Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation
Abstract
:1. Introduction
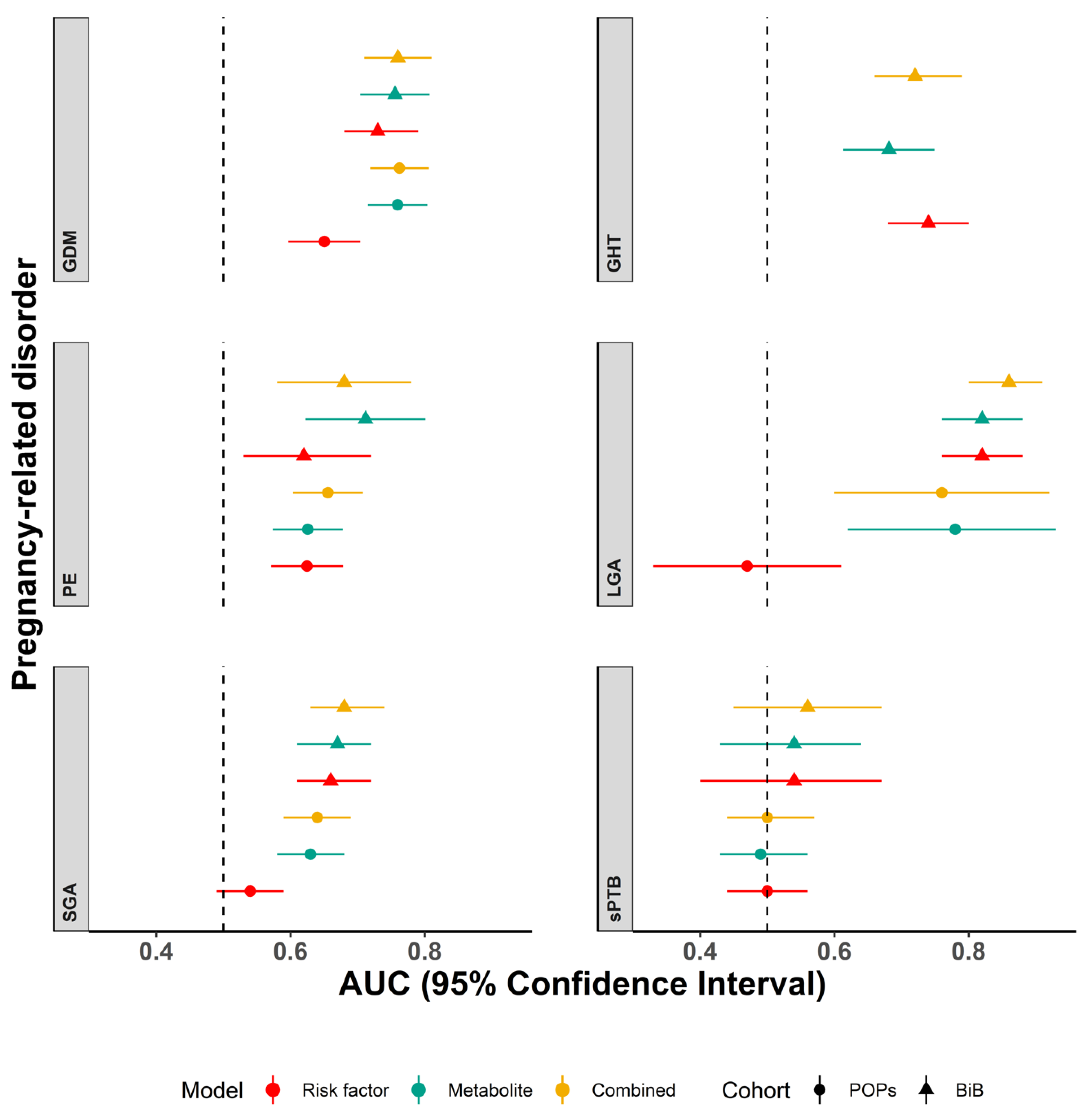
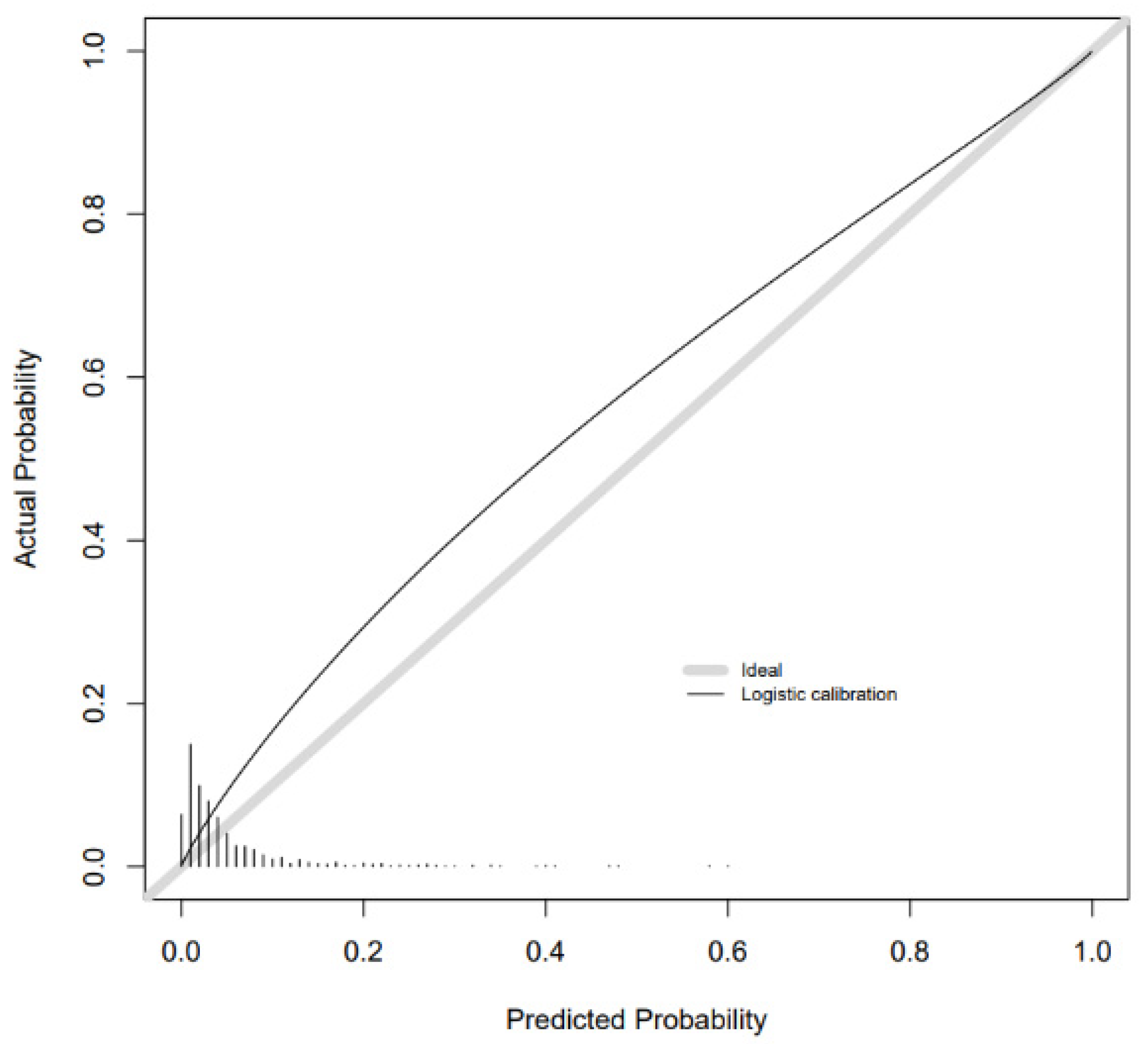
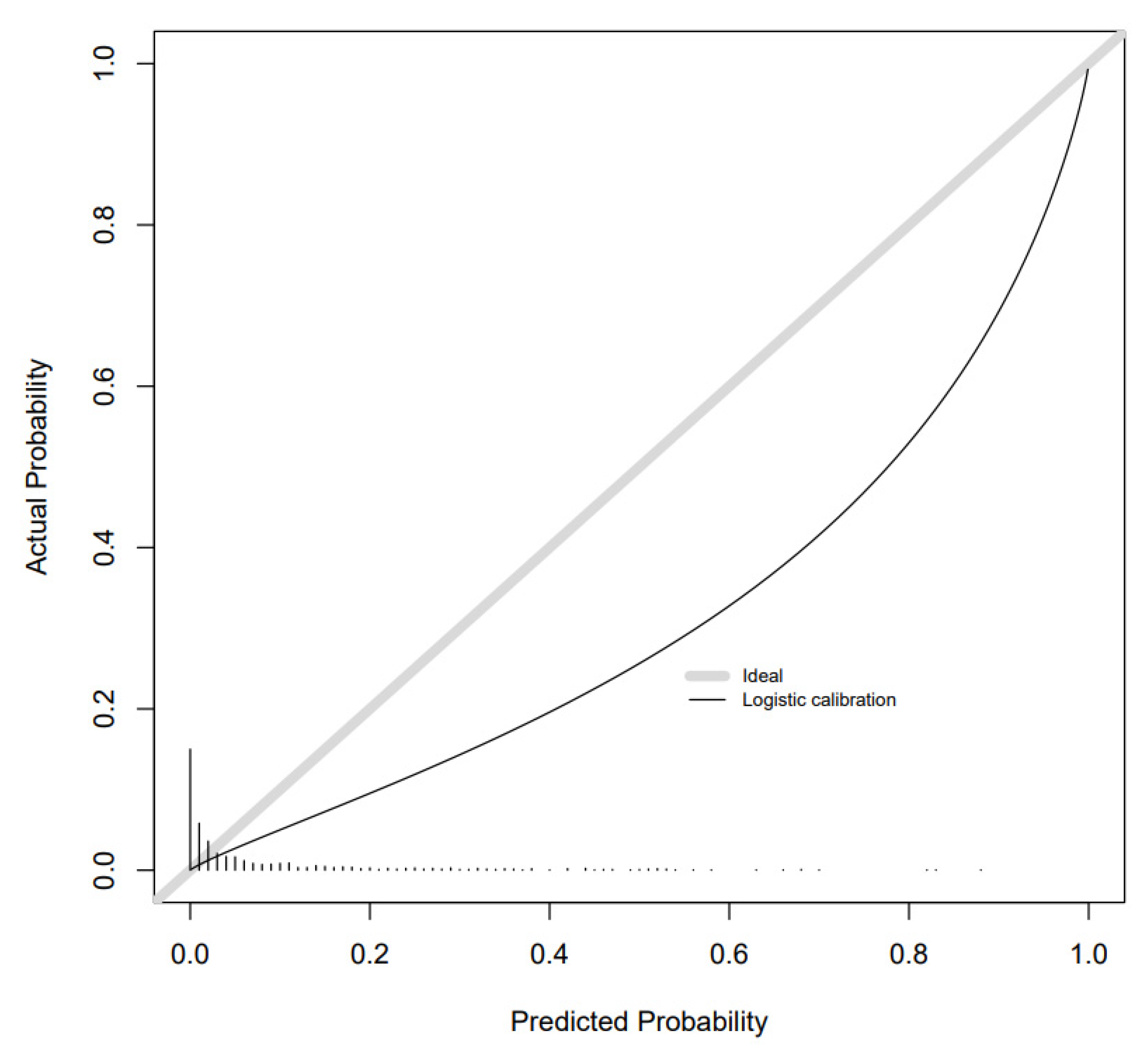
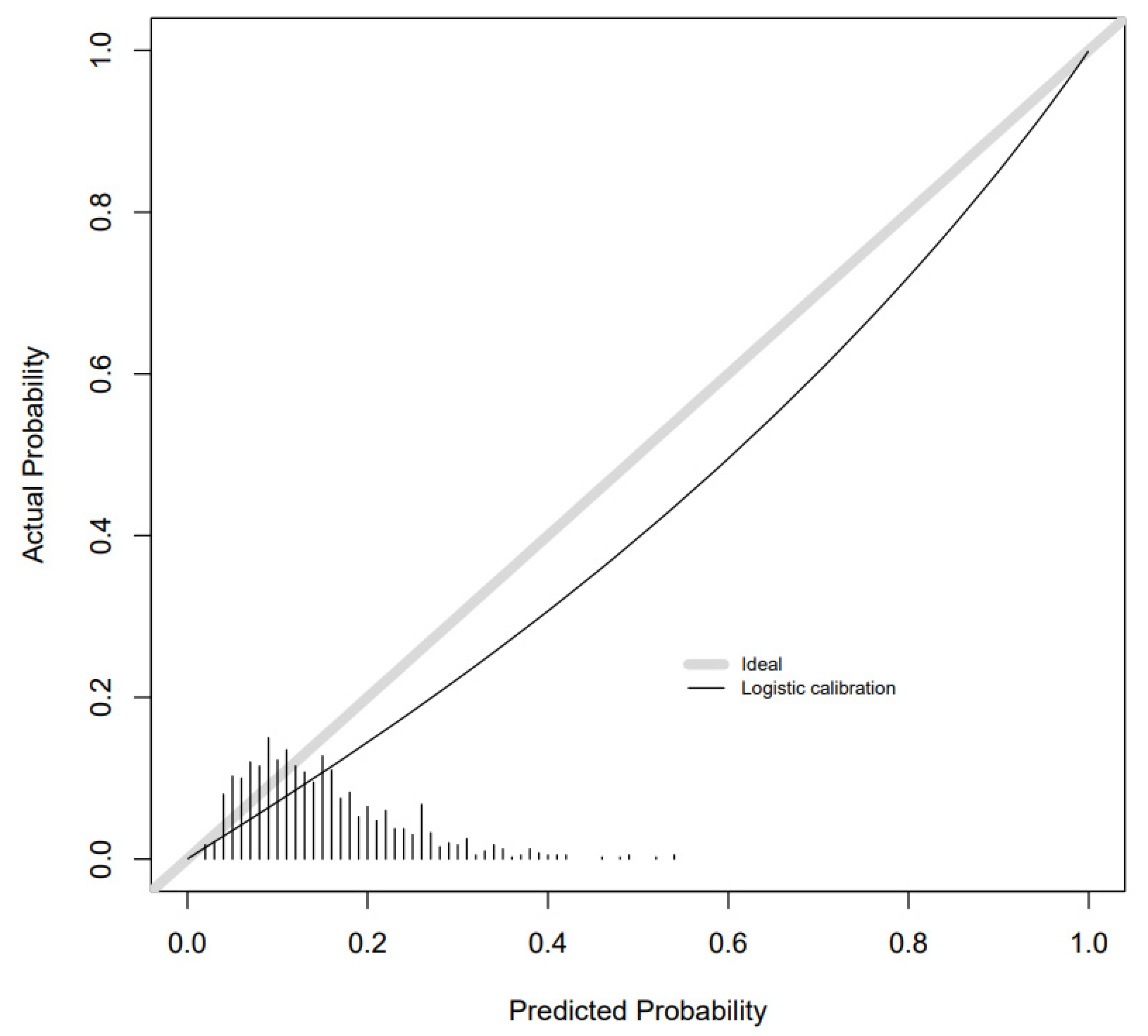
2. Results
Additional Analyses
3. Discussion
4. Materials and Methods
4.1. Participants
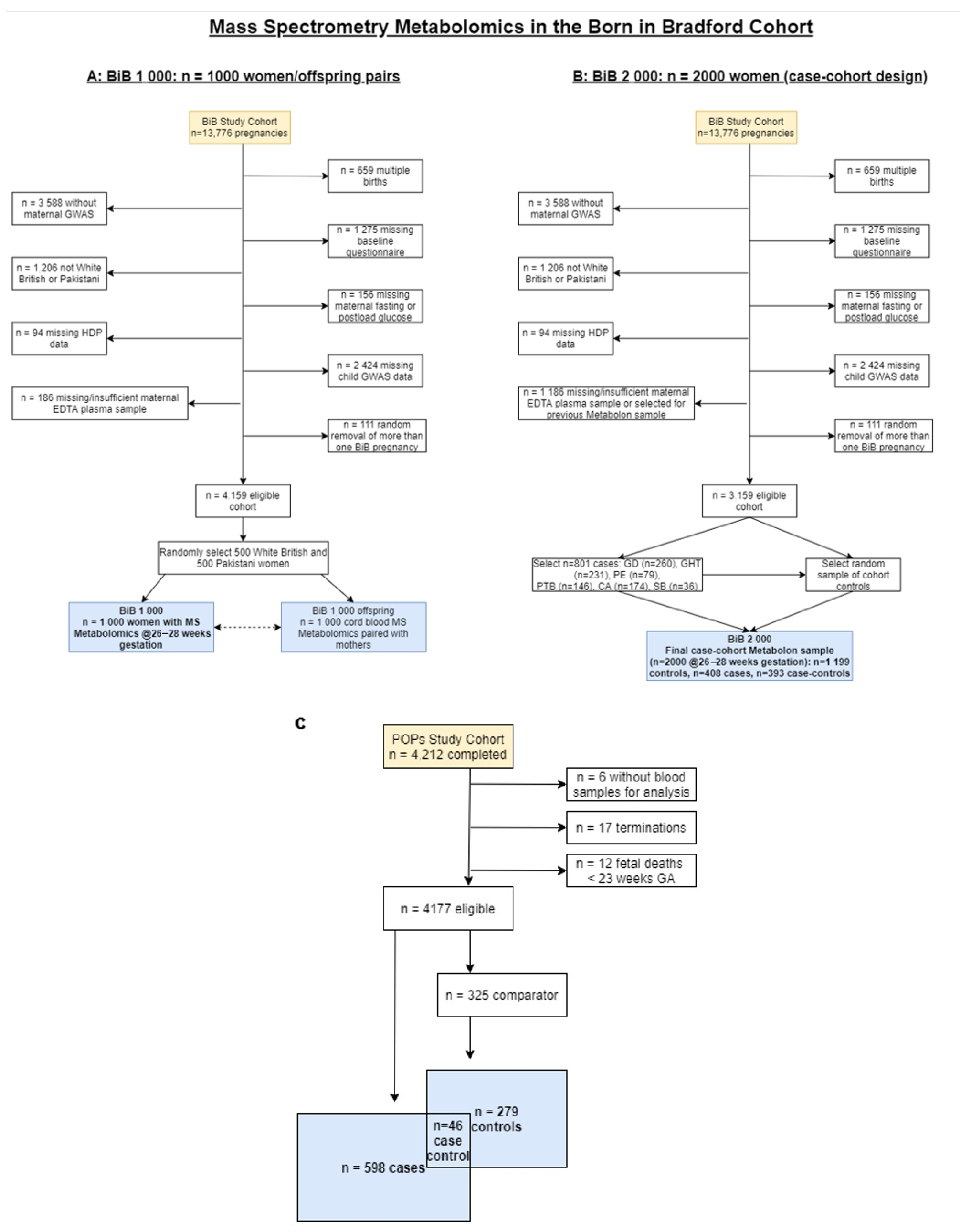
4.2. Metabolomic Predictors
4.3. Risk Factor Predictors
4.4. Outcomes
4.5. Statistical Analysis
4.6. Comparison Groups in the Case-Cohort BiB 2000 and POPs
4.7. Main Analyses
4.8. Additional Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald-Wallis, C.; Lawlor, D.A.; Fraser, A.; May, M.; Nelson, S.; Tilling, K. Blood Pressure Change in Normotensive, Gestational Hypertensive, Preeclamptic, and Essential Hypertensive Pregnancies. Hypertension 2012, 59, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Macdonald-Wallis, C.; Tilling, K.; Fraser, A.; Nelson, S.; Lawlor, D.A. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2013, 209, 327.e1–327.e17. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Silverwood, R.J.; de Stavola, B.L.; Inskip, H.; Cooper, C.; Godfrey, K.M.; Crozier, S.; Fraser, A.; Nelson, S.M.; Lawlor, D.A.; et al. Antenatal blood pressure for prediction of pre-eclampsia, preterm birth, and small for gestational age babies: Development and validation in two general popu-lation cohorts. BMJ 2015, 351, h5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, D.; Santorelli, G.; Lawlor, D.A.; Tuffnell, D.; Sheldon, T.A.; West, J.; Macdonald-Wallis, C. Blood pressure change across pregnancy in white British and Pakistani women: Analysis of data from the Born in Bradford cohort. Sci. Rep. 2019, 9, 13199. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D.; Simmonds, M.; Bryant, M.; Lawlor, D.A.; Dunne, F.; Tuffnell, D.; Sheldon, T.A. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: A systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE 2017, 6, e0175288. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Griffin, S.; Duarte, A.; Lawlor, D.A.; Sculpher, M.; Fairley, L.; Golder, S.; Tuffnell, D.; Bland, M.; et al. The identification and treatment of women with hyperglycaemia in pregnancy: An analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol. Assess. 2016, 20, 1–348. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Fraser, A.; Lawlor, D.A.; Catov, J.M. Pregnancy Characteristics and Women’s Future Cardiovascular Health: An Underused Opportunity to Improve Women’s Health? Epidemiol. Rev. 2014, 36, 57–70. [Google Scholar] [CrossRef]
- Barrett, P.M.; McCarthy, F.P.; Kublickiene, K.; Cormican, S.; Judge, C.; Evans, M.; Kublickas, M.; Perry, I.J.; Stenvinkel, P.; Khashan, A.S. Adverse Pregnancy Outcomes and Long-term Maternal Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020, 3, e1920964. [Google Scholar] [CrossRef] [Green Version]
- Neiger, R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major risk factors for stillbirth in high-income countries: A systematic review and meta-analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef]
- Fitzpatrick, K.E.; Tuffnell, D.; Kurinczuk, J.J.; Knight, M. Pregnancy at very advanced maternal age: A UK population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; Ali, N.; Papadopoulos, C.; Randhawa, G. Specific antenatal interventions for Black, Asian and Minority Ethnic (BAME) pregnant women at high risk of poor birth outcomes in the United Kingdom: A scoping review. BMC Pregnancy Childbirth 2015, 15, 226. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.L.; Edwards, S.E.; Myers, E.R. Adverse Birth Outcomes among Nulliparous vs. Multiparous Women. Public Health Rep. 2011, 126, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.; Valente, O. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.C.; Seed, P.T.; Myers, J.; Taylor, R.S.; Kenny, L.C.; Dekker, G.A.; Walker, J.J.; McCowan, L.M.E.; North, R.A.; Poston, L. Exploration and confirmation of factors associated with uncomplicated pregnancy in nulliparous women: Prospective cohort study. BMJ Clin. Res. 2013, 347, f6398. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.C.; White, S.L.; Patel, N.; Seed, P.T.; Briley, A.L.; Sandall, J.; Welsh, P.; Sattar, N.; Nelson, S.M. Prediction of uncomplicated pregnancies in obese women: A prospective multicentre study. BMC Med. 2017, 15, 194. [Google Scholar] [CrossRef] [Green Version]
- Danilack, V.A.; Nunes, A.; Phipps, M.G. Unexpected complications of low-risk pregnancies in the United States. Am. J. Obstet. Gynecol. 2015, 212, 809.e1–809.e6. [Google Scholar] [CrossRef] [Green Version]
- Gaccioli, F.; Lager, S.; Sovio, U.; Charnock-Jones, D.S.; Smith, G.C. The pregnancy outcome prediction (POP) study: Investigating the relationship between serial prenatal ultrasonography, biomarkers, placental phenotype and adverse pregnancy outcomes. Placenta 2017, 59, S17–S25. [Google Scholar] [CrossRef] [Green Version]
