Metabolomic Recovery as a Result of Ischemic Preconditioning Was More Pronounced in Hippocampus than in Cortex That Appeared More Sensitive to Metabolomic Blood Components
Abstract
:1. Introduction
- (i)
- cerebral cortex
- (ii)
- hippocampus,
- (iii)
- blood plasma, as well as,
- (iv)
- heart as brain’s remote organ, due to its close cerebro-cardial connections.
2. Results
- In cortex: succinate, alanine, aspartate, myo-inositol, creatine, taurine, ascorbate.
- In hippocampus: isoleucine, valine, alanine, glutamine, phenylalanine, fumarate.
- In heart: isoleucine, valine, alanine, glutamine, succinate, creatine, taurine, phenylalanine, tyrosine, fumarate, inosine.
- In blood plasma: tyrosine.
3. Discussion
3.1. Cerebral Cortex and Hippocampus
3.1.1. Neurotransmitters and Related Metabolites
3.1.2. Acorbate as Antioxidant
3.1.3. Metabolites Known from In Vivo MRS
3.2. Protective Effect of Ischemic Preconditioning in Brain Tissue Extracts
3.3. Heart and Blood Plasma
3.3.1. Glutamate in Heart Tissue
3.3.2. Metabolites Participating in Energy Metabolism
3.4. Brain and Plasma Metabolites—Correlation
3.5. PCA and Groups’ Proximity
3.6. Random Forest towards Biomarkers
3.7. Methodological Note to Four Vessels Occlusion
4. Materials and Methods
4.1. Induction of Ischemic Preconditioning and Ischemia
4.2. Organ Collection and Blood Plasma Samples Preparation
4.3. NMR Data Acquisition
4.4. Data Acquisition, Processing and Evaluation
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehotský, J.; Burda, J.; Danielisová, V.; Gottlieb, M.; Kaplán, P.; Saniová, B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anat. Rec. 2009, 292, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T. Ischemic Tolerance. Br. J. Pharmacol. 2002, 22, 1283–1296. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Xin, M.; Feng, L.; Wang, X.; Wang, X.; Ma, D.; Feng, J. Review Cerebral Ischemic Tolerance and Preconditioning: Methods, Mechanisms, Clinical Applications, and Challenges. Front. Neurol. 2020, 11, 812. [Google Scholar] [CrossRef]
- Liang, J.; Han, R.; Zhou, B. Metabolic Reprogramming: Strategy for Ischemic Stroke Treatment by Ischemic Preconditioning. Biology 2021, 10, 424. [Google Scholar] [CrossRef]
- Pulsinelli, W.; Buchan, A. The four-vessel occlusion rat model: Method for complete occlusion of vertebral arteries and control of collateral circulation. Stroke 1988, 19, 913–914. [Google Scholar] [CrossRef] [Green Version]
- McBean, D.E.; Kelly, P.A. Rodent models of global cerebral ischemia: A comparison of two-vessel occlusion and four-vessel occlusion. Gen. Pharmacol. 1998, 30, 431–434. [Google Scholar] [CrossRef]
