Google Scholar] [CrossRef]
- Government of Canada. StatisticsCanada. Overweight and Obese Adults (Self-Reported). 2016. Available online: http://www.statcan.gc.ca/eng/help/bb/info/obesity (accessed on 11 October 2016).
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.; Mägi, R.; Strawbridge, R.; Pers, T.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S.J.; Lee, I.-M. Age-specific physical activities and life style patterns as related to all-causes mortality and to longevity. In Exercise for Preventing Common Diseases; Tanaka, H., Shindo, M., Eds.; Springer: Tokyo, Japan, 1999; pp. 121–130. [Google Scholar]
- Paffenbarger, R.S., Jr. Factors predisposing to fatal stroke in longshoremen. Prev. Med. 1972, 1, 522–528. [Google Scholar] [CrossRef]
- Després, J.P.; Pouliot, M.C.; Moorjani, S.; Nadeau, A.; Tremblay, A.; Lupien, P.J.; Thériault, G.; Bouchard, C. Loss of abdominal fat and metabolic response to exercise training in obese women. Am. J. Physiol. Metab. 1991, 261 (Pt 1), E159–E167. [Google Scholar] [CrossRef]
- Pérusse, L.; Bouchard, C. Genotype-environment interaction in human obesity. Nutr. Rev. 1999, 57, S31–S37. [Google Scholar] [CrossRef]
- Pérusse, L.; Bouchard, C. Gene-diet interactions in obesity. Am. J. Clin. Nutr. 2000, 72, 1285s–1290s. [Google Scholar] [CrossRef] [PubMed][Green Version]
- St-Amand, J.; Prud’Homme, D.; Moorjani, S.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Lupien, P.J.; Després, J.P. Apolipoprotein E polymorphism and the relationships of physical fitness to plasma lipoprotein-lipid levels in men and women. Med. Sci. Sports Exerc. 1999, 31, 692–697. [Google Scholar] [CrossRef] [PubMed]
- St-Amand, J.; Moorjani, S.; Lupien, P.J.; Prud’Homme, D.; Després, J.P. The relation of plasma triglyceride, apolipoprotein B and high-density lipoprotein-cholesterol to postheparin lipoprotein lipase activity is dependent upon apolipoprotein E polymorphism. Metabolism 1996, 45, 261–268. [Google Scholar] [CrossRef]
- St-Amand, J.; Okamura, K.; Matsumoto, K.; Shimizu, S.; Sogawa, Y. Characterization of control and immobilized skeletal muscle: An overview from genetic engineering. FASEB J. 2001, 15, 684–692. [Google Scholar] [CrossRef]
- Lissner, L.; Levitsky, D.A.; Strupp, B.J.; Kalkwarf, H.J.; Roe, D.A. Dietary fat and the regulation of energy intake in human subjects. Am. J. Clin. Nutr. 1987, 46, 886–892. [Google Scholar] [CrossRef]
- Flatt, J.P.; Ravussin, E.; Acheson, K.J.; Jéquier, E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J. Clin. Investig. 1985, 76, 1019–1024. [Google Scholar] [CrossRef]
- Schutz, Y.; Flatt, J.P.; Jéquier, E. Failure of dietary fat intake to promote fat oxidation: A factor favoring the development of obesity. Am. J. Clin. Nutr. 1989, 50. [Google Scholar] [CrossRef]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Will an obesity pandemic replace the coronavirus disease-2019 (COVID-19) pandemic? Med. Hypotheses 2020, 144, 110042. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis: Losing Our Immunity When We Need It the Most. Biology 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Abdominal obesity and the risk of coronary artery disease. Can. J. Cardiol. 1992, 8, 561–562. [Google Scholar] [PubMed]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Mathewson, F.A.; Hsu, P.-H. Relation of body weight to development of ischemic heart disease in a cohort of young north American men after a 26 year observation period: The manitoba study. Am. J. Cardiol. 1977, 39, 452–458. [Google Scholar] [CrossRef]
- Mobley, D.; Baum, N. The Obesity Epidemic and Its Impact on Urologic Care. Rev. Urol. 2015, 17, 165–170. [Google Scholar]
- Corey, K.E.; Kaplan, L.M. Obesity and liver disease: The epidemic of the twenty-first century. Clin. Liver Dis. 2014, 18, 1–18. [Google Scholar] [CrossRef]