- Smith, G. Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia. PLoS Med. 2012, 9, e1001274. [Google Scholar] [CrossRef]
- Wang, Q.; Würtz, P.; Auro, K.; Mäkinen, V.-P.; Kangas, A.J.; Soininen, P. Metabolic profiling of pregnancy: Cross-sectional and lon-gitudinal evidence. BMC Med. 2016, 14, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Ferreira, D.L.; Williams, D.M.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Smith, G.D.; Jarvelin, M.-R.; Lawlor, D.A. Association of pre-pregnancy body mass index with offspring metabolic profile: Analyses of 3 European prospective birth cohorts. PLoS Med. 2017, 14, e1002376. [Google Scholar] [CrossRef] [Green Version]
- White, S.L.; on behalf of the UPBEAT Consortium; Pasupathy, D.; Sattar, N.; Nelson, S.; Lawlor, D.A.; Briley, A.L.; Seed, P.T.; Welsh, P.; Poston, L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetology 2017, 60, 1903–1912. [Google Scholar] [CrossRef] [Green Version]
- Grieger, J.A.; Bianco-Miotto, T.; Grzeskowiak, L.E.; Leemaqz, S.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.; Walker, J.J.; Dekker, G.A.; et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med. 2018, 15, e1002710. [Google Scholar] [CrossRef] [PubMed]
- McBride, N.; Yousefi, P.; White, S.L.; Poston, L.; Farrar, D.; Sattar, N.; Nelson, S.M.; Wright, J.; Mason, D.; Suderman, M.; et al. Do nuclear magnetic resonance (NMR)-based metabo-lomics improve the prediction of pregnancy-related disorders? Findings from a UK birth cohort with independent validation. BMC Med. 2020, 18, 366. [Google Scholar] [CrossRef]
