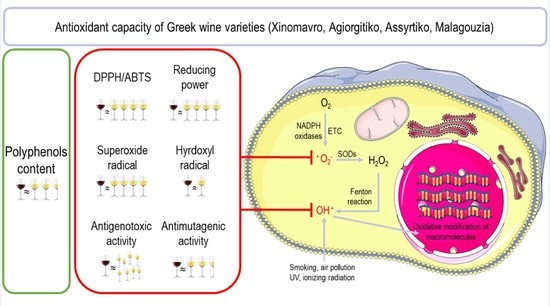
Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro
Abstract
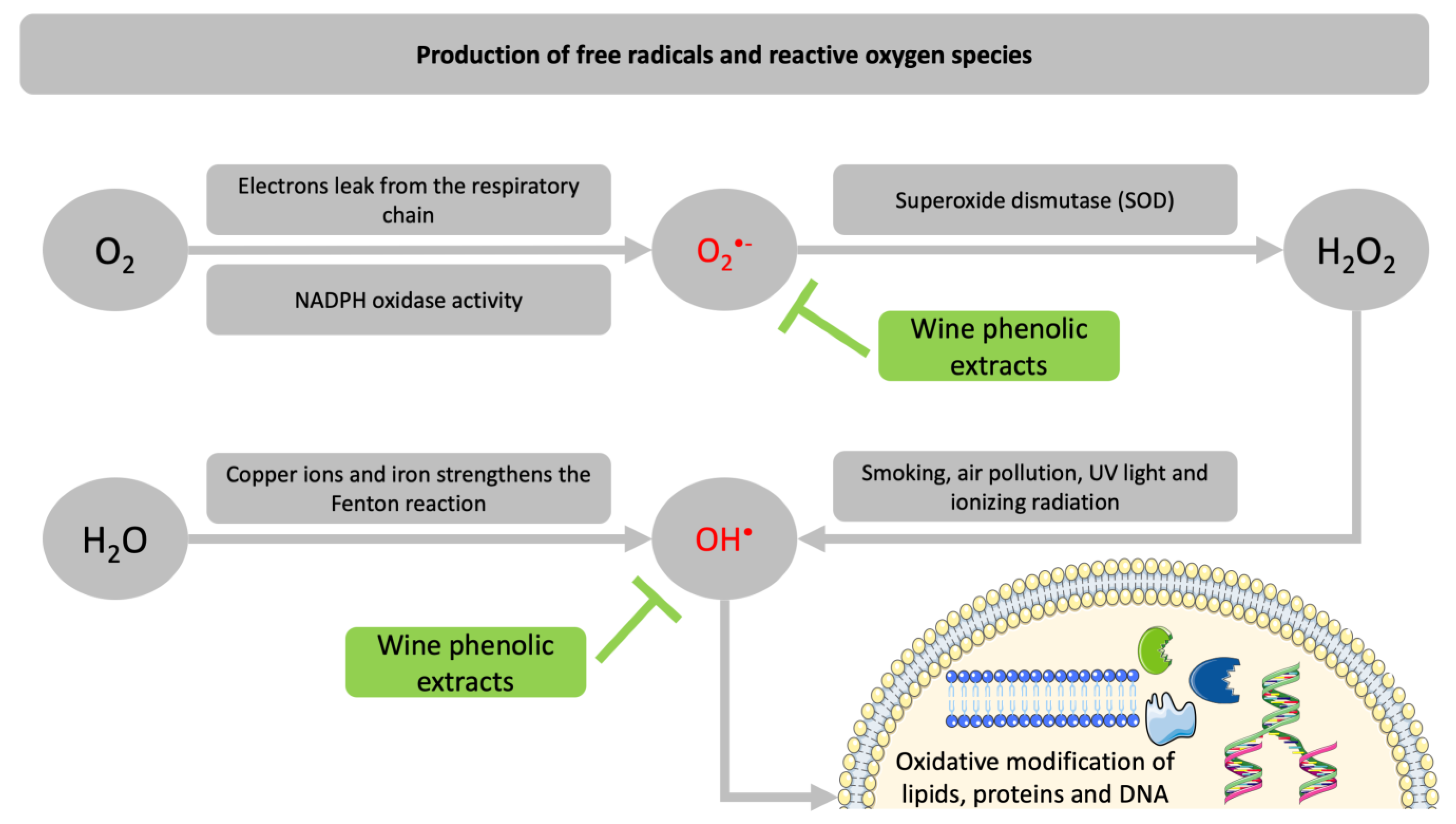
:1. Introduction
2. Results
2.1. UHPLC-ESI-TripleTOF-HRMS Analysis and Metabolites Comparison
2.2. In Vitro Measurements for the Assessment of the Wine Extracts’ Antioxidant Activity
2.2.1. Total Phenolic Content of Wine Varieties (TPC)
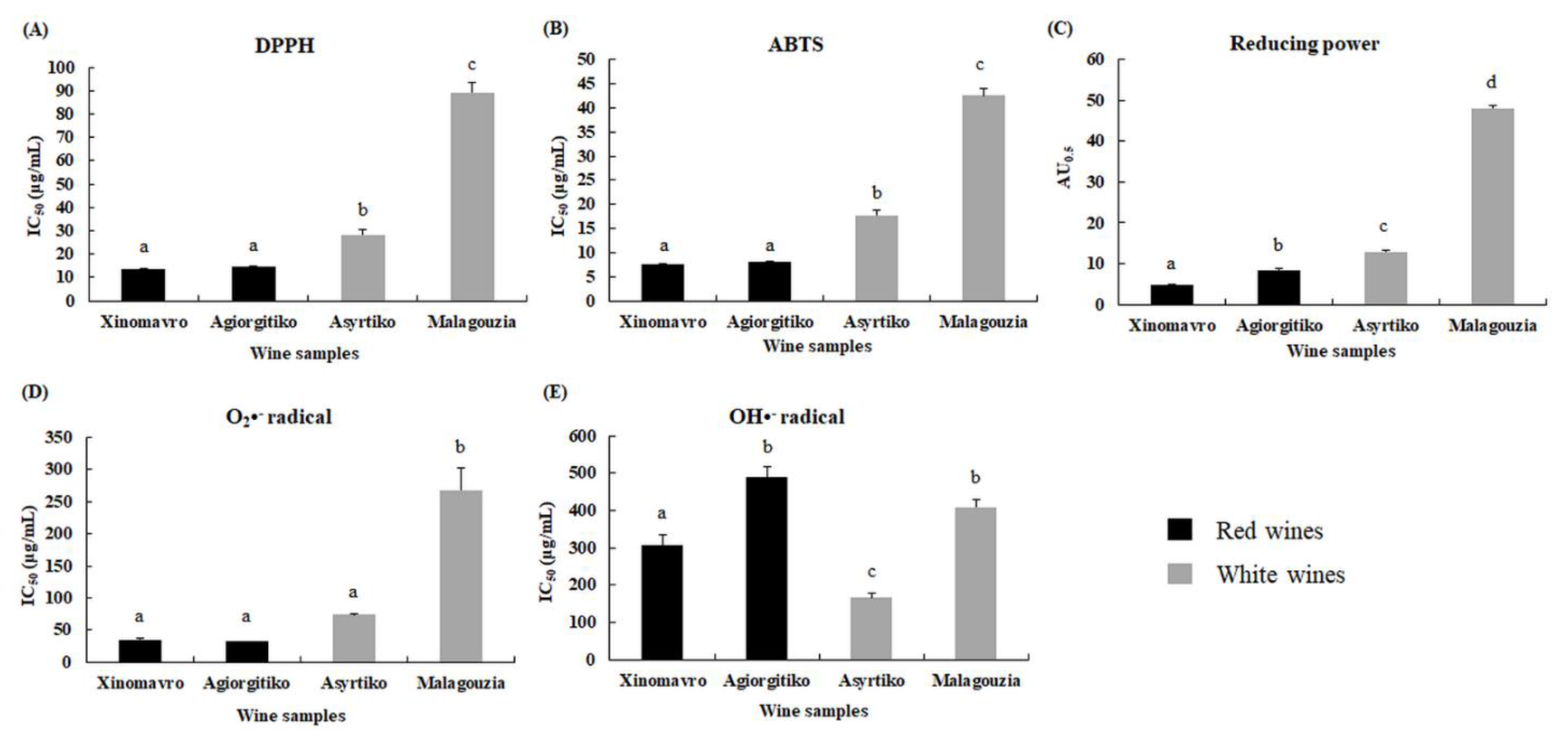
2.2.2. Determination of IC50 Values of Extracts in DPPH•, ABTS•+, Reducing Power, Superoxide, and Hydroxyl Radical Scavenging Activity Assays
2.2.3. Antigenotoxic Activity of Wine Extracts via a Plasmid Relaxation Assay
2.2.4. Antimutagenic Capacity of Wine Extracts through an Ames Test
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. UHPLC-ESI-TripleTOF-HRMS Analysis
4.4. In Vitro Biomarkers for the Assessment of the Wine Extracts’ Antioxidant Activity
4.4.1. Determination of Total Polyphenolic Content (TPC)
4.4.2. Determination of DPPH• Radical Scavenging Activity
4.4.3. Determination of ABTS Radical Scavenging Activity
4.4.4. Determination of the Reducing Power Assay
4.4.5. Determination of Superoxide Radical (O2•−) Scavenging Activity
4.4.6. Determination of Hydroxyl Radical (OH•) Scavenging Activity
4.4.7. Determination of Peroxyl Radical-Induced DNA Plasmid Strand Cleavage
4.4.8. Determination of Antimutagenic Capacity Using an Ames Test
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sies, H. Oxidative Stress: Introductory Remarks. In Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 1985; pp. 1–8. [Google Scholar]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Kerasioti, E.; Terzopoulou, Z.; Komini, O.; Kafantaris, I.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Anisimov, N.Y.N.Y.; Tsatsakis, A.M.A.M.; Kouretas, D. Tissue specific effects of feeds supplemented with grape pomace or olive oil mill wastewater on detoxification enzymes in sheep. Toxicol. Reports 2017, 4, 364–372. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Bidlack, W.R.; Omaye, S.T.; Meