Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation
Abstract
1. Introduction
2. Arcuate Nucleus Involvement in Metabolic Regulation
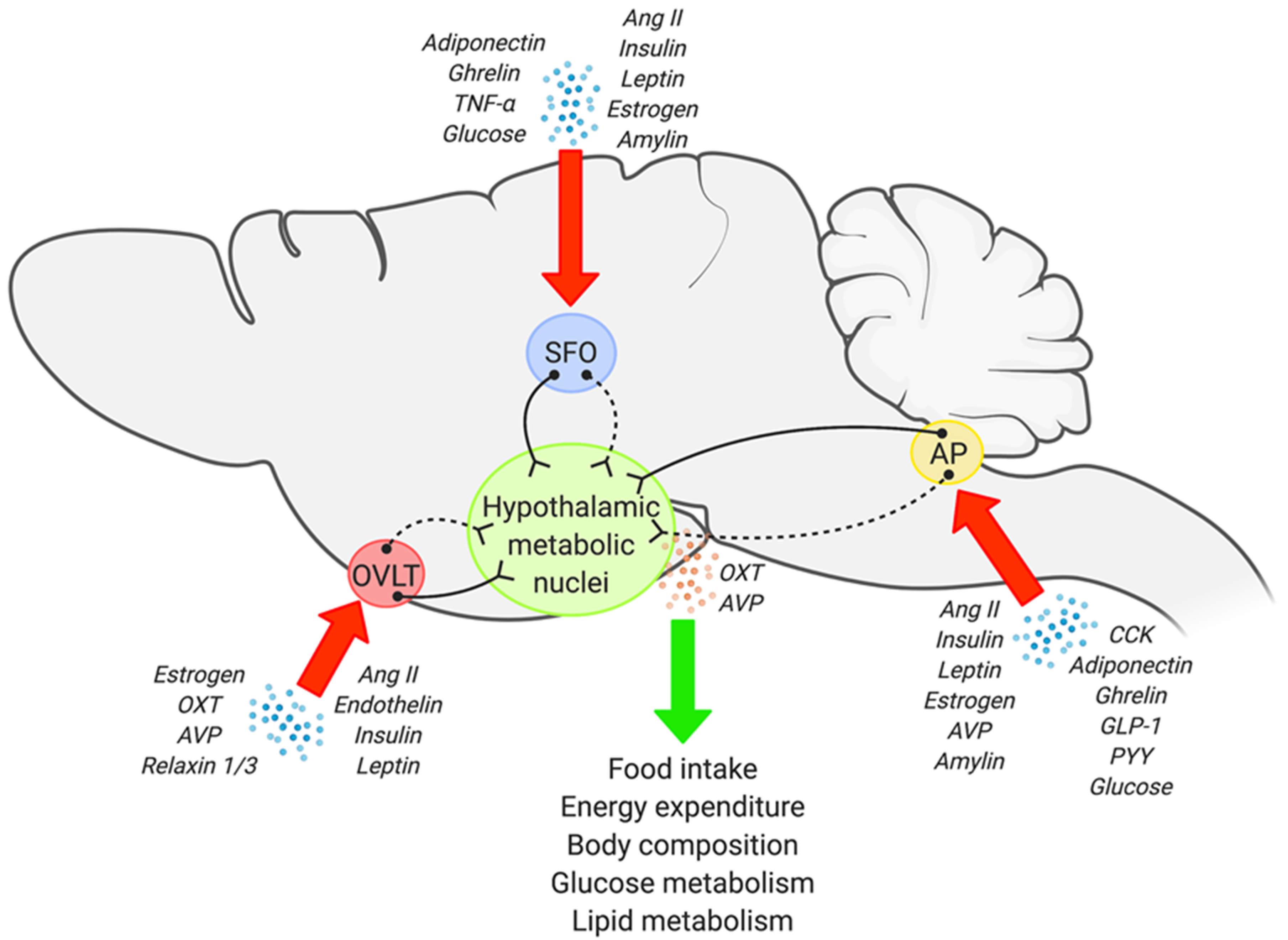
3. Anatomy and Potential Metabolic Role of the Sensory CVOs
3.1. The SFO
3.2. The OVLT
3.3. The AP
4. Sensory CVOs and Hypothalamic Circuits in Metabolic Regulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, J.K.; Horwath, J.A.; Simonyan, H.; Blackmore, K.A.; Butler, S.D.; Young, C.N. Subfornical organ insulin receptors tonically modulate cardiovascular and metabolic function. Physiol. Genom. 2019, 51, 333–341. [Google Scholar] [CrossRef]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef]
- Jeong, J.K.; Kim, J.G.; Lee, B.J. Participation of the central melanocortin system in metabolic regulation and energy homeostasis. Cell. Mol. Life Sci. 2014, 71, 3799–3809. [Google Scholar] [CrossRef]
- Silva, S.C.; Cavadas, C. Hypothalamic Dysfunction in Obesity and Metabolic Disorders. Adv. Neurobiol. 2017, 19, 73–116. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Suyama, S.; Koch, M.; Jin, S.; Arizón, P.A.; Argente, J.; Liu, Z.-W.; Zimmer, M.R.; Jeong, J.K.; Szigeti-Buck, K.; et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr. Metabolic sensing and regulation by the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E809. [Google Scholar] [CrossRef]
- Morita, S.; Furube, E.; Mannari, T.; Okuda, H.; Tatsumi, K.; Wanaka, A.; Miyata, S. Heterogeneous vascular permeability and alternative diffusion barrier in sensory circumventricular organs of adult mouse brain. Cell Tissue Res. 2015, 363, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B. Development of the blood-brain barrier. Cell Tissue Res. 2003, 314, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Okano, H.J.; Li, C.; Lee, G.-H.; Zhao, C.; Darnell, R.; Friedman, J.M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 7001–7005. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Bing, C.; Cai, X.J.; Harrold, J.A.; King, P.J.; Liu, X.H. The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiol. Behav. 2001, 74, 683–701. [Google Scholar] [CrossRef]
- Ciofi, P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci. Lett. 2011, 487, 187–190. [Google Scholar] [CrossRef]
- M Smith, P.; V Ferguson, A. Metabolic Signaling to the Central Nervous System: Routes Across the Blood Brain Barrier. Curr. Pharm. Des. 2014, 20, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gallet, S.; Klemm, P.; Scholl, P.; Folz-Donahue, K.; Altmüller, J.; Alber, J.; Heilinger, C.; Kukat, C.; Loyens, A.; et al. MCH Neurons Regulate Permeability of the Median Eminence Barrier. Neuron 2020, 107, 306–319. [Google Scholar] [CrossRef]
- Balland, E.; Dam, J.; Langlet, F.; Caron, E.; Steculorum, S.; Messina, A.; Rasika, S.; Falluel-Morel, A.; Anouar, Y.; Dehouck, B.; et al. Hypothalamic Tanycytes Are an ERK-Gated Conduit for Leptin into the Brain. Cell Metab. 2014, 19, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Development of hypothalamic circuits that control food intake and energy balance. In Appetite and Food Intake: Central Control, 2nd ed.; Harris, R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 135–154. [Google Scholar]
- Nuzzaci, D.; Laderrière, A.; Lemoine, A.; Nédélec, E.; Pénicaud, L.; Rigault, C.; Benani, A. Plasticity of the Melanocortin System: Determinants and Possible Consequences on Food Intake. Front. Endocrinol. 2015, 6, 143. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Zhao, Z.; Feng, Q.; Lin, R.; Sun, Y.; Ding, T.; Xu, F.; Luo, M.; Zhan, C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 2015, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Prevot, V.; Dehouck, B.; Sharif, A.; Ciofi, P.; Giacobini, P.; Clasadonte, J. The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr. Rev. 2018, 39, 333–368. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.A. The circumventricular organs. Histol. Histopathol. 2017, 32, 879–892. [Google Scholar] [CrossRef]
- Ganong, W.F. Circumventricular Organs: Definition And Role In The Regulation Of Endocrine And Autonomic Function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.K.; Gross, P.M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993, 7, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Hoyda, T.D.; Ferguson, A.V. Making sense of it: Roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp. Biol. Med. 2007, 232, 14–26. [Google Scholar]
- McKinley, M.J.; McAllen, R.; Davern, P.; Giles, M.E.; Penschow, J.; Sunn, N.; Uschakov, A.; Oldfield, B. The Sensory Circumventricular Organs of the Mammalian Brain. Adv. Anat. Embryol. Cell Biol. 2003, 172, 1–122. [Google Scholar] [CrossRef]