- Sovio, U.; McBride, N.; Wood, A.M.; Masconi, K.L.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G.C.S. 4-Hydroxyglutamate is a novel predictor of pre-eclampsia. Int. J. Epidemiol. 2020, 49, 301–311. [Google Scholar] [CrossRef]
- Sovio, U.; Goulding, N.; McBride, N.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G. A maternal serum metabolite ratio predicts fetal growth restriction at term. Nat. Med. 2020, 26, 348–353. [Google Scholar] [CrossRef]
- Bahado-Singh, R.; Poon, L.C.; Yilmaz, A.; Syngelaki, A.; Turkoglu, O.; Kumar, P.; Kirma, J.; Allos, M.; Accurti, V.; Li, J.; et al. Integrated Proteomic and Metabolomic pre-diction of Term Preeclampsia. Sci. Rep. 2017, 7, 16189. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R.P.; Broadhurst, D.I.; Walsh, S.K.; Dunn, W.B.; Brown, M.; Roberts, C.T.; North, R.A.; McCowan, L.M.; Kell, D.B.; Baker, P.N.; et al. Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy. J. Proteome Res. 2011, 10, 3660–3673. [Google Scholar] [CrossRef]
- Considine, E.C.; Khashan, A.S.; Kenny, L.C. Screening for Preterm Birth: Potential for a Metabolomics Biomarker Panel. Metabolites 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.; Small, N.; Raynor, P.; Tuffnell, D.; Bhopal, R.; Cameron, N.; Fairley, L.; Lawlor, D.A.; Parslow, R.; Petherick, E.; et al. Cohort Profile: The Born in Bradford multi-ethnic family cohort study. Int. J. Epidemiol. 2013, 42, 978–991. [Google Scholar] [CrossRef] [Green Version]