- McElroy, S.L. The epidemic of depression with obesity. J. Clin. Psychiatry 2015, 76. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Regeneration during Obesity: An Impaired Homeostasis. Animals 2020, 10, 2344. [Google Scholar] [CrossRef]
- D’Apuzzo, M.R.; Novicoff, W.M.; Browne, J.A. The John Insall Award: Morbid Obesity Independently Impacts Complications, Mortality, and Resource Use After TKA. Clin. Orthop. Relat. Res. 2015, 473, 57–63. [Google Scholar] [CrossRef]
- Nasraway, S.A.; Albert, M.; Donnelly, A.M.; Ruthazer, R.; Shikora, S.A.; Saltzman, E. Morbid obesity is an independent determinant of death among surgical critically ill patients. Crit. Care Med. 2006, 34, 964–970, quiz 971. [Google Scholar] [CrossRef]
- Wood, P.D.; Stefanick, M.L.; Dreon, D.M.; Frey-Hewitt, B.; Garay, S.C.; Williams, P.T.; Superko, H.R.; Fortmann, S.P.; Albers, J.J.; Vranizan, K.M.; et al. Changes in Plasma Lipids and Lipoproteins in Overweight Men during Weight Loss through Dieting as Compared with Exercise. N. Engl. J. Med. 1988, 319, 1173–1179. [Google Scholar] [CrossRef]
- Anderson, J.W.; Brinkman-Kaplan, V.L.; Lee, H.; Wood, C.L. Relationship of weight loss to cardiovascular risk factors in morbidly obese individuals. J. Am. Coll. Nutr. 1994, 13, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Mauriège, P.; Bergeron, J.; Despres, J.P.; Prud’homme, D. Effect of a six month gemfibrozil treatment and dietary recommendations on the metabolic risk profile of visceral obese men. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Greenway, F.L. Current and potential drugs for treatment of obesity. Endocr. Rev. 1999, 20, 805–875. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E. Pathways to obesity. Int. J. Obes. Relat. Metab. Disord. 2002, 26 (Suppl. S2), S12–S17. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting Appetite-Regulating Pathways in the Hypothalamic Regulation of Body Weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Bolduc, C.; Raymond, V.; St-Amand, J. High-fat Meal-induced Changes in the Duodenum Mucosa Transcriptome. Obesity 2008, 16, 2302–2307. [Google Scholar] [CrossRef]
- De Giorgio, M.R.; Yoshioka, M.; St-Amand, J. Feeding Regulates the Expression of Pancreatic Genes in Gastric Mucosa. J. Obes. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Bolduc, C.; Yoshioka, M.; St-Amand, J. Acute Molecular Mechanisms Responsive to Feeding and Meal Constitution in Mesenteric Adipose Tissue. Obesity 2010, 18, 410–413. [Google Scholar] [CrossRef]
- De Giorgio, M.R.; Yoshioka, M.; St-Amand, J. Feeding induced changes in the hypothalamic transcriptome. Clin. Chim. Acta 2009, 406, 103–107. [Google Scholar] [CrossRef]
- Melouane, A.; Ghanemi, A.; Aubé, S.; Yoshioka, M.; St-Amand, J. Differential gene expression analysis in ageing muscle and drug discovery perspectives. Ageing Res. Rev. 2018, 41, 53–63. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef]
- Melouane, A.; Ghanemi, A.; Yoshioka, M.; St-Amand, J. Functional genomics applications and therapeutic implications in sarcopenia. Mutat. Res. 2019, 781, 175–185. [Google Scholar] [CrossRef]
- Mucunguzi, O.; Melouane, A.; Ghanemi, A.; Yoshioka, M.; Boivin, A.; Calvo, E.-L.; St-Amand, J. Identification of the principal transcriptional regulators for low-fat and high-fat meal responsive genes in small intestine. Nutr. Metab. 2017, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Thim, L.; May, F.E. Structure of mammalian trefoil factors and functional insights. Cell Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W.; Wiede, A. Molecular medicine of TFF-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334. [Google Scholar] [PubMed]
- Gajhede, M.; Petersen, T.; Henriksen, A.; Petersen, J.; Dauter, Z.; Wilson, K.; Thim, L. Pancreatic spasmolytic polypeptide: First three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure 1993, 1, 253–262. [Google Scholar] [CrossRef]
- Dignass, A.U.; Sturm, A. Peptide growth factors in the intestine. Eur. J. Gastroenterol. Hepatol. 2001, 13, 763–770. [Google Scholar] [CrossRef]
- Beld, J.; Woycechowsky, K.J.; Hilvert, D. Diselenides as universal oxidative folding catalysts of diverse proteins. J. Biotechnol. 2010, 150, 481–489. [Google Scholar] [CrossRef]
- Hua, Q.X.; Mayer, P.J.; Jia, W.; Zhang, J.; Weiss, A.M. The folding nucleus of the insulin superfamily: A flexible peptide model foreshadows the native state. J. Biol. Chem. 2006, 281, 28131–28142. [Google Scholar] [CrossRef] [PubMed]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. 1997, 53, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Marchbank, T.; Chinery, R.; Evison, R.; Pignatelli, M.; Boulton, R.A.; Thim, L.; Hanby, A.M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology 1995, 108, 108–116. [Google Scholar] [CrossRef]
- Tomasetto, C.; Masson, R.; Linares, J.; Wendling, C.; Lefebvre, O.; Chenard, M.; Rio, M. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology 2000, 118, 70–80. [Google Scholar] [CrossRef]
- Westley, B.R.; Griffin, S.M.; May, F.E.B. Interaction between TFF1, a Gastric Tumor Suppressor Trefoil Protein, and TFIZ1, a Brichos Domain-Containing Protein with Homology to SP-C. Biochemistry 2005, 44, 7967–7975. [Google Scholar] [CrossRef]
- Madsen, J.; Nielsen, O.; Tornøe, I.; Thim, L.; Holmskov, U. Tissue Localization of Human Trefoil Factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.S.; Thulesen, J.; Christensen, L.; Nexo, E.; Thim, L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut 1999, 45, 516–522. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Lu, H.; Wu, L.; Wang, D.; Zhang, Q.; Ding, H. Serum trefoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: A monocentric cohort study in China. BMC Gastroenterol. 2014, 14, 74. [Google Scholar] [CrossRef]
- De Giorgio, M.R.; Yoshioka, M.; Riedl, I.; Moreault, O.; Cherizol, R.-G.; Shah, A.A.; Blin, N.; Richard, D.; St-Amand, J. Trefoil factor family member 2 (Tff2) KO mice are protected from high-fat diet-induced obesity. Obesity 2013, 21, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Baus-Loncar, M.; Schmid, J.; Lalani, E.-N.; Rosewell, I.; Goodlad, R.; Stamp, G.W.H.; Blin, N.; Kayademir, T. Trefoil Factor 2 (Tff2) Deficiency in Murine Digestive Tract Influences the Immune System. Cell. Physiol. Biochem. 2005, 16, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Baus-Loncar, M.; Schmid, J.; Lalani, E.N.; Rosewell, I.; Goodlad, R.; Stamp, G.; Blin, N.; Kayademir, T. Trefoil factor family 2 deficiency and immune response. Cell Mol. Life Sci. 2005, 62, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjöberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil Family Factor 2 Is Expressed in Murine Gastric and Immune Cells and Controls both Gastrointestinal Inflammation and Systemic Immune Responses. Infect. Immun. 2007, 75, 471–480. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor. Animals 2020, 10, 1646. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. High-Fat Diet-Induced Trefoil Factor Family Member 2 (TFF2) to Counteract the Immune-Mediated Damage in Mice. Animals 2021, 11, 258. [Google Scholar] [CrossRef]
- Vowinkel, T.; Mori, M.; Krieglstein, C.F.; Russell, J.; Saijo, F.; Bharwani, S.; Turnage, R.H.; Davidson, W.; Tso, P.; Granger, D.N.; et al. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Investig. 2004, 114, 260–269. [Google Scholar] [CrossRef]
- Okumura, T.; Fukagawa, K.; Tso, P.; Taylor, I.L.; Pappas, T. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology 1994, 107, 1861–1864. [Google Scholar] [CrossRef]