- Sisó, S.; Jeffrey, M.; González, L. Sensory circumventricular organs in health and disease. Acta Neuropathol. 2010, 120, 689–705. [Google Scholar] [CrossRef]
- Sunn, N.; McKinley, M.J.; Oldfield, B.J. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J. Neuroendocr. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Young, C.N.; Morgan, D.A.; Butler, S.D.; Rahmouni, K.; Gurley, S.B.; Coffman, T.M.; Mark, A.L.; Davisson, R.L. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol. Metab. 2015, 4, 337–343. [Google Scholar] [CrossRef]
- de Kloet, A.D.; Pioquinto, D.J.; Nguyen, D.; Wang, L.; Smith, J.A.; Hiller, H.; Sumners, C. Obesity induces neuroinflammation mediated by altered expression of the renin–angiotensin system in mouse forebrain nuclei. Physiol. Behav. 2014, 136, 31–38. [Google Scholar] [CrossRef]
- Matsuda, T.; Hiyama, T.Y.; Kobayashi, K.; Kobayashi, K.; Noda, M. Distinct CCK-positive SFO neurons are involved in persistent or transient suppression of water intake. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.; Li, A.; Dong, F.N.; Horwath, J.A.; Clark, C.G.; Davisson, R.L. Endoplasmic reticulum and oxidant stress mediate nuclear factor-κB activation in the subfornical organ during angiotensin II hypertension. Am. J. Physiol. Cell Physiol. 2015, 308, C803–C812. [Google Scholar] [CrossRef]
- Zimmerman, C.; Lin, Y.C.; Leib, D.E.; Guo, L.; Huey, E.L.; Daly, G.E.; Chen, Y.; Knight, Z.A. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 2016, 537, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Nation, H.L.; Nicoleau, M.; Kinsman, B.J.; Browning, K.N.; Stocker, S.D. DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite. J. Neurophysiol. 2016, 115, 3123–3129. [Google Scholar] [CrossRef]
- Alim, I.; Fry, M.; Walsh, M.H.; Ferguson, A.V. Actions of adiponectin on the excitability of subfornical organ neurons are altered by food deprivation. Brain Res. 2010, 1330, 72–82. [Google Scholar] [CrossRef]
- Lakhi, S.; Snow, W.; Fry, M. Insulin modulates the electrical activity of subfornical organ neurons. NeuroReport 2013, 24, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Wang, G.; Waters, E.; Gonzales, K.; Speth, R.; Van Kempen, T.; Marques-Lopes, J.; Young, C.; Butler, S.; Davisson, R.; et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 2012, 226, 489–509. [Google Scholar] [CrossRef]
- Hindmarch, C.; Ferguson, A.V. Physiological roles for the subfornical organ: A dynamic transcriptome shaped by autonomic state. J. Physiol. 2016, 594, 1581–1589. [Google Scholar] [CrossRef]
- Peterson, C.S.; Huang, S.; Lee, S.A.; Ferguson, A.; Fry, W.M. The transcriptome of the rat subfornical organ is altered in response to early postnatal overnutrition. IBRO Rep. 2018, 5, 17–23. [Google Scholar] [CrossRef]
- Smith, P.M.; Rozanski, G.; Ferguson, A.V. Acute electrical stimulation of the subfornical organ induces feeding in satiated rats. Physiol. Behav. 2010, 99, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Riediger, T.; Rauch, M.; Schmid, H.A. Actions of amylin on subfornical organ neurons and on drinking behavior in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R514–R521. [Google Scholar] [CrossRef]
- Pulman, K.J.; Fry, W.M.; Cottrell, G.T.; Ferguson, A.V. The Subfornical Organ: A Central Target for Circulating Feeding Signals. J. Neurosci. 2006, 26, 2022–2030. [Google Scholar] [CrossRef]
- Paes-Leme, B.; Dos-Santos, R.C.; Mecawi, A.S.; Ferguson, A.V. Interaction between angiotensinIIand glucose sensing at the subfornical organ. J. Neuroendocr. 2018, 30, e12654. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.J.; Ferguson, A.V. Tumor necrosis factor-α potentiates the effects of angiotensin II on subfornical organ neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R425–R433. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.J.; Ferguson, A.V. The proinflammatory cytokine tumor necrosis factor-α excites subfornical organ neurons. J. Neurophysiol. 2017, 118, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Brzezinska, P.; Hubert, F.; Mimee, A.; Maurice, D.H.; Ferguson, A.V. Leptin influences the excitability of area postrema neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R440–R448. [Google Scholar] [CrossRef]
- Medeiros, N.; Dai, L.; Ferguson, A. Glucose-responsive neurons in the subfornical organ of the rat—A novel site for direct CNS monitoring of circulating glucose. Neuroscience 2012, 201, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Vallières, L.; Lacroix, S.; Rivest, S. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: An effect depending upon the route of administration. Eur. J. Neurosci. 1997, 9, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Jiang, M.; Tamvakopoulos, C.C.; Shen, X.; Yu, H.; Mock, S.; Fenyk-Melody, J.; Van der Ploeg, L.H.; Guan, X.-M. Exploring the site of anorectic action of peripherally administered synthetic melanocortin peptide MT-II in rats. Brain Res. 2003, 977, 221–230. [Google Scholar] [CrossRef]
- Cruz, J.C.; Flôr, A.F.L.; França-Silva, M.S.; Balarini, C.M.; Braga, V.D.A. Reactive Oxygen Species in the Paraventricular Nucleus of the Hypothalamus Alter Sympathetic Activity During Metabolic Syndrome. Front. Physiol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Horwath, J.A.; Hurr, C.; Butler, S.D.; Guruju, M.; Cassell, M.D.; Mark, A.L.; Davisson, R.L.; Young, C.N. Obesity-induced hepatic steatosis is mediated by endoplasmic reticulum stress in the subfornical organ of the brain. JCI Insight 2017, 2, 2. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Bourque, C.W. Anatomical organization of the rat organum vasculosum laminae terminalis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R324–R337. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, B.J.; Simmonds, S.S.; Browning, K.N.; Wenner, M.M.; Farquhar, W.B.; Stocker, S.D. Integration of Hypernatremia and Angiotensin II by the Organum Vasculosum of the Lamina Terminalis Regulates Thirst. J. Neurosci. 2020, 40, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Somponpun, S.J.; Johnson, A.K.; Beltz, T.; Sladek, C.D. Estrogen receptor-α expression in osmosensitive elements of the lamina terminalis: Regulation by hypertonicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R661–R669. [Google Scholar] [CrossRef]
- Lenkei, Z.; Corvol, P.; Llorens-Cortès, C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Mol. Brain Res. 1995, 30, 53–60. [Google Scholar] [CrossRef]
- Phillips, M.I.; Shen, L.; Richards, E.M.; Raizada, M.K. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul. Pept. 1993, 44, 95–107. [Google Scholar] [CrossRef]
- Nakano, Y.; Furube, E.; Morita, S.; Wanaka, A.; Nakashima, T.; Miyata, S. Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J. Neuroimmunol. 2015, 278, 144–158. [Google Scholar] [CrossRef]
- Nomura, K.; Hiyama, T.; Sakuta, H.; Matsuda, T.; Lin, C.-H.; Kobayashi, K.; Kobayashi, K.; Kuwaki, T.; Takahashi, K.; Matsui, S.; et al. [Na+] Increases in Body Fluids Sensed by Central Nax Induce Sympathetically Mediated Blood Pressure Elevations via H+-Dependent Activation of ASIC1a. Neuron 2019, 101, 60–75.e6. [Google Scholar] [CrossRef]
- Mannari, T.; Morita, S.; Furube, E.; Tominaga, M.; Miyata, S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 2013, 61, 957–971. [Google Scholar] [CrossRef]
- Gebke, E.; Müller, A.R.; Jurzak, M.; Gerstberger, R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience 1998, 85, 509–520. [Google Scholar] [CrossRef]
- Gebke, E.; Müller, A.R.; Pehl, U.; Gerstberger, R. Astrocytes in sensory circumventricular organs of the rat brain express functional binding sites for endothelin. Neuroscience 2000, 97, 371–381. [Google Scholar] [CrossRef]
- Jurzak, M.; Müller, A.R.; Gerstberger, R. Characterization of vasopressin receptors in cultured cells derived from the region of rat brain circumventricular organs. Neuroscience 1995, 65, 1145–1159. [Google Scholar] [CrossRef]
- Jurzak, M.; Müller, A.R.; Gerstberger, R. AVP-fragment peptides induce Ca2+ transients in cells cultured from rat circumventricular organs. Brain Res. 1995, 673, 349–355. [Google Scholar] [CrossRef]
- Voisin, D.; Simonian, S.; Herbison, A. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience 1997, 78, 215–228. [Google Scholar] [CrossRef]
- Osheroff, P.L.; Phillips, H.S. Autoradiographic localization of relaxin binding sites in rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 6413–6417. [Google Scholar] [CrossRef]
- De Ávila, C.; Chometton, S.; Lenglos, C.; Calvez, J.; Gundlach, A.L.; Timofeeva, E. Differential effects of relaxin-3 and a selective relaxin-3 receptor agonist on food and water intake and hypothalamic neuronal activity in rats. Behav. Brain Res. 2018, 336, 135–144. [Google Scholar] [CrossRef]
- Alsuhaymi, N.; Habeeballah, H.; Stebbing, M.J.; Badoer, E. High Fat Diet Decreases Neuronal Activation in the Brain Induced by Resistin and Leptin. Front. Physiol. 2017, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, B.J.; Browning, K.N.; Stocker, S.D. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J. Physiol. 2017, 595, 6187–6201. [Google Scholar] [CrossRef]
- Kinsman, B.J.; Simmonds, S.S.; Browning, K.N.; Stocker, S.D. Organum Vasculosum of the Lamina Terminalis Detects NaCl to Elevate Sympathetic Nerve Activity and Blood Pressure. Hypertension 2017, 69, 163–170. [Google Scholar] [CrossRef]
- Sunn, N.; McKinley, M.J.; Oldfield, B.J. Identification of Efferent Neural Pathways from the Lamina Terminalis Activated by Blood-Borne Relaxin. J. Neuroendocr. 2001, 13, 432–437. [Google Scholar] [CrossRef]
