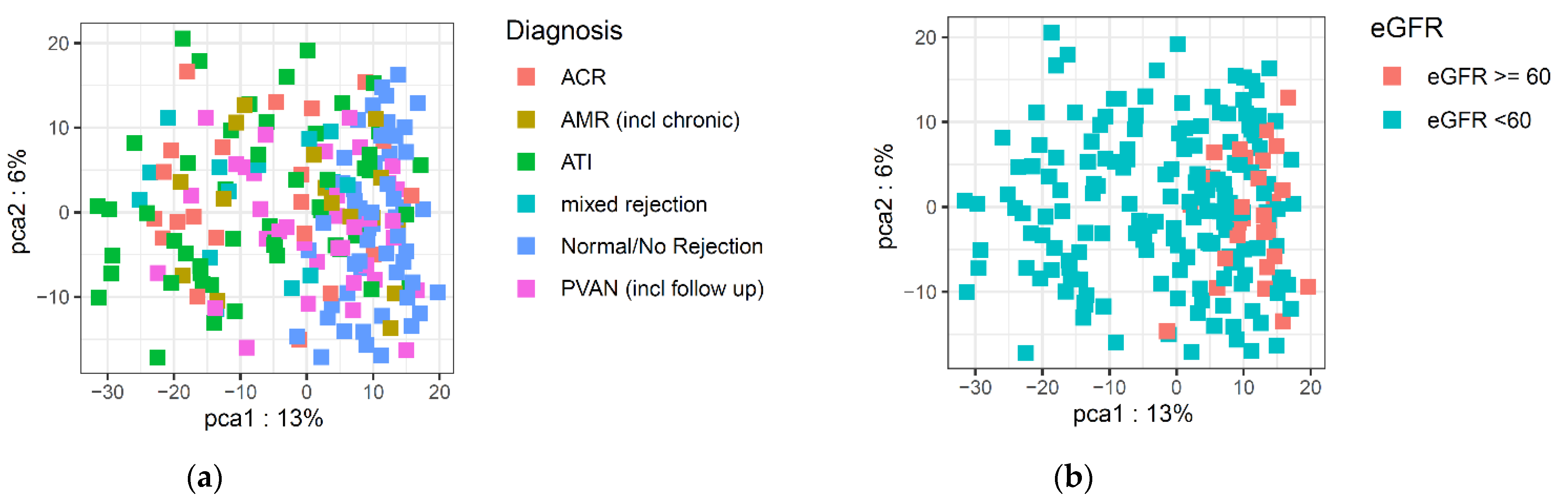
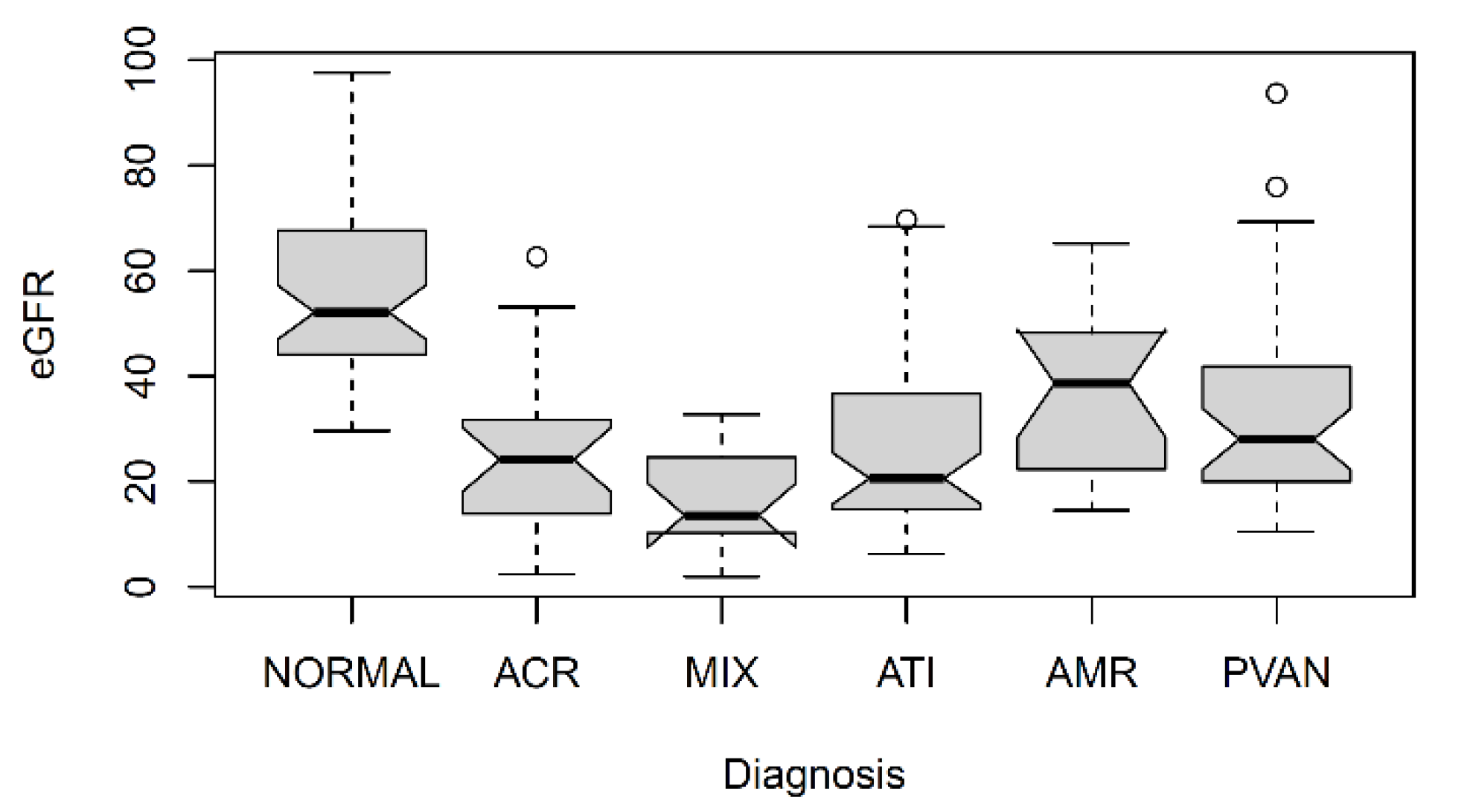
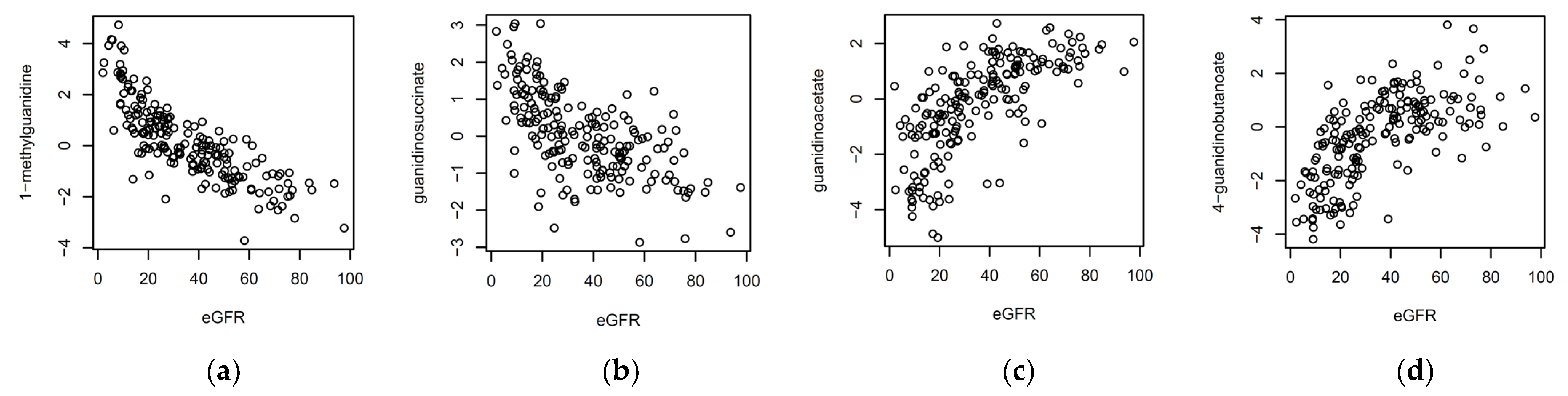
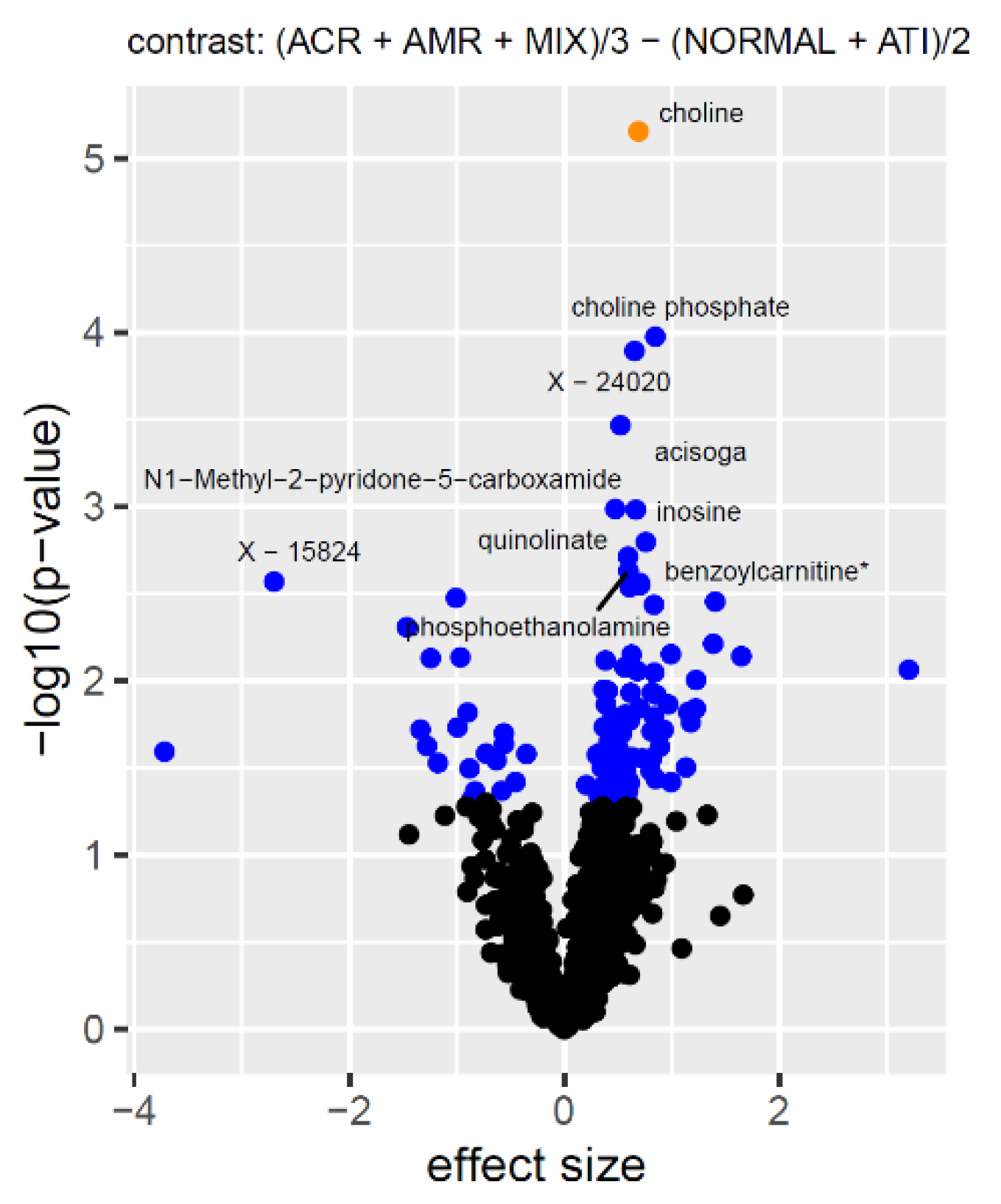
1. Introduction
Kidney transplantation is the treatment of choice for patients who are diagnosed with irreversible kidney failure. However, the full benefits of kidney transplantation are undermined by both immune factors and non-immune complications. Among the immune factors, allograft rejection is the foremost complication, and is a major contributor to allograft failure and the death of the transplant recipient. Among the non-immune factors, infections and malignancy are life-threatening complications. With the introduction of potent immunosuppressive regimens, viral infections have emerged as a major threat, with infection with the cytomegalovirus (CMV) and BK virus being the two leading viral infections in the post-transplant period. Effective anti-viral therapy for treating CMV, but not for BK virus, is currently available. Thus, BK virus-associated nephropathy (BKVN) is an important cause of kidney allograft dysfunction and failure.
The kidney allograft biopsy is the current diagnostic tool for identifying kidney allograft dysfunction. This invasive procedure has become safer over the years, and the interpretation of biopsy findings has been standardized by almost yearly updates of the Banff classification schema. Nevertheless, bleeding, and even death, are documented complications of kidney allograft biopsy, and biopsy interpretation still suffers from its semi-quantitative nature and inter-observer variability, even among experienced pathologists. Importantly, the immune response that is directed at the allograft is dynamic, and repeat biopsies to capture the kinetics of the anti-allograft repertoire are neither practical nor safe.
The kidney is a major excretory organ, and the glomerular filtration rate (GFR) is either measured or estimated to evaluate kidney function. Multiple factors, including water-soluble drugs and metabolites, are filtered and excreted by the kidneys, and the dosages of drugs are adjusted based on the GFR. Indeed, plasma metabolite concentration may be a more sensitive indicator of the GFR than the clinically used serum creatinine [
1].
Noninvasive assessment of kidney allograft status is a major goal in kidney transplantation. Our laboratory pioneered urinary cell mRNA profiling, and demonstrated that the urinary cell levels of mRNAs encoding immunoregulatory proteins and mRNAs encoding cytotoxic proteins are biomarkers of acute cellular rejection (ACR). In the earlier investigation, and in the current one, ACR or acute T-cell-mediated rejection are used as interchangeable terms. Our single-center studies led to a multicenter clinical trials of transplantation (CTOT)-04 study of 485 prospectively enrolled kidney allograft recipients [
2]. In the CTOT-04 study, we discovered and validated a urinary cell three-gene signature, consisting of CD3E mRNA, IP-10 mRNA, and 18S rRNA (CTOT-04 signature), which is diagnostic and prognostic of ACR. In a multimodal interrogation of the CTOT-04 study cohort, we identified that a composite signature of the CTOT-04 three-gene signature, and ratios of 3-sialyllactose to xanthosine (3-SL/X) and quinolinate to X-16397, outperform either the mRNA signature or the metabolite signature in diagnosing ACR [
3].
Metabolite profiles have also been associated with kidney allograft status by others. Ho et al. [
4] analyzed samples from adult kidney transplant recipients, using a targeted metabolomics platform, and demonstrated that urinary metabolites distinguished patients with no rejection, sub-clinical rejection, or clinical rejection from each other. Sigdel et al. [
5] applied gas chromatography–mass spectrometry (GC-MS) to analyze biopsy-matched urine samples from a pediatric cohort of kidney allograft recipients, and generated metabolite panels to detect graft injury phenotypes.