- KaM, T.; Goulding, N.J.; Burrows, N.; Mason, K.; Pembrey, D.; Yang, L.; Azad, T.; Wright, R.; Lawlor, J.A. Metabolomics datasets in the Born in Bradford cohort. Wellcome Open Res. 2020, 5, 264. [Google Scholar]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Sovio, U.; Aye, I.L.; Gaccioli, F.; Dopierala, J.; Johnson, M.D.; Wood, A.M.; Cook, E.; Jenkins, B.J.; Koulman, A.; et al. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight 2018, 3, e120723. [Google Scholar] [CrossRef] [Green Version]
- Gaccioli, F.; Sovio, U.; Cook, E.; Hund, M.; Charnock-Jones, D.S.; Smith, G.C.S. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: A prospective cohort study. Lancet Child Adolesc. Health 2018, 2, 569–581. [Google Scholar] [CrossRef]
- Sovio, U.; White, I.; Dacey, A.; Pasupathy, D.; Smith, G. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: A prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, D.; Dacey, A.; Cook, E.; Charnock-Jones, D.S.; White, I.R.; Smith, G.C. Study protocol. A prospective cohort study of unselected primiparous women: The pregnancy outcome prediction study. BMC Pregnancy Childbirth 2008, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.M.; August, P.A.; Bakris, G.; Barton, J.R.; Bernstein, I.M.; Druzin, M.; Gaiser, R.R.; Granger, J.P.; Jeyabalan, A.; Johnson, D.D. Hypertension in Pregnancy: Executive Summary. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Sovio, U.; Murphy, H.R.; Smith, G. Accelerated Fetal Growth Prior to Diagnosis of Gestational Diabetes Mellitus: A Prospective Cohort Study of Nulliparous Women. Diabetes Care 2016, 39, 982–987. [Google Scholar] [CrossRef] [Green Version]