- Sinnayah, P.; Burns, P.; Wade, J.D.; Weisinger, R.S.; McKinley, M.J. Water Drinking in Rats Resulting from Intravenous Relaxin and Its Modification by Other Dipsogenic Factors. Endocrinology 1999, 140, 5082–5086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGowan, B.; Stanley, S.A.; Smith, K.L.; White, N.E.; Connolly, M.M.; Thompson, E.L.; Gardiner, J.; Murphy, K.; Ghatei, M.A.; Bloom, S.R. Central Relaxin-3 Administration Causes Hyperphagia in Male Wistar Rats. Endocrinology 2005, 146, 3295–3300. [Google Scholar] [CrossRef]
- Thornton, S.; Sirinathsinghji, D.; Delaney, C. The effects of a reversible colchicine-induced lesion of the anterior ventral region of the third cerebral ventricle in rats. Brain Res. 1987, 437, 339–344. [Google Scholar] [CrossRef]
- Zimmerman, C.; Leib, D.; Knight, Z. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 2017, 18, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.L.; Meza, E.; Morgado, E.; Juárez, C.; Ramos-Ligonio, A.; Ortega, A.; Caba, M. Activation of Organum Vasculosum of Lamina Terminalis, Median Preoptic Nucleus, and Medial Preoptic Area in Anticipation of Nursing in Rabbit Pups. Chronobiol. Int. 2013, 30, 1272–1282. [Google Scholar] [CrossRef]
- Boschmann, M.; Steiniger, J.; Franke, G.; Birkenfeld, A.L.; Luft, F.; Jordan, J. Water Drinking Induces Thermogenesis through Osmosensitive Mechanisms. J. Clin. Endocrinol. Metab. 2007, 92, 3334–3337. [Google Scholar] [CrossRef]
- Stookey, J.J.D. Negative, Null and Beneficial Effects of Drinking Water on Energy Intake, Energy Expenditure, Fat Oxidation and Weight Change in Randomized Trials: A Qualitative Review. Nutrients 2016, 8, 19. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- Enhörning, S.; Melander, O. The Vasopressin System in the Risk of Diabetes and Cardiorenal Disease, and Hydration as a Potential Lifestyle Intervention. Ann. Nutr. Metab. 2018, 72, 21–27. [Google Scholar] [CrossRef]
- Chang, D.C.; Basolo, A.; Piaggi, P.; Votruba, S.B.; Krakoff, J. Hydration biomarkers and copeptin: Relationship with ad libitum energy intake, energy expenditure, and metabolic fuel selection. Eur. J. Clin. Nutr. 2020, 74, 158–166. [Google Scholar] [CrossRef]
- Roth, G.I.; Yamamoto, W.S. The microcirculation of the area postrema in the rat. J. Comp. Neurol. 1968, 133, 329–340. [Google Scholar] [CrossRef]
- Dempsey, E.W. Neural and vascular ultrastructure of the area postrema in the rat. J. Comp. Neurol. 1973, 150, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Hoyda, T.D.; Ferguson, A.V. The Area Postrema: A Brain Monitor and Integrator of Systemic Autonomic State. Neuroscientist 2007, 14, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Simerly, R.B.; Swanson, L.W.; Chang, C.; Muramatsu, M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Briggs, D.B. Insulin excites neurons of the area postrema and causes emesis. Neurosci. Lett. 1986, 68, 85–89. [Google Scholar] [CrossRef]
- Van Houten, M.; Posner, B.I.; Kopriwa, B.M.; Brawer, J.R. Insulin-Binding Sites in the Rat Brain: In Vivo Localization to the Circumventricular Organs by Quantitative Radioautography. Endocrinology 1979, 105, 666–673. [Google Scholar] [CrossRef]
- Van Houten, M.; Posner, B.I. Specific Binding and Internalization of Blood-Borne [125I]-Iodoinsulin by Neurons of the Rat Area Postrema. Endocrinology 1981, 109, 853–859. [Google Scholar] [CrossRef]
- Liberini, C.G.; Boyle, C.N.; Cifani, C.; Venniro, M.; Hope, B.T.; Lutz, T.A. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur. J. Neurosci. 2016, 43, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Smith, P.M.; Hoyda, T.D.; Duncan, M.; Ahima, R.S.; Sharkey, K.A.; Ferguson, A.V. Area Postrema Neurons Are Modulated by the Adipocyte Hormone Adiponectin. J. Neurosci. 2006, 26, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hara, Y.; Anrather, J.; Speth, R.; Iadecola, C.; Pickel, V. Angiotensin II subtype 1A (AT1A) receptors in the rat sensory vagal complex: Subcellular localization and association with endogenous angiotensin. Neuroscience 2003, 122, 21–36. [Google Scholar] [CrossRef]
- Cork, S.; Richards, J.E.; Holt, M.; Gribble, F.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef]
- Mercer, L.D.; Beart, P.M. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci. Lett. 2004, 359, 109–113. [Google Scholar] [CrossRef]
- Sugeta, S.; Hirai, Y.; Maezawa, H.; Inoue, N.; Yamazaki, Y.; Funahashi, M. Presynaptically mediated effects of cholecystokinin-8 on the excitability of area postrema neurons in rat brain slices. Brain Res. 2015, 1618, 83–90. [Google Scholar] [CrossRef]
- Covasa, M.; Ritter, R.C. Reduced CCK-induced Fos expression in the hindbrain, nodose ganglia, and enteric neurons of rats lacking CCK-1 receptors. Brain Res. 2005, 1051, 155–163. [Google Scholar] [CrossRef]