In this investigation, we examined whether the metabolites that we previously identified as being associated with ACR are also associated with ACR when urine cell-free supernatants from an independent cohort of kidney allograft recipients are profiled with a new metabolomics platform that is capable of identifying a boarder spectrum of metabolites than in our earlier study (1276 metabolites vs. 749 metabolites). We also investigated whether the metabolite profiles that are associated with ACR are also associated with the other major type of acute rejection, which is the antibody-mediated rejection (AMR). In view of the clinical significance of polyomavirus-associated nephropathy (PVAN), it might of be of interest to explore the metabolite profile of PVAN. While we include all the available samples from this new study in the statistical analysis, to increase the statistical power, we shall address the detailed biomarker analysis of PVAN in a future paper, as its biology is quite different.
In our earlier investigation, we observed a weak association between the metabolite signature and eGFR, but did not have access to the required phenotype data to further investigate this link [
3]. In this investigation, we explored this relationship further, including whether eGFR confounds the association between the metabolites and acute rejection. First, we computed linear models for each metabolite with all the available and potentially relevant covariates, including, in particular, eGFR and the CTOT-04 mRNA signature. Then, we computed a range of relevant contrasts, which were defined as differences between the combined estimated means of the selected rejection types, and we have provided the full statistics as
supplementary data, so that everything that we do not cover in this paper can be looked up by the interested reader. To include the largest possible number of samples, we focus our discussion on the difference between acute rejection samples, which are a combination of ACR, AMR, and mixed rejection, and non-rejection samples, which are a combination of ATI and normal biopsy samples. As there are more analyses that could be conducted with this dataset than what can be covered in a single paper, we also make the metabolomics data freely available for further analysis by other investigators. Finally, we evaluate the potential of a multi-parameter model to predict the presence of acute rejection in human kidney allografts.
3. Discussion
In this investigation, we asked whether metabolites that were measured in biopsy-matched cell-free urine supernatants distinguish kidney allograft recipient patients with acute-rejection biopsies from patients with biopsies without histological features of acute rejection. We found that kidney function, as measured by eGFR, is an important confounding factor of metabolite profiles. Sekula et al. investigated the blood-based markers of kidney function, using a non-targeted metabolomics platform [
1]. Many of the metabolites that they reported in that study were also found here, in urine. The metabolites that were most strongly associated with eGFR were from the guanidino and guanine pathways. Our examination of the replicability of our previously identified associations, from the multi-center CTOT-04 study, identified quinolinate as a metabolite that remains significant, even after accounting for all the available sources of variation, including ethnicity, age, sex, diabetes, and medication used for the induction and maintenance of immunosuppression and, importantly, eGFR. While the association of the ratio quinolinate/X—16397 also replicated, it should be noted that the
p-gain, that is, the increase in the strength of association when using ratios [
9], does not.
The validated increase in quinolinate in patients with acute rejection biopsies is of interest, since this metabolite has been considered a “double-edge” sword. Quinolinate is an intermediate metabolite in the tryptophan metabolism contributing to the de novo biosynthesis of nicotinamide adenine dinucleotide (NAD
+), which is pivotal to energy and critical cellular processes [
10]. Importantly, de novo NAD
+ biosynthesis impairment has been linked to acute kidney injury in humans [
11]. Thus, higher quinolinate levels may reflect impaired NAD
+ biosynthesis during an episode of acute rejection, and restoration of NAD
+ synthesis may be of benefit in this setting. It is also possible that the accumulation of quinolinate, which is considered a toxic metabolite, may be a contributory factor to graft dysfunction during an episode of acute rejection.
The current study was not designed to identify mechanisms for the alterations in metabolite concentration, such as the increase in quinolinate. However, in our ongoing RNA sequencing studies of human kidney allografts undergoing acute rejection, we identified that the mRNA for quinolinate phosphoribosyltransferase (QPRT) is significantly reduced in acute rejection biopsies compared to biopsies without acute rejection changes. The reduced expression of QPRT in the rejecting allograft is a biologically plausible mechanism for increased quinolinate, since QPRT is central to NAD+ synthesis from quinolinate.