- Wahul, A.; Joshi, P.C.; Kumar, A.; Chakravarty, S. Transient global cerebral ischemia differentially affects cortex, striatum and hippocampus in Bilateral Common Carotid Arterial occlusion (BCCAo) mouse model. J. Chem. Neuroanat. 2018, 92, 1–15. [Google Scholar] [CrossRef]
- Klacanova, K.; Kovalska, M.; Chomova, M.; Pilchova, I.; Tatarkova, Z.; Kaplan, P.; Racay, P. Global brain ischemia in rats is associated with mitochondrial release and downregulation of Mfn2 in the cerebral cortex, but not the hippocampus. Int. J. Mol. Med. 2019, 43, 2420–2428. [Google Scholar] [CrossRef] [Green Version]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules 2020, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Kondo, F.; Kondo, Y.; Makino, H.; Ogawa, N. Delayed neuronal death in hippocampal CA1 pyramidal neurons after forebrain ischemia in hyperglycemic gerbils: Amelioration by indomethacin. Brain Res. 2000, 853, 93–98. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by -Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Kauppinen, A.; Hiltunen, M.; Kaarniranta, K. Krebs cycle intermediates regulate DNA and histone methylation: Epigenetic impact on the aging process. Ageing Res. Rev. 2014, 16, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Dai, Z.; Xuan, Y.; Wu, R. Early metabolic changes following ischemia onset in rats: An in vivo diffusion-weighted imaging and 1H-magnetic resonance spectroscopy study at 7.0 T. Mol. Med. Rep. 2015, 11, 4109–4114. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Honmou, O.; Liu, H.; Bando, M.; Houkin, K.; Kocsis, J.D. Magnetic resonance lactate and lipid signals in rat brain after middle cerebral artery occlusion model. Brain Res. 2007, 1134, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Xarrié, E.; Davila, M.; Candiota, A.P.; Delgado-Mederos, R.; Ortega-Martorell, S.; Julià-Sapé, M.; Arús, C.; Martí-Fàbregas, J. Brain metabolic pattern analysis using a magnetic resonance spectra classification software in experimental stroke. BMC Neurosci. 2017, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, W.; Huang, J.; Liu, X.; Zhang, H.; Zhang, N. Metabolomic investigation of regional brain tissue dysfunctions induced by global cerebral ischemia. BMC Neurosci. 2016, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Xia, H.; Yang, Y.; Zheng, H.; Zhao, L.; Chen, Y.; Zhuge, Q.; Xia, N.; Gao, H.; Chen, W. Metabolic alterations in the rat cerebellum following acute middle cerebral artery occlusion, as determined by 1H NMR spectroscopy. Mol. Med. Rep. 2017, 17, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, K.; Berressem, D.; Konietzka, J.; Thinnes, A.; Eckert, G.P.; Klein, J. Hepatic Ketogenesis Induced by Middle Cerebral Artery Occlusion in Mice. J. Am. Heart Assoc. 2017, 6, e005556. [Google Scholar] [CrossRef] [PubMed]
- Baranovicova, E.; Kalenska, D.; Tomascova, A.; Lehotsky, J. Metabolomic study of altered energy metabolism during global forebrain ischemia and ischemic precoditioning in blood plasma in homocysteine treated rats. J. Physiol. Pharmacol. 2019, 69, 901–909. [Google Scholar] [CrossRef]
- Baranovičová, E.; Grendár, M.; Kalenská, D.; Tomascova, A.; Čierny, D.; Lehotský, J. NMR metabolomic study of blood plasma in ischemic and ischemically preconditioned rats: An increased level of ketone bodies and decreased content of glycolytic products 24 h after global cerebral ischemia. J. Physiol. Biochem. 2018, 74, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Baranovicova, E.; Kalenska, D.; Tomascova, A.; Holubcikova, S.; Lehotsky, J. Time-related metabolomics study in the rat plasma after global cerebral ischemia and reperfusion: Effect of ischemic preconditioning. IUBMB Life 2020, 72, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.; Xu, Y.; Correa, E.; Turner, M.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2012, 9, 280–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipton, P. Ischemic Cell Death in Brain Neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef] [PubMed]
- Kirdajova, D.B.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001, 69, 369–381. [Google Scholar] [CrossRef]
- Jachova, J.; Gottlieb, M.; Nemethova, M.; Bona, M.; Bonova, P. Brain to blood efflux as a mechanism underlying the neuroprotection mediated by rapid remote preconditioning in brain ischemia. Mol. Biol. Rep. 2020, 47, 5385–5395. [Google Scholar] [CrossRef]
- Huang, Q.; Li, C.; Xia, N.; Zhao, L.; Wang, D.; Yang, Y.; Gao, H. Neurochemical changes in unilateral cerebral hemisphere during the subacute stage of focal cerebral ischemia-reperfusion in rats: An ex vivo 1H magnetic resonance spectroscopy study. Brain Res. 2018, 1684, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.-Z.; Ruan, L.; Wang, Y.; Chen, S.-C.; Zhao, T.; Huang, Q.; Shu-Chao, C.; Xia, N.-Z.; Liu, J.-J.; et al. Metabolite changes in the ipsilateral and contralateral cerebral hemispheres in rats with middle cerebral artery occlusion. Neural Regen. Res. 2017, 12, 931–937. [Google Scholar] [CrossRef]