- Tso, P.; Sun, W.; Liu, M. Gastrointestinal satiety signals IV. Apolipoprotein A-IV. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G885–G890. [Google Scholar] [CrossRef]
- Johns, C.E.; Newton, J.L.; Westley, B.R.; May, F.E. Human pancreatic polypeptide has a marked diurnal rhythm that is affected by ageing and is associated with the gastric TFF2 circadian rhythm. Peptides 2006, 27, 1341–1348. [Google Scholar] [CrossRef]
- Shimada, T.; Fujii, Y.; Koike, T.; Tabei, K.; Namatame, T.; Yamagata, M.; Tajima, A.; Yoneda, M.; Trano, A.; Hiraishi, H. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates trefoil factor family 2 (TFF2) expression in gastric epithelial cells. Int. J. Biochem. Cell Biol. 2007, 39, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.; Silvestre, J.S.; Prous, J.R. Experimental approaches to study PPAR gamma agonists as antidiabetic drugs. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Dubeykovskaya, Z.; Dubeykovskiy, A.; Solal-Cohen, J.; Wang, T.C. Secreted Trefoil Factor 2 Activates the CXCR4 Receptor in Epithelial and Lymphocytic Cancer Cell Lines. J. Biol. Chem. 2009, 284, 3650–3662. [Google Scholar] [CrossRef] [PubMed]
- Chinery, R.; Cox, H.M. Immunoprecipitation and characterization of a binding protein specific for the peptide, intestinal trefoil factor. Peptides 1995, 16, 749–755. [Google Scholar] [CrossRef]
- Tan, X.-D.; Hsueh, W.; Chang, H.; Wei, K.R.; Crussi, F.G. Characterization of a Putative Receptor for Intestinal Trefoil Factor in Rat Small Intestine: Identification byin SituBinding and Ligand Blotting. Biochem. Biophys. Res. Commun. 1997, 237, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Thim, L.; Mørtz, E. Isolation and characterization of putative trefoil peptide receptors. Regul. Pept. 2000, 90, 61–68. [Google Scholar] [CrossRef]
- Otto, W.R.; Patel, K.; McKinnell, I.; Evans, M.D.; Lee, C.-Y.; Frith, D.; Hanrahan, S.; Blight, K.; Blin, N.; Kayademir, T.; et al. Identification of blottin: A novel gastric trefoil factor family-2 binding protein. Proteomics 2006, 6, 4235–4245. [Google Scholar] [CrossRef]
- Rimland, J.; Xin, W.; Sweetnam, P.; Saijoh, K.; Nestler, E.J.; Duman, R.S. Sequence and expression of a neuropeptide Y receptor cDNA. Mol. Pharmacol. 1991, 40, 869–875. [Google Scholar]
- Banisadr, G.; Fontanges, P.; Haour, F.; Kitabgi, P.; Rostene, W.; Parsadaniantz, S.M. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002, 16, 1661–1671. [Google Scholar] [CrossRef]
- Vucetic, Z.; Reyes, T.M. Central dopaminergic circuitry controlling food intake and reward: Implications for the regulation of obesity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 577–593. [Google Scholar] [CrossRef]
- Richard, D. Energy expenditure: A critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol. 2007, 32, 173–183. [Google Scholar]
- Trecki, J.; Unterwald, E. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience 2009, 161, 13–22. [Google Scholar] [CrossRef][Green Version]
- Pervin, B.; Aydın, G.; Visser, T.; Uçkan-Çetinkaya, D.; Aerts-Kaya, F.S.F. CXCR4 expression by mesenchymal stromal cells is lost after use of enzymatic dissociation agents, but preserved by use of non-enzymatic methods. Int. J. Hematol. 2021, 113, 5–9. [Google Scholar] [CrossRef]
- Mousessian, A.S.; da Silva, C.P.N.; Oba-Shinjo, S.M.; Kolias, A.G.; Paiva, W.S.; Marie, S.K.N. CXCR7, CXCR4, and Their Ligand Expression Profile in Traumatic Brain Injury. World Neurosurg. 2021, 147, e16–e24. [Google Scholar] [CrossRef]