- Cabral, A.; Cornejo, M.P.; Fernandez, G.; De Francesco, P.N.; Romero, G.G.; Uriarte, M.; Zigman, J.M.; Portiansky, E.; Reynaldo, M.; Perello, M. Circulating Ghrelin Acts on GABA Neurons of the Area Postrema and Mediates Gastric Emptying in Male Mice. Endocrinology 2017, 158, 1436–1449. [Google Scholar] [CrossRef]
- Nambu, Y.; Ohira, K.; Morita, M.; Yasumoto, H.; Kurganov, E.; Miyata, S. Effects of leptin on proliferation of astrocyte- and tanycyte-like neural stem cells in the adult mouse medulla oblongata. Neurosci. Res. 2021. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Kaur, C.; Ling, E. Response of neurons and microglia/macrophages in the area postrema of adult rats following exposure to hypobaric hypoxia. Neurosci. Lett. 2003, 346, 77–80. [Google Scholar] [CrossRef]
- Wuchert, F.; Ott, D.; Murgott, J.; Rafalzik, S.; Hitzel, N.; Roth, J.; Gerstberger, R. Rat area postrema microglial cells act as sensors for the toll-like receptor-4 agonist lipopolysaccharide. J. Neuroimmunol. 2008, 204, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, G.K.; Lam, B.Y.; Tadross, J.A.; Cimino, I.; Rimmington, D.; Coll, A.P.; Polex-Wolf, J.; Knudsen, L.B.; Pyke, C.; Yeo, G.S. A survey of the mouse hindbrain in the fed and fasted states using single-nucleus RNA sequencing. Mol. Metab. 2021, 53, 101240. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, R.; Fahrenholz, F. Autoradiographic localization of V1 vasopressin binding sites in rat brain and kidney. Eur. J. Pharmacol. 1989, 167, 105–116. [Google Scholar] [CrossRef]
- Kishi, T.; Aschkenasi, C.J.; Choi, B.J.; Lopez, M.E.; Lee, C.E.; Liu, H.; Hollenberg, A.N.; Friedman, J.M.; Elmquist, J.K. Neuropeptide Y Y1 receptor mRNA in rodent brain: Distribution and colocalization with melanocortin-4 receptor. J. Comp. Neurol. 2004, 482, 217–243. [Google Scholar] [CrossRef]
- Riediger, T.; Zuend, D.; Becskei, C.; Lutz, T.A. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R114–R122. [Google Scholar] [CrossRef] [PubMed]
- Rinaman, L.; Verbalis, J.G.; Stricker, E.M.; Hoffman, G.E. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J. Comp. Neurol. 1993, 338, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Taylor, I.; Taché, Y. Peripheral peptide YY induces c-fos-like immunoreactivity in the rat brain. Neurosci. Lett. 1993, 163, 77–80. [Google Scholar] [CrossRef]
- Fukuda, T.; Hirai, Y.; Maezawa, H.; Kitagawa, Y.; Funahashi, M. Electrophysiologically identified presynaptic mechanisms underlying amylinergic modulation of area postrema neuronal excitability in rat brain slices. Brain Res. 2013, 1494, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Riediger, T.; Schmid, H.A.; Lutz, T.A.; Simon, E. Amylin and glucose co-activate area postrema neurons of the rat. Neurosci. Lett. 2002, 328, 121–124. [Google Scholar] [CrossRef]
- Fry, M.; Ferguson, A.V. Ghrelin modulates electrical activity of area postrema neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R485–R492. [Google Scholar] [CrossRef]
- Miller, A.D.; Leslie, R.A. The Area Postrema and Vomiting. Front. Neuroendocr. 1994, 15, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Mollet, A.; Gilg, S.; Riediger, T.; Lutz, T.A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol. Behav. 2004, 81, 149–155. [Google Scholar] [CrossRef]
- Cox, J.E.; Randich, A. Enhancement of feeding suppression by PYY3-36 in rats with area postrema ablations. Peptides 2004, 25, 985–989. [Google Scholar] [CrossRef]
- Lutz, T.; Del Prete, E.; Scharrer, E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol. Behav. 1994, 55, 891–895. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.-W.; Manandhar, R.; Jørgensen, S.B.; Lee-Ng, K.K.M.; Zhang, H.P.; Marquis, C.; Jiang, L.; Husaini, Y.; Lin, S.; Sainsbury, A.; et al. The Anorectic Actions of the TGFβ Cytokine MIC-1/GDF15 Require an Intact Brainstem Area Postrema and Nucleus of the Solitary Tract. PLoS ONE 2014, 9, e100370. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Vaughan, C.H.; Bartness, T.J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1049–R1058. [Google Scholar] [CrossRef]
- Coester, B.; Le Foll, C.; Lutz, T.A. Viral depletion of calcitonin receptors in the area postrema: A proof-of-concept study. Physiol. Behav. 2020, 223, 112992. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Erratum: Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 551, 398. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Ye, M.; Zuker, C.S. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 2015, 520, 349–352. [Google Scholar] [CrossRef]