- Figlewski, K.; Andersen, H.; Stærmose, T.; Von Weitzel-Mudersbach, P.; Nielsen, J.F.; Blicher, J.U. Decreased GABA levels in the symptomatic hemisphere in patients with transient ischemic attack. Heliyon 2018, 4, e00790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz, L. The Glutamate–Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. 2013, 4, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCombe, P.A.; Read, S. Immune and Inflammatory Responses to Stroke: Good or Bad? Int. J. Stroke 2008, 3, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Herring, B.E.; Silm, K.; Edwards, R.H.; Nicoll, R.A. Is Aspartate an Excitatory Neurotransmitter? J. Neurosci. 2015, 35, 10168–10171. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Kašparová, S.; Brezová, V.; Valko, M.; Horecký, J.; Mlynarik, V.; Liptaj, T.; Vančová, O.; Uličná, O.; Dobrota, D. Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem. Int. 2005, 46, 601–611. [Google Scholar] [CrossRef]
- Duarte, J.M.; Lei, H.; Mlynárik, V.; Gruetter, R. The neurochemical profile quantified by in vivo1H NMR spectroscopy. NeuroImage 2012, 61, 342–362. [Google Scholar] [CrossRef] [Green Version]
- Brulatout, S.; Méric, P.; Loubinoux, I.; Borredon, J.; Corrèze, J.L.; Roucher, P.; Gillet, B.; Bérenger, G.; Beloeil, J.C.; Tiffon, B.; et al. A One-Dimensional (Proton and Phosphorus) and Two-Dimensional (Proton) In Vivo NMR Spectroscopic Study of Reversible Global Cerebral Ischemia. J. Neurochem. 2002, 66, 2491–2499. [Google Scholar] [CrossRef]
- Demougeot, C.; Bertrand, N.; Prigent-Tessier, A.; Garnier, P.; Mossiat, C.; Giroud, M.; Marie, C.; Beley, A. Reversible Loss of N-Acetyl-Aspartate in Rats Subjected to Long-Term Focal Cerebral Ischemia. Br. J. Pharmacol. 2003, 23, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Brands, S.; Schein, P.; Castro-Ochoa, K.F.; Galinski, E.A. Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch. Biochem. Biophys. 2019, 674, 108097. [Google Scholar] [CrossRef] [PubMed]
- Villalba, H.; Shah, K.; Albekairi, T.H.; Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Potential role of myo-inositol to improve ischemic stroke outcome in diabetic mouse. Brain Res. 2018, 1699, 166–176. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.-H.; Wang, H.; Long, C.-L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ei-Batah, P.N.; Yamashita, L.F.; Santana, A.D.S.; Lopes, A.C.; Freymüller-Haapalainen, E.; Coimbra, C.G.; Sinigaglia-Coimbra, R. Neuroprotective effect of oral choline administration after global brain ischemia in rats. Nutr. Neurosci. 2015, 18, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Samary, C.S.; Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Ischaemic stroke-induced distal organ damage: Pathophysiology and new therapeutic strategies. Intensive Care Med. Exp. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Pulido, O.M.; Mueller, R.W.; McGuire, P.F. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res. Bull. 1998, 46, 429–434. [Google Scholar] [CrossRef]
- Gill, S.S.; Pulido, O.M.; Mueller, R.W.; McGuire, P.F. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res. Bull. 1999, 48, 143–146. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, 124079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Venkatesh, B. Clinical review: Ketones and brain injury. Crit. Care 2011, 15, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Suzuki, M.; Sato, K.; Dohi, S.; Sato, T.; Matsuura, A.; Hiraide, A. Effect of β-Hydroxybutyrate, a Cerebral Function Improving Agent, on Cerebral Hypoxia, Anoxia and Ischemia in Mice and Rats. Jpn. J. Pharmacol. 2001, 87, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Kassner, A.; Merali, Z. Assessment of Blood–Brain Barrier Disruption in Stroke. Stroke 2015, 46, 3310–3315. [Google Scholar] [CrossRef]
- Pulsinelli, W.A.; Levy, D.E.; Duffy, T.E. Cerebral blood flow in the four-vessel occlusion rat model. Stroke 1983, 14, 832–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, G.A.N.; Gowda, Y.N.; Raftery, D. Expanding the Limits of Human Blood Metabolite Quantitation Using NMR Spectroscopy. Anal. Chem. 2015, 87, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Allison, P.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]

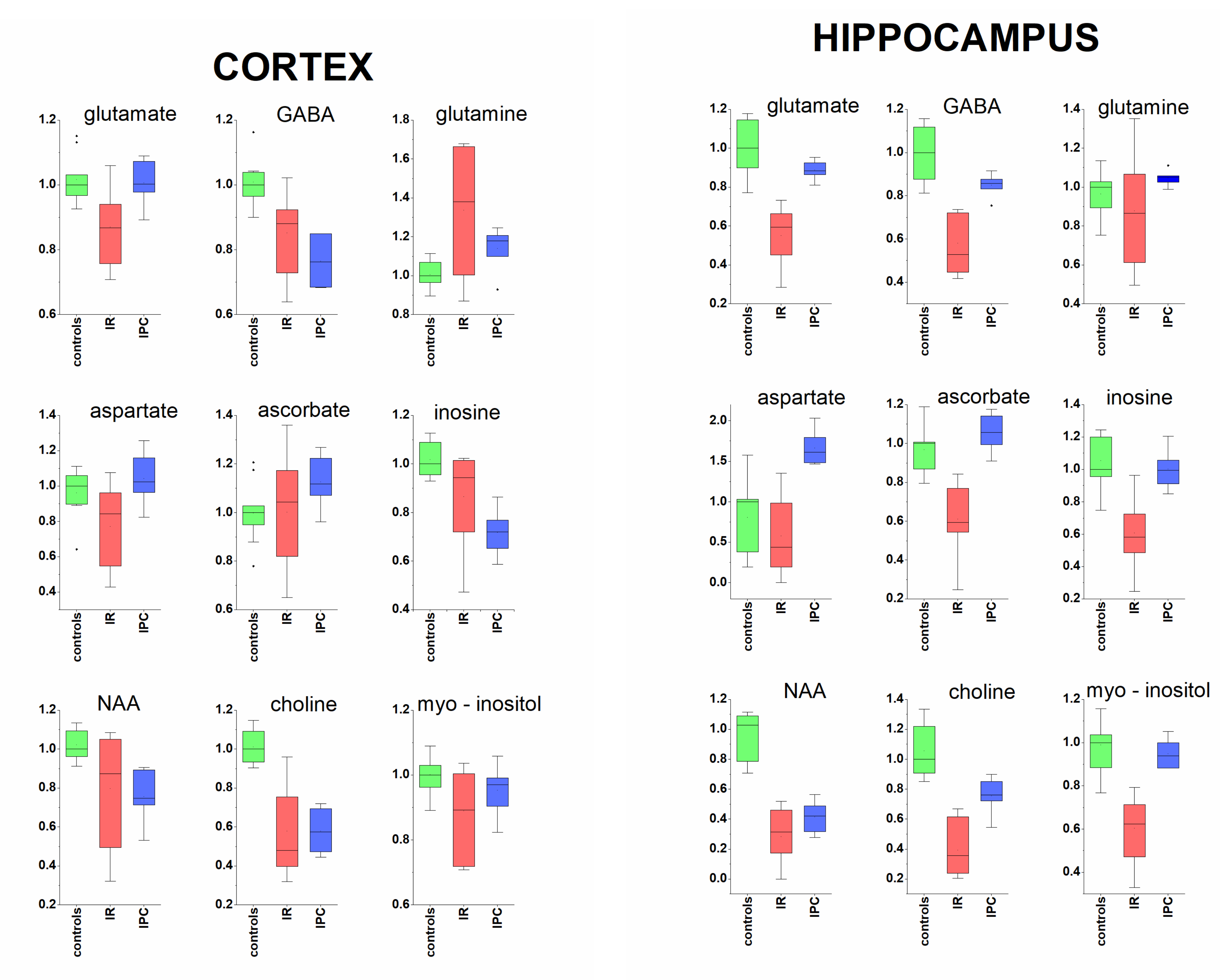

| Cortex | Kruskal-Wallis | IR/C | IR/IPC | IPC/C | |||
|---|---|---|---|---|---|---|---|
| p Value | p Value | FC | p Value | FC | p Value | FC | |
| isoleucine | 0.042 | 0.003 | 0.53 | 0.027 | −0.31 | 0.423 | 0.07 |
| valine | 0.042 | 0.003 | 0.61 | 0.027 | −0.35 | 0.423 | 0.04 |
| glutamine | 0.009 | 0.009 | 0.38 | 0.223 | −0.15 | 0.126 | 0.17 |
| glutamate | 0.073 | 0.015 | −0.16 | 0.017 | 0.19 | 0.943 | 0.00 |
| 4-aminobutyrate | <0.001 | 0.015 | −0.14 | 0.503 | −0.11 | 0.001 | −0.24 |
| niacineamide | 0.009 | 0.014 | −0.15 | 0.635 | 0.02 | 0.022 | −0.13 |
| phenylalanine | 0.005 | 0.013 | 0.64 | 0.005 | −0.41 | 0.402 | −0.04 |
| tyrosine | 0.016 | 0.762 | 0.03 | 0.007 | −0.22 | 0.007 | −0.20 |
| fumarate | 0.042 | 0.003 | 0.40 | 0.039 | −0.27 | 0.345 | 0.03 |
| inosine | 0.003 | 0.084 | −0.06 | 0.289 | −0.24 | 0.004 | −0.28 |
| ascorbate | 0.061 | 0.850 | 0.03 | 0.299 | 0.07 | 0.282 | 0.10 |
| NAA | 0.003 | 0.004 | −0.16 | 0.963 | −0.12 | 0.004 | −0.25 |