- Milner, J.; Arezina, J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: A systematic review. Ultrasound 2018, 26, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Hadlock, F.P.; Harrist, R.F.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Altman, D.G.; Cadarette, S.; Collins, G.; Evans, S.; Sekula, P.; Williamson, E.; Woodward, M. Design choices for observational studies of the effect of exposure on disease incidence. BMJ Open 2019, 9, e031031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Soft. 2008, 28, 26. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Born in Bradford 2000 | Born in Bradford 1000 n = 915 | Pregnancy Outcome Prediction Study n = 827 |
|---|---|---|---|
| Gestational diabetes (case/comparator) or n (%) | 245/1350 | 84 (9.2) | 172/295 |
| Gestational hypertension (case/comparator) or n (%) | 217/1375 | 64 (7.0) | 6/300 |
| Pre-eclampsia (case/comparator) or n (%) | 74/1494 | 24 (2.6) | 175/286 |
| Large for gestational age (case/comparator) or n (%) | 76/1425 | 37 (4.0) | 12/294 |
| Small for gestational age (case/comparator) or n (%) | 260/1275 | 102 (11.1) | 188/279 |
| Spontaneous preterm birth (case/comparator) or n (%) | 87/1441 | 21 (2.3) | 98/297 |
| BMI kg/m2 (mean (SD)) | 26.8 (5.8) | 26.7 (6.0) | 26.0 (5.3) |
| Age (mean years) | 27.5 (5.6) | 27.4 (5.7) | 30.3 (5.3) |
| Pregnancy smoking, n (%) | 378 (18.9) | 159 (17.4) | 119 (14.4) |
| Multiparous, n (%) | 1213 (60.7) | 581 (63.5) | 0 (0) |
| White ethnicity, n (%) | 933 (46.7) | 456 (49.8) | 787 (95.2) |
| Outcome | Model (Retained Predictors/Total Number of Predictors Possible (%)) |
|---|---|
| Gestational diabetes | Risk factor (4/5 (80%)) |
| Metabolite (81/718 (11%)) | |
| Combined (82/723 (11%)) | |
| Gestational hypertension | Risk factor (4/5 (80%)) |