- Matsuda, T.; Hiyama, T.; Niimura, F.; Matsusaka, T.; Fukamizu, A.; Kobayashi, K.; Kobayashi, K.; Noda, M. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat. Neurosci. 2017, 20, 230–241. [Google Scholar] [CrossRef]
- Kawano, H.; Masuko, S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience 2010, 169, 1227–1234. [Google Scholar] [CrossRef]
- Yeo, S.H.; Kyle, V.; Blouet, C.; Jones, S.; Colledge, W.H. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PLoS ONE 2019, 14, e0213927. [Google Scholar] [CrossRef]
- Kolaj, M.; Renaud, L.P. Metabotropic Glutamate Receptors in Median Preoptic Neurons Modulate Neuronal Excitability and Glutamatergic and GABAergic Inputs From the Subfornical Organ. J. Neurophysiol. 2010, 103, 1104–1113. [Google Scholar] [CrossRef]
- Abbott, S.B.; Machado, N.L.; Geerling, J.C.; Saper, C.B. Reciprocal Control of Drinking Behavior by Median Preoptic Neurons in Mice. J. Neurosci. 2016, 36, 8228–8237. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.G.; Kawano, H.; Masuko, S. Collateral projections from the subfornical organ to the median preoptic nucleus and paraventricular hypothalamic nucleus in the rat. Brain Res. 2008, 1198, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H. Synaptic contact between median preoptic neurons and subfornical organ neurons projecting to the paraventricular hypothalamic nucleus. Exp. Brain Res. 2017, 235, 1053–1062. [Google Scholar] [CrossRef]
- McKinley, M.J.; Denton, D.A.; Ryan, P.; Yao, S.; Stefanidis, A.; Oldfield, B.J. From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger. J. Neuroendocr. 2019, 31, e12689. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Honda, E.; Ono, K.; Inenaga, K. Muscarinic modulation of GABAergic transmission to neurons in the rat subfornical organ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1657–R1664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Herbert, J. Calcium channels mediate angiotensin II-induced drinking behaviour and c-fos expression in the brain. Brain Res. 1997, 778, 206–214. [Google Scholar] [CrossRef]
- Shi, P.; Martinez, M.A.; Calderon, A.S.; Chen, Q.; Cunningham, J.T.; Toney, G.M. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J. Physiol. 2008, 586, 5231–5245. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kooy, D.; Koda, L.Y. Organization of the projections of a circumventricular organ: The area postrema in the rat. J. Comp. Neurol. 1983, 219, 328–338. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Miselis, R.R. The central neural connections of the area postrema of the rat. J. Comp. Neurol. 1985, 234, 344–364. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.H.; Collister, J.P.; Osborn, J.W. The area postrema modulates hypothalamic fos responses to intragastric hypertonic saline in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1921–R1927. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, B.A.; Lemus, M.; Montero, S.; Melnikov, V.; Luquín, S.; García-Estrada, J.; De Álvarez-Buylla, E.R. Nitric oxide in the nucleus of the tractus solitarius is involved in hypoglycemic conditioned response. Brain Res. 2017, 1667, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Filippi, B.M.; Yang, C.S.; Tang, C.; Lam, T.K. Insulin Activates Erk1/2 Signaling in the Dorsal Vagal Complex to Inhibit Glucose Production. Cell Metab. 2012, 16, 500–510. [Google Scholar] [CrossRef]
- Tsai, J.P. The association of serum leptin levels with metabolic diseases. Tzu-Chi Med. J. 2017, 29, 192–196. [Google Scholar] [CrossRef]
- Filippi, B.M.; Bassiri, A.; Abraham, M.A.; Duca, F.A.; Yue, J.T.; Lam, T.K. Insulin Signals Through the Dorsal Vagal Complex to Regulate Energy Balance. Diabetes 2014, 63, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lemus, M.B.; Stark, R.; Bayliss, J.A.; Reichenbach, A.; Lockie, S.; Andrews, Z.B. The Temporal Pattern of cfos Activation in Hypothalamic, Cortical, and Brainstem Nuclei in Response to Fasting and Refeeding in Male Mice. Endocrinology 2014, 155, 840–853. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Lyons, D.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; DiLeone, R.; et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife 2016, 5, e12225. [Google Scholar] [CrossRef]
- Herman, J.P. Regulation of Hypothalamo-Pituitary-Adrenocortical Responses to Stressors by the Nucleus of the Solitary Tract/Dorsal Vagal Complex. Cell. Mol. Neurobiol. 2018, 38, 25–35. [Google Scholar] [CrossRef]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef]
- Maniscalco, J.W.; Rinaman, L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol. Behav. 2013, 121, 35–42. [Google Scholar] [CrossRef]