| choline | <0.001 | 0.001 | −0.56 | 0.413 | 0.31 | 0.008 | −0.42 |
| Hippocampus | |||||||
| glutamate | 0.002 | 0.000 | −0.41 | 0.032 | 0.49 | 0.194 | −0.12 |
| 4-aminobutyrate | 0.002 | 0.000 | −0.47 | 0.045 | 0.62 | 0.123 | −0.14 |
| succinate | 0.005 | 0.004 | −0.33 | 0.012 | 0.37 | 0.783 | −0.08 |
| aspartate | 0.016 | 0.793 | −0.49 | 0.005 | 2.16 | 0.005 | 0.61 |
| myo-inositol | 0.005 | 0.002 | −0.38 | 0.008 | 0.51 | 0.709 | −0.06 |
| creatine | 0.005 | 0.004 | −0.29 | 0.007 | 0.44 | 0.948 | 0.02 |
| taurine | 0.005 | 0.003 | −0.25 | 0.029 | 0.31 | 0.465 | −0.02 |
| tyrosine | 0.042 | 0.009 | −0.32 | 0.208 | 0.28 | 0.197 | −0.13 |
| inosine | 0.042 | 0.004 | −0.42 | 0.028 | 0.71 | 0.537 | −0.01 |
| ascorbate | 0.001 | 0.005 | −0.41 | 0.001 | 0.78 | 0.355 | 0.06 |
| NAA | 0.009 | 0.008 | −0.68 | 0.612 | 0.30 | 0.030 | −0.58 |
| choline | p < 0.0001 | 0.000 | −0.64 | 0.085 | 1.14 | 0.050 | −0.24 |
| Heart | |||||||
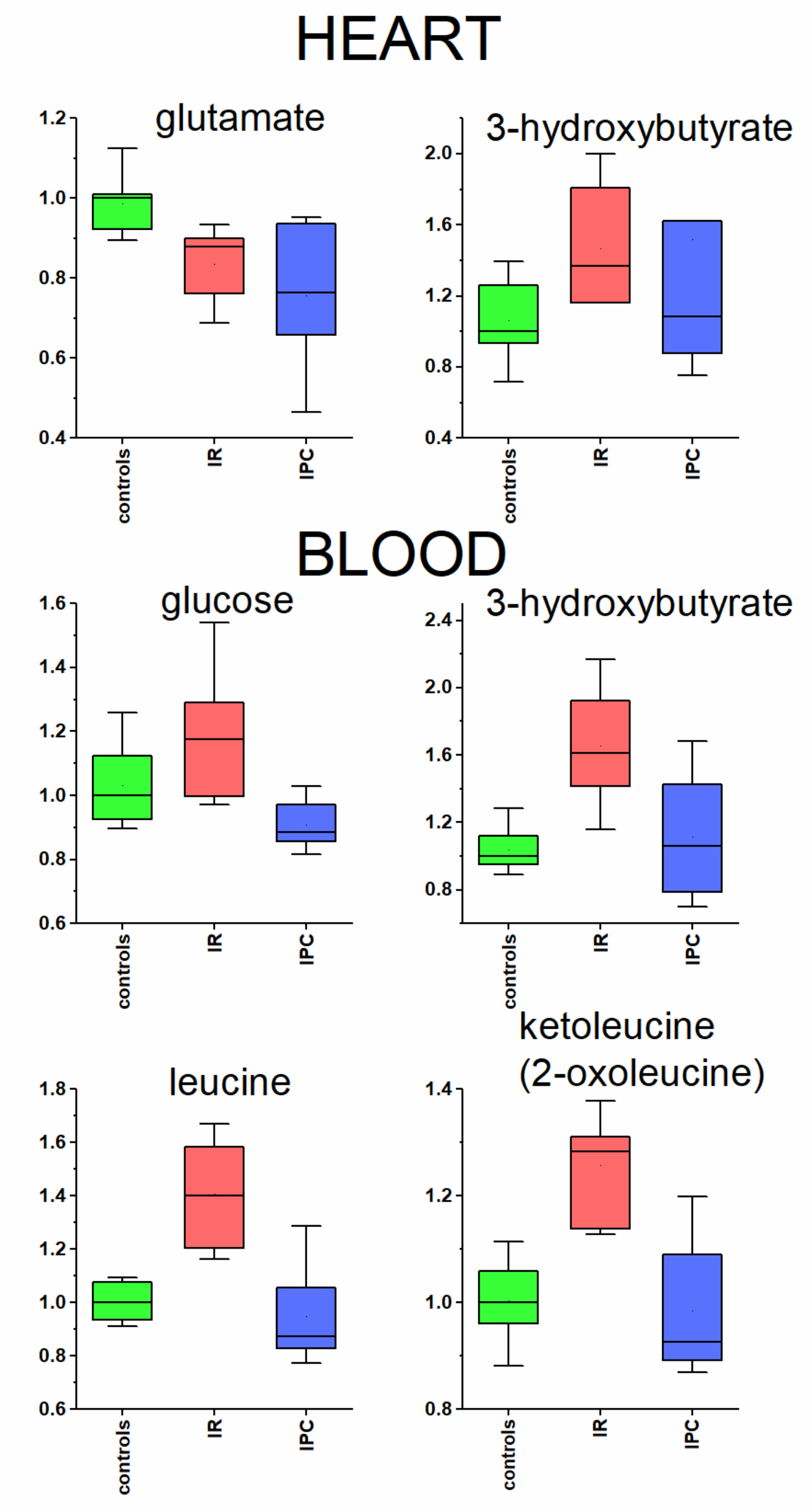
| glutamate | 0.2 | 0.008 | −0.11 | 0.830 | −0.13 | 0.008 | −0.23 |
| 3-hydroxybutyrate | 0.055 | 0.048 | 0.34 | 0.253 | −0.20 | 0.413 | 0.07 |
| Blood Plasma | |||||||
| lactate | 0.009 | 0.004 | −0.26 | 0.766 | 0.01 | 0.011 | −0.25 |
| alanine | 0.001 | 0.004 | −0.34 | 0.341 | −0.10 | 0.000 | −0.40 |
| valine | 0.007 | 0.016 | 0.28 | 0.004 | −0.33 | 0.552 | −0.15 |
| glucose | 0.017 | 0.123 | 0.19 | 0.001 | −0.27 | 0.064 | −0.13 |
| leucine | 0.007 | 0.008 | 0.41 | 0.004 | −0.38 | 0.746 | −0.13 |
| isoleucine | 0.006 | 0.006 | 0.40 | 0.003 | −0.39 | 0.771 | −0.14 |
| acetate | 0.001 | 0.001 | −0.32 | 0.861 | 0.08 | 0.002 | −0.27 |
| acetoacetate | 0.001 | 0.000 | 2.27 | 0.421 | −0.23 | 0.003 | 1.53 |
| pyruvate | 0.001 | 0.000 | −0.44 | 0.661 | 0.07 | 0.002 | −0.40 |
| citrate | 0.013 | 0.005 | −0.30 | 0.811 | −0.09 | 0.004 | −0.37 |
| phenylalanine | 0.042 | 0.004 | 0.31 | 0.020 | −0.21 | 0.636 | 0.04 |
| glutamine | 0.013 | 0.084 | −0.14 | 0.369 | −0.03 | 0.018 | −0.16 |
| lysine | 0.686 | 0.929 | −0.03 | 0.329 | −0.08 | 0.494 | −0.10 |
| 3-hydroxybutyrate | 0.002 | 0.003 | 0.66 | 0.007 | −0.34 | 0.849 | 0.09 |
| ketoleucine | 0.006 | 0.003 | 0.30 | 0.001 | −0.28 | 0.636 | −0.07 |
| ketoisoleucine | 0.007 | 0.008 | 0.21 | 0.005 | −0.22 | 0.827 | −0.05 |
| ketovaline | 0.007 | 0.009 | 0.22 | 0.004 | −0.26 | 0.695 | −0.10 |
| tryptophan | 0.013 | 0.027 | −0.11 | 0.464 | −0.03 | 0.006 | −0.14 |
| Cortex IR/Controls | Hippocampus IR/Controls | Blood IR/Controls | Cortex/Blood R Score/p Value/ p Value Adj (IR Rats) | Hippocampus/Blood R Score/p Value/ p Value Adj (IR Rats) | |