- Singh, A.K.; Arya, R.K.; Trivedi, A.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.J.; Ng, B.H.; Smits, M.M.; Wang, J.; Jasavala, R.J.; Martinez, H.D.; Lee, J.; Alston, J.J.; Misonou, H.; Trimmer, J.S.; et al. Androgen receptor and chemokine receptors 4 and 7 form a signaling axis to regulate CXCL12-dependent cellular motility. BMC Cancer 2015, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Sun, Y.; Liu, X. CXCR4 as a prognostic biomarker in gastrointestinal cancer: A meta-analysis. Biomarkers 2019, 24, 510–516. [Google Scholar] [CrossRef]
- Li, L.-N.; Jiang, K.-T.; Tan, P.; Wang, A.-H.; Kong, Q.-Y.; Wang, C.-Y.; Lu, H.-R.; Wang, J. Prognosis and Clinicopathology of CXCR4 in Colorectal Cancer Patients: A Meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 4077–4080. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Ahmad, Y.; Barlogie, B.; Jagannath, S.; Teruya-Feldstein, J.; Richter, J. Increased Muscle CXCR4 Expression in the Setting of Rare Muscle-invasive Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, e341–e344. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, W.; Dai, G.; Huang, Y. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4. Int. J. Mol. Med. 2020, 45, 141–150. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Jiang, Z.; Ge, C.; Zhao, F.; Jiang, J.; Tian, H.; Chen, T.; Xie, H.; Cui, Y.; et al. TCF12 promotes the tumorigenesis and metastasis of hepatocellular carcinoma via upregulation of CXCR4 expression. Theranostics 2019, 9, 5810–5827. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, E.; Baraboi, E.D.; Richard, D. Contribution of the vagus nerve and lamina terminalis to brain activation induced by refeeding. Eur. J. Neurosci. 2005, 22, 1489–1501. [Google Scholar] [CrossRef]
- Guesdon, B.; Samson, P.; Richard, D. Effects of intracerebroventricular and intra-accumbens melanin-concentrating hormone agonism on food intake and energy expenditure. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R469–R475. [Google Scholar] [CrossRef]
- Arvaniti, K.; Huang, Q.; Richard, D. Effects of leptin and corticosterone on the expression of corticotropin-releasing hormone, agouti-related protein, and proopiomelanocortin in the brain of ob/ob mouse. Neuroendocrinology 2001, 73, 227–236. [Google Scholar] [CrossRef]
- Huang, Q.; Rivest, R.; Richard, D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology 1998, 139, 1524–1532. [Google Scholar] [CrossRef]
- Timofeeva, E.; Picard, F.; Duclos, M.; Deshaies, Y.; Richard, D. Neuronal activation and corticotropin-releasing hormone expression in the brain of obese (fa/fa) and lean (fa/?) Zucker rats in response to refeeding. Eur. J. Neurosci. 2002, 15, 1013–1029. [Google Scholar] [CrossRef]
- Coppola, A.; Liu, Z.-W.; Andrews, Z.B.; Paradis, E.; Roy, M.-C.; Friedman, J.M.; Ricquier, D.; Richard, D.; Horvath, T.L.; Gao, X.-B.; et al. A Central Thermogenic-like Mechanism in Feeding Regulation: An Interplay between Arcuate Nucleus T3 and UCP2. Cell Metab. 2007, 5, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nadreau, E.; Baraboi, E.-D.; Samson, P.; Blouin, A.; Hould, F.-S.; Marceau, P.; Biron, S.; Richard, D. Effects of the biliopancreatic diversion on energy balance in the rat. Int. J. Obes. 2006, 30, 419–429. [Google Scholar] [CrossRef]
- Cabanac, M.; Richard, D. Acute intraventricular CRF lowers the hoarding threshold in male rats. Physiol. Behav. 1995, 57, 705–710. [Google Scholar] [CrossRef]
- Dagnault, A.; Ouerghi, D.; Richard, D. Treatment with alpha-helical-CRF(9-41) prevents the anorectic effect of 17-beta-estradiol. Brain Res. Bull. 1993, 32, 689–692. [Google Scholar] [CrossRef]
- Richard, D.; Rivest, R.; Naimi, N.; Timofeeva, E.; Rivest, S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology 1996, 137, 4786–4795. [Google Scholar] [CrossRef]
- Rivest, S.; Richard, D. Hypothalamic paraventricular nucleus lesions do not prevent anorectic effect of exercise in male rats. Am. J. Physiol. Integr. Comp. Physiol. 1990, 259 (Pt 2), R579–R584. [Google Scholar] [CrossRef]