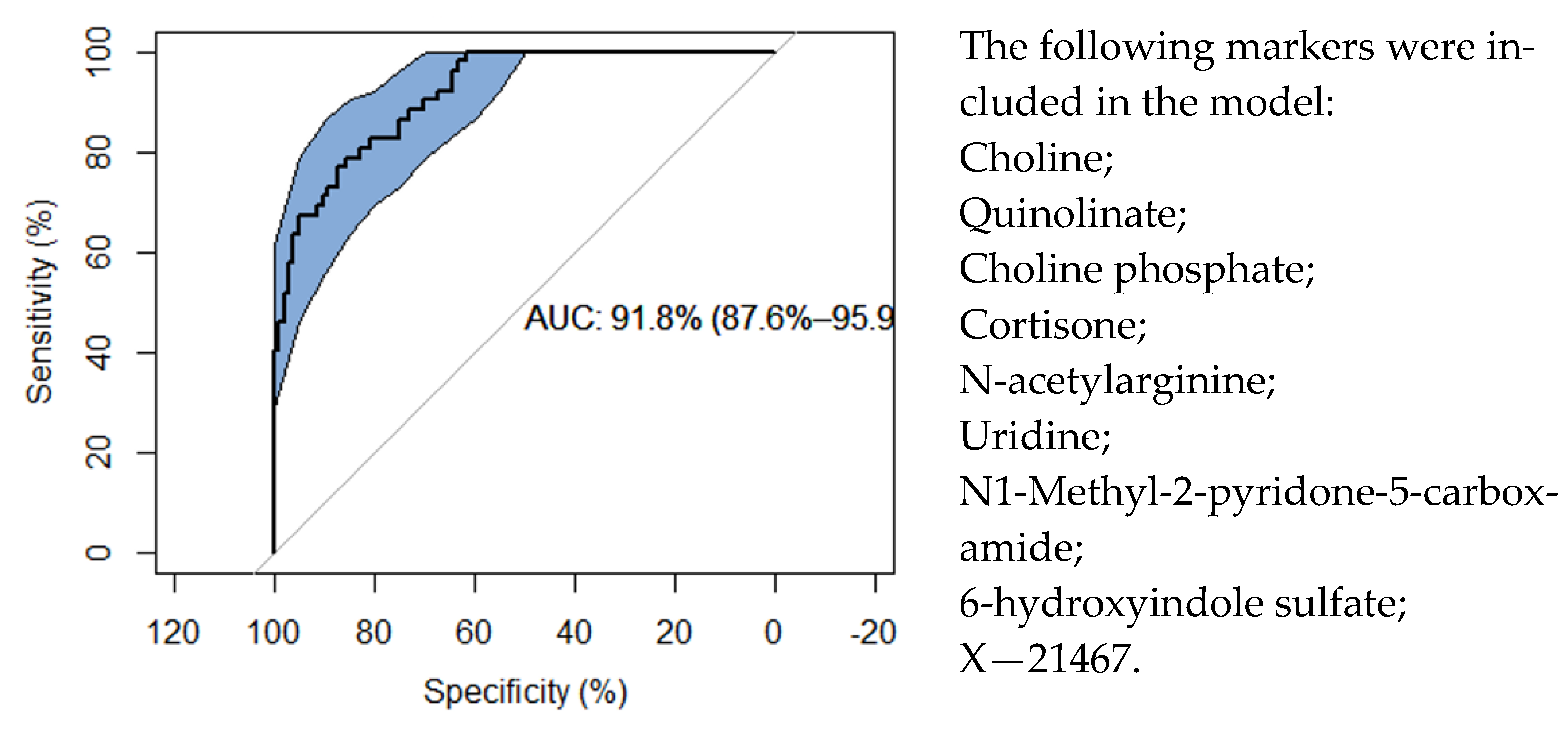
This current study differs from our previous study, in considering both ACR and AMR rejection instead of ACR alone, and using an updated version of the Metabolon (Durham, NC) platform that includes metabolites that have not been investigated in the context of kidney rejection before. We could therefore identify new associations that had not been observed before, and assure that these metabolite profiles were most likely not the result of impaired kidney function alone. Using a multivariate analysis with variable selection, we affirm that the metabolites that were included in the prediction model discriminate acute- from no-rejection samples, with an AUC of 91.8%.