- Ito, H.; Seki, M. Ascending Projections from the Area Postrema and the Nucleus of the Solitary Tract of Suncus Murinus: Anterograde tracing study using Phaseolus vulgaris leucoagglutinin. Okajimas Folia Anat. Jpn. 1998, 75, 9–31. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Krause, E.; Scott, K.; Foster, M.T.; Herman, J.; Sakai, R.R.; Seeley, R.; Woods, S.C. Central angiotensin II has catabolic action at white and brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1081–E1091. [Google Scholar] [CrossRef]
- McKinley, M.J.; Burns, P.; Colvill, L.M.; Oldfield, B.J.; Wade, J.D.; Weisinger, R.S.; Tregear, G.W. Distribution of Fos immunoreactivity in the lamina terminalis and hypothalamus induced by centrally administered relaxin in conscious rats. J. Neuroendocr. 1997, 9, 431–437. [Google Scholar] [CrossRef]
- Wilson, B.C.; Summerlee, A.J.S. Effects of exogenous relaxin on oxytocin and vasopressin release and the intramammary pressure response to central hyperosmotic challenge. J. Endocrinol. 1994, 141, 75–80. [Google Scholar] [CrossRef]
- Zhice, X.; Herbert, J. Regional suppression by water intake of c-fos expression induced by intraventricular infusions of angiotensin II. Brain Res. 1994, 659, 157–168. [Google Scholar] [CrossRef]
- Ueno, H.; Yoshimura, M.; Tanaka, K.; Nishimura, K.; Sonoda, S.; Motojima, Y.; Saito, R.; Maruyama, T.; Miyamoto, T.; Serino, R.; et al. Up-regulation of hypothalamic arginine vasopressin by peripherally administered furosemide in transgenic rats expressing arginine vasopressin-enhanced green fluorescent protein. J. Neuroendocr. 2018, 30, e12603. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.V.; Kasting, N.W. Activation of subfornical organ efferents stimulates oxytocin secretion in the rat. Regul. Pept. 1987, 18, 93–100. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Kasting, N.W. Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986, 251, R425–R428. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Pekarek, E.; Ge, J.; Yao, J. Functional relationship between subfornical organ cholinergic stimulation and cellular activation in the hypothalamus and AV3V region. Brain Res. 2001, 922, 191–200. [Google Scholar] [CrossRef]
- Thrasher, T.N.; Keil, L.C.; Ramsay, D.J. Lesions of the organum vasculosum of the lamina terminalis (ovlt) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology 1982, 110, 1837–1839. [Google Scholar] [CrossRef]
- Russell, J.; Blackburn, R.; Leng, G. The role of the AV3V region in the control of magnocellular oxytocin neurons. Brain Res. Bull. 1988, 20, 803–810. [Google Scholar] [CrossRef]
- Carter, D.; Lightman, S. A role for the area postrema in mediating cholecystokinin-stimulated oxytocin secretion. Brain Res. 1987, 435, 327–330. [Google Scholar] [CrossRef]
- Larsen, P.J.; Tang-Christensen, M.; Jessop, D.S. Central Administration of Glucagon-Like Peptide-1 Activates Hypothalamic Neuroendocrine Neurons in the Rat. Endocrinology 1997, 138, 4445–4455. [Google Scholar] [CrossRef]
- Kublaoui, B.M.; Gemelli, T.; Tolson, K.; Wang, Y.; Zinn, A.R. Oxytocin Deficiency Mediates Hyperphagic Obesity of Sim1 Haploinsufficient Mice. Mol. Endocrinol. 2008, 22, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E134–E144. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Graham, J.; Morton, G.J.; Bales, K.L.; Schwartz, M.W.; Baskin, D.G.; Havel, P. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R431–R438. [Google Scholar] [CrossRef]
- Ott, V.; Finlayson, G.; Lehnert, H.; Heitmann, B.L.; Heinrichs, M.; Born, J.; Hallschmid, M. Oxytocin Reduces Reward-Driven Food Intake in Humans. Diabetes 2013, 62, 3418–3425. [Google Scholar] [CrossRef]
- Thienel, M.; Fritsche, A.; Heinrichs, M.; Peter, A.; Ewers, M.; Lehnert, H.; Born, J.; Hallschmid, M. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. 2016, 40, 1707–1714. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.-L.; Caillon, A.; Arsenijevic, D.; Veyrat-Durebex, C.; Lyautey, J.; Dulloo, A.; Rohner-Jeanrenaud, F. Divergent Effects of Oxytocin Treatment of Obese Diabetic Mice on Adiposity and Diabetes. Endocrinology 2014, 155, 4189–4201. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Camerino, C. Low Sympathetic Tone and Obese Phenotype in Oxytocin-deficient Mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Zhu, Y.; Sutton, A.K.; Zhao, R.; Lowell, B.B.; Olson, D.P.; Tong, Q. An Obligate Role of Oxytocin Neurons in Diet Induced Energy Expenditure. PLoS ONE 2012, 7, e45167. [Google Scholar] [CrossRef]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.-H.; Kawada, T.; Inoue, N.; Ikeda, I.; et al. Oxytocin Receptor in the Hypothalamus Is Sufficient to Rescue Normal Thermoregulatory Function in Male Oxytocin Receptor Knockout Mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocr. 2020, 32, e12805. [Google Scholar] [CrossRef]