- Mailhot, G.; Ravid, Z.; Barchi, S.; Moreau, A.; Rabasa-Lhoret, R.; Levy, E. CFTR knockdown stimulates lipid synthesis and transport in intestinal Caco-2/15 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1239–G1249. [Google Scholar] [CrossRef]
- Baraboi, E.-D.; Michel, C.; Smith, P.; Thibaudeau, K.; Ferguson, A.V.; Richard, D. Effects of albumin-conjugated PYY on food intake: The respective roles of the circumventricular organs and vagus nerve. Eur. J. Neurosci. 2010, 32, 826–839. [Google Scholar] [CrossRef]
- Levy, E.; Harmel, E.; Laville, M.; Sanchez, R.; Emonnot, L.; Sinnett, D.; Ziv, E.; Delvin, E.; Couture, P.; Marcil, V.; et al. Expression of Sar1b Enhances Chylomicron Assembly and Key Components of the Coat Protein Complex II System Driving Vesicle Budding. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2692–2699. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obese Animals as Models for Numerous Diseases: Advantages and Applications. Medicina 2021, 57, 399. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.-M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A protein intimately involved in metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Khalil, H.; Ellwood, L.; Lord, H.; Fernandez, R. Pharmacological Treatment for Obesity in Adults: An Umbrella Review. Ann. Pharmacother. 2020, 54, 691–705. [Google Scholar] [CrossRef]
- Al-Azzeh, E.; Dittrich-Breiholz, O.; Vervoorts, J.; Blin, N.; Gött, P.; Lüscher, B. Gastroprotective peptide trefoil factor family 2 gene is activated by upstream stimulating factor but not by c-Myc in gastrointestinal cancer cells. Gut 2002, 51, 685–690. [Google Scholar] [CrossRef][Green Version]
- Shah, A.A.; Leidinger, P.; Keller, A.; Wendschlag, A.; Meese, E.; Blin, N. Altered miRNA expression patterns in Tff2 knock-out mice correlate with cellular pathways of neoplastic development and caloric metabolism. Int. J. Mol. Med. 2012, 29, 637–643. [Google Scholar] [CrossRef]
- Shah, A.A.; Mihalj, M.; Ratkaj, I.; Lubka-Pathak, M.; Balogh, P.; Klingel, K.; Bohn, E.; Blin, N.; Baus-Loncar, M. Increased Susceptibility to Yersinia enterocolitica Infection of Tff2 Deficient Mice. Cell. Physiol. Biochem. 2012, 30, 853–862. [Google Scholar] [CrossRef]
- Chao, L.C.; Zhang, Z.; Pei, L.; Saito, T.; Tontonoz, P.; Pilch, P. Nur77 Coordinately Regulates Expression of Genes Linked to Glucose Metabolism in Skeletal Muscle. Mol. Endocrinol. 2007, 21, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.A.; Cleasby, M.E.; Harding, A.; Stark, A.; Cooney, G.J.; Muscat, G.E. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J. Biol. Chem. 2005, 280, 12573–12584. [Google Scholar] [CrossRef] [PubMed]
- Pearen, M.A.; Muscat, G.E.O. Minireview: Nuclear Hormone Receptor 4A Signaling: Implications for Metabolic Disease. Mol. Endocrinol. 2010, 24, 1891–1903. [Google Scholar] [CrossRef]
- Guerremillo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18. [Google Scholar] [CrossRef]
- Siersbaek, R.; Nielsen, R.; Mandrup, S. PPARgamma in adipocyte differentiation and metabolism—Novel insights from genome-wide studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target. Metabolites 2021, 11, 536. https://doi.org/10.3390/metabo11080536
Ghanemi A, Yoshioka M, St-Amand J. Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target. Metabolites. 2021; 11(8):536. https://doi.org/10.3390/metabo11080536
Chicago/Turabian StyleGhanemi, Abdelaziz, Mayumi Yoshioka, and Jonny St-Amand. 2021. "Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target" Metabolites 11, no. 8: 536. https://doi.org/10.3390/metabo11080536
APA StyleGhanemi, A., Yoshioka, M., & St-Amand, J. (2021). Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target. Metabolites, 11(8), 536. https://doi.org/10.3390/metabo11080536