|---|---|---|---|---|---|
| isoleucine | ↑ * | ↑ | ↑ * | 0.7654/0.045/0.089 | 0.073/0.87/1 |
| valine | ↑ * | ↑ | ↑ * | 0.9066/0.012/0.048 | 0.056/0.90/0.9 |
| phenylalanine | ↑ * | ↑ | ↑ * | 0.7147/0.071/0.071 | 0.257/0.57/1 |
| tyrosine | no change | ↓ * | no change | 0.7379/0.058/0.077 | −0.246/0.59/1 |
| Sample | Groups | Metabolites with Individual AUC Values |
|---|---|---|
| cortex | IR/controls | fumarate 1, choline 0.998, phenylalanine 0.997, valine 0.995, isoleucine 0.925, glutamine 0.923, alanine 0.842, GABA 0.809 |
| cortex | IPC/controls | GABA 1, inosine 1, choline 1, tyrosine 0.996, NAA 0.990, ascorbate 0.943, glutamine 0.804 |
| cortex | IPC/IR | phenylalanine 1, valine 0.995, isoleucine 0.958, fumarate 0.939, tyrosine 0.924, alanine 0.906, ascorbate 0.901, glutamine 0.829 |
| hippocampus | IR/controls | glutamate 1, GABA 1, choline 1, myo-inositol 0.978, ascorbate 0.963, inosine 0.919, NAA 0.918, creatine 0.894, succinate 0.878 |
| hippocampus | IPC/controls | aspartate 0.936, choline 0.912, NAA 0.931 |
| hippocampus | IPC/IR | glutamate 1, myo-inositol 1, ascorbate 1, succinate 0.999, aspartate 0.991, GABA 0.979, creatine 0.997, choline 0.941, inosine 0.913 |
| heart | IR/controls | glutamate 0.898 |
| heart | IPC/controls | glutamate 0.882 |
| heart | IPC/IR | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranovicova, E.; Kalenska, D.; Grendar, M.; Lehotsky, J. Metabolomic Recovery as a Result of Ischemic Preconditioning Was More Pronounced in Hippocampus than in Cortex That Appeared More Sensitive to Metabolomic Blood Components. Metabolites 2021, 11, 516. https://doi.org/10.3390/metabo11080516
Baranovicova E, Kalenska D, Grendar M, Lehotsky J. Metabolomic Recovery as a Result of Ischemic Preconditioning Was More Pronounced in Hippocampus than in Cortex That Appeared More Sensitive to Metabolomic Blood Components. Metabolites. 2021; 11(8):516. https://doi.org/10.3390/metabo11080516
Chicago/Turabian StyleBaranovicova, Eva, Dagmar Kalenska, Marian Grendar, and Jan Lehotsky. 2021. "Metabolomic Recovery as a Result of Ischemic Preconditioning Was More Pronounced in Hippocampus than in Cortex That Appeared More Sensitive to Metabolomic Blood Components" Metabolites 11, no. 8: 516. https://doi.org/10.3390/metabo11080516
APA StyleBaranovicova, E., Kalenska, D., Grendar, M., & Lehotsky, J. (2021). Metabolomic Recovery as a Result of Ischemic Preconditioning Was More Pronounced in Hippocampus than in Cortex That Appeared More Sensitive to Metabolomic Blood Components. Metabolites, 11(8), 516. https://doi.org/10.3390/metabo11080516