Our investigation has several strengths. The biospecimens were collected from kidney transplant patients who all received their kidney transplants at a single center and were managed with standardized immunosuppressive protocols, thereby minimizing the variabilities that are intrinsic to studies involving a multicenter study cohort. Each cell-free urine supernatant that was analyzed in this study was matched to a kidney allograft biopsy, ensuring “ground truth” about the kidney allograft status, where each biopsy was interpreted by highly experienced kidney pathologists and classified using the Banff classification schema, thereby minimizing the inter-observer variability in biopsy interpretation. The urine cell-free supernatants were stored in a −80 °C biorepository, and were not thawed prior to metabolomic profiling. It is noteworthy that unbiased metabolite profiling was performed, using the highly robust and state-of-the-art Metabolon platform.
Our study also has several limitations. Kidney allograft function was defined using estimated GFR, rather than measured GFR, and the “best” formula to be used to estimate GFR is an area of evolving science. Importantly, the inclusion of race in the eGFR measurement is being vigorously addressed by the professional societies, and in this study, a substantial number of kidney transplant recipients were black recipients and the eGFR was calculated using race as one the variables [
12]. Then, there is always the issue of association analysis, that is, whether the metabolite profile is altered by GFR or whether the GFR is altered by metabolites, or both. With respect to the metabolites measured, the measurements are relative in nature, and the translation of any marker signature would benefit from the development of a targeted assay for absolute quantification, followed by a reevaluation of the model coefficients using absolute values. While we included all the covariates that might explain part of the variation, and that were available to us, other non-identified factors are likely to co-exist. The variable selection methods depend on multiple parameters, and may yield differing results. The prediction model we developed here should, therefore, be interpreted as one of many possible ones. In order to allow others to build on our study, and potentially develop additional accurate models for prediction, we have provided the full dataset in
Tables S3–S5.
As a correlate, the unknown metabolites that are associated here with the drugs used for the maintenance of immunosuppression, are likely to be metabolites of immunosuppressive drugs. This information could be used in the future, to facilitate the identification of unidentified molecules using a mass-spectrometry method, following the approach suggested by Krumsiek et al. [
13].
In summary, our investigation of the association between metabolite profiles and acute rejection in human kidney allografts, in addition to replicating the directionality of the 22 of the 24 metabolites that have previously been associated with ACR [
3], identified a candidate signature model consisting of 9 metabolites that discriminated kidney allograft recipients, with the biopsies showing acute rejection from the recipients without acute rejection. The current study also emphasizes the importance of considering GFR in linking metabolite concentrations to kidney allograft biopsy findings. We hypothesize that metabolites, besides serving as biomarkers, may suggest novel therapeutics, such as the use of nicotinamide adenine mononucleotide to promote NAD+ biosynthesis and improve allograft function.
Author Contributions
Conceptualization, K.S. and M.S.; data analysis, K.S. and J.R.L.; data curation, D.M.D., T.M., Q.C., and S.S.G.; writing, K.S. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. This work was also supported, in part, by awards from the NIH to MS (An Administrative Supplement to NIH MERIT Award, R37AI051652-13), and Weill Cornell Medical College (Clinical and Translational Science Center Award UL1TR000457) and an award from the Mendez National Institute of Transplantation Foundation to MS.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Weill Cornell Medicine (IRB Protocol number 9402002786 and 1207012730).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data used in this investigation are provided as
Tables S3–S5 in Excel format.