| Metabolite (28/718 (4%)) | |
| Combined (75/723 (10%)) | |
| Pre-eclampsia | Risk factor (4/5 (80%)) |
| Metabolite (154/718 (21%)) | |
| Combined (28/723 (4%)) | |
| Small for gestational age | Risk factor (5/5 (100%)) |
| Metabolite (66/718 (9%)) | |
| Combined (65/723 (8%)) | |
| Large for gestational age | Risk factor (5/5 (100%)) |
| Metabolite (490/718 (68%)) | |
| Combined (360/723 (50%)) | |
| Spontaneous preterm birth | Risk factor (4/5 (80%)) |
| Metabolite (587/718 (83%)) | |
| Combined (328/723 (45%)) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McBride, N.; Yousefi, P.; Sovio, U.; Taylor, K.; Vafai, Y.; Yang, T.; Hou, B.; Suderman, M.; Relton, C.; Smith, G.C.S.; et al. Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. Metabolites 2021, 11, 530. https://doi.org/10.3390/metabo11080530
McBride N, Yousefi P, Sovio U, Taylor K, Vafai Y, Yang T, Hou B, Suderman M, Relton C, Smith GCS, et al. Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. Metabolites. 2021; 11(8):530. https://doi.org/10.3390/metabo11080530
Chicago/Turabian StyleMcBride, Nancy, Paul Yousefi, Ulla Sovio, Kurt Taylor, Yassaman Vafai, Tiffany Yang, Bo Hou, Matthew Suderman, Caroline Relton, Gordon C. S. Smith, and et al. 2021. "Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation" Metabolites 11, no. 8: 530. https://doi.org/10.3390/metabo11080530
APA StyleMcBride, N., Yousefi, P., Sovio, U., Taylor, K., Vafai, Y., Yang, T., Hou, B., Suderman, M., Relton, C., Smith, G. C. S., & Lawlor, D. A. (2021). Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. Metabolites, 11(8), 530. https://doi.org/10.3390/metabo11080530