- Roberts, Z.S.; Wolden-Hanson, T.; Matsen, M.E.; Ryu, V.; Vaughan, C.H.; Graham, J.L.; Havel, P.J.; Chukri, D.W.; Schwartz, M.W.; Morton, G.J.; et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R357–R371. [Google Scholar] [CrossRef]
- Ding, C.; Leow, M.K.S.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2018, 20, 22–40. [Google Scholar] [CrossRef]
- Hanif, K.; Goren, H.J.; Hollenberg, M.D.; Lederis, K. Oxytocin action: Lipid metabolism in adipocytes from homozygous diabetes insipidus rats (Brattleboro strain). Can. J. Physiol. Pharmacol. 1982, 60, 993–997. [Google Scholar] [CrossRef]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.L.; Petrosino, S.; Piscitelli, F.; Legros, J.J.; Geenen, V.; Foti, M.; et al. Mechanisms of the Anti-Obesity Effects of Oxytocin in Diet-Induced Obese Rats. PLoS ONE 2011, 6, e25565. [Google Scholar] [CrossRef]
- Lee, E.S.; Uhm, K.O.; Lee, Y.M.; Kwon, J.; Park, S.H.; Soo, K.H. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium–CaMKK–AMPK pathway. Regul. Pept. 2008, 151, 71–74. [Google Scholar] [CrossRef]
- Yoshimura, M.; Conway-Campbell, B.; Ueta, Y. Arginine vasopressin: Direct and indirect action on metabolism. Peptides 2021, 142, 170555. [Google Scholar] [CrossRef]
- Shido, O.; Kifune, A.; Nagasaka, T. Baroreflexive suppression of heat production and fall in body temperature following peripheral administration of vasopressin in rats. Jpn. J. Physiol. 1984, 34, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Sutton, A.K.; Burnett, K.H.; Fuller, P.M.; Olson, D.P. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol. Metab. 2014, 3, 209–215. [Google Scholar] [CrossRef]
- Yoshimura, M.; Nishimura, K.; Nishimura, H.; Sonoda, S.; Ueno, H.; Motojima, Y.; Saito, R.; Maruyama, T.; Nonaka, Y.; Ueta, Y. Activation of endogenous arginine vasopressin neurons inhibit food intake: By using a novel transgenic rat line with DREADDs system. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Na Kim, Y.; Kim, I.Y.; Pak, S.-I.; Lee, I.S.; Seong, J.K.; Yoon, Y.S. Enhanced Expressions of Arginine Vasopressin (Avp) in the Hypothalamic Paraventricular and Supraoptic Nuclei of Type 2 Diabetic Rats. Neurochem. Res. 2007, 33, 833–841. [Google Scholar] [CrossRef]
- Aoyagi, T.; Birumachi, J.-I.; Hiroyama, M.; Fujiwara, Y.; Sanbe, A.; Yamauchi, J.; Tanoue, A. Alteration of Glucose Homeostasis in V1a Vasopressin Receptor-Deficient Mice. Endocrinology 2007, 148, 2075–2084. [Google Scholar] [CrossRef]
- Nakamura, K.; Velho, G.; Bouby, N. Vasopressin and metabolic disorders: Translation from experimental models to clinical use. J. Intern. Med. 2017, 282, 298–309. [Google Scholar] [CrossRef]
- Nakamura, K.; Aoyagi, T.; Hiroyama, M.; Kusakawa, S.; Mizutani, R.; Sanbe, A.; Yamauchi, J.; Kamohara, M.; Momose, K.; Tanoue, A. Both V1A and V1B vasopressin receptors deficiency result in impaired glucose tolerance. Eur. J. Pharmacol. 2009, 613, 182–188. [Google Scholar] [CrossRef]
- Hiroyama, M.; Aoyagi, T.; Fujiwara, Y.; Birumachi, J.; Shigematsu, Y.; Kiwaki, K.; Tasaki, R.; Endo, F.; Tanoue, A. Hypermetabolism of Fat in V1a Vasopressin Receptor Knockout Mice. Mol. Endocrinol. 2007, 21, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Velho, G.; Bouby, N.; Hadjadj, S.; Matallah, N.; Mohammedi, K.; Fumeron, F.; Potier, L.; Bellili-Munoz, N.; Taveau, C.; Alhenc-Gelas, F.; et al. Plasma Copeptin and Renal Outcomes in Patients With Type 2 Diabetes and Albuminuria. Diabetes Care 2013, 36, 3639–3645. [Google Scholar] [CrossRef]
- Velho, G.; El Boustany, R.; Lefèvre, G.; Mohammedi, K.; Fumeron, F.; Potier, L.; Bankir, L.; Bouby, N.; Hadjadj, S.; Marre, M.; et al. Plasma Copeptin, Kidney Outcomes, Ischemic Heart Disease, and All-Cause Mortality in People With Long-standing Type 1 Diabetes. Diabetes Care 2016, 39, 2288–2295. [Google Scholar] [CrossRef]
- Roussel, R.; El Boustany, R.; Bouby, N.; Potier, L.; Fumeron, F.; Mohammedi, K.; Balkau, B.; Tichet, J.; Bankir, L.; Marre, M.; et al. Plasma Copeptin, AVP Gene Variants, and Incidence of Type 2 Diabetes in a Cohort From the Community. J. Clin. Endocrinol. Metab. 2016, 101, 2432–2439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.K.; Dow, S.A.; Young, C.N. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites 2021, 11, 494. https://doi.org/10.3390/metabo11080494
Jeong JK, Dow SA, Young CN. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites. 2021; 11(8):494. https://doi.org/10.3390/metabo11080494
Chicago/Turabian StyleJeong, Jin Kwon, Samantha A. Dow, and Colin N. Young. 2021. "Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation" Metabolites 11, no. 8: 494. https://doi.org/10.3390/metabo11080494
APA StyleJeong, J. K., Dow, S. A., & Young, C. N. (2021). Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites, 11(8), 494. https://doi.org/10.3390/metabo11080494