Acknowledgments
We thank Catherine Snopkowski and Caroline Li for the superb technical assistance. We thank all study patients for their participation in this study and thank the Weill Cornell Transplant team for their contributions.
Conflicts of Interest
K.S. and M.S. are named as inventors on patent application US15/577,977 (“Urine metabolite profiles identify kidney allograft status”). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sekula, P.; Goek, O.N.; Quaye, L.; Barrios, C.; Levey, A.S.; Römisch-Margl, W.; Menni, C.; Yet, I.; Gieger, C.; Inker, L.A.; et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. J. Am. Soc. Nephrol. JASN 2016, 27, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Suthanthiran, M.; Schwartz, J.E.; Ding, R.; Abecassis, M.; Dadhania, D.; Samstein, B.; Knechtle, S.J.; Friedewald, J.; Becker, Y.T.; Sharma, V.K.; et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N. Engl. J. Med. 2013, 369, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhre, K.; Schwartz, J.E.; Sharma, V.K.; Chen, Q.; Lee, J.R.; Muthukumar, T.; Dadhania, D.M.; Ding, R.; Ikle, D.N.; Bridges, N.D.; et al. Urine Metabolite Profiles Predictive of Human Kidney Allograft Status. J. Am. Soc. Nephrol. JASN 2016, 27, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.; Sharma, A.; Mandal, R.; Wishart, D.S.; Wiebe, C.; Storsley, L.; Karpinski, M.; Gibson, I.W.; Nickerson, P.W.; Rush, D.N. Detecting Renal Allograft Inflammation Using Quantitative Urine Metabolomics and CXCL10. Transplant. Direct 2016, 2, e78. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Schroeder, A.W.; Yang, J.Y.C.; Sarwal, R.D.; Liberto, J.M.; Sarwal, M.M. Targeted Urine Metabolomics for Monitoring Renal Allograft Injury and Immunosuppression in Pediatric Patients. J. Clin. Med. 2020, 9, 2341. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Orita, Y.; Ando, A.; Mikami, H.; Fujii, M.; Okada, A.; Abe, H. Liquid-chromatographic determination of guanidino compounds in plasma and erythrocyte of normal persons and uremic patients. Clin. Chem. 1981, 27, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, I.; Schultheiss, U.T.; Kotsis, F.; Schlosser, P.; Stockmann, H.; Mohney, R.P.; Schmid, M.; Oefner, P.J.; Eckardt, K.U.; Köttgen, A.; et al. Urine Metabolite Levels, Adverse Kidney Outcomes, and Mortality in CKD Patients: A Metabolome-wide Association Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Hofner, B.; Boccuto, L.; Göker, M. Controlling false discoveries in high-dimensional situations: Boosting with stability selection. BMC Bioinform. 2015, 16, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, A.K.; Krumsiek, J.; Wagele, B.; Theis, F.J.; Wichmann, H.E.; Gieger, C.; Suhre, K. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinform. 2012, 13, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.-B.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyan Mehr, A.; Tran, M.T.; Ralto, K.M.; Leaf, D.E.; Washco, V.; Messmer, J.; Lerner, A.; Kher, A.; Kim, S.H.; Khoury, C.C.; et al. De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat. Med. 2018, 24, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.A.; Inker, L.A.; Levey, A.S.; Tighiouart, H.; Powe, N.R.; Manrai, A.K. In Search of a Better Equation—Performance and Equity in Estimates of Kidney Function. N. Engl. J. Med. 2021, 384, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Suhre, K.; Evans, A.M.; Mitchell, M.W.; Mohney, R.P.; Milburn, M.V.; Wägele, B.; Römisch-Margl, W.; Illig, T.; Adamski, J.; et al. Mining the unknown: A systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet. 2012, 8, e1003005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wagele, B.; Altmaier, E.; Deloukas, P.; Erdmann, J.; Grundberg, E.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).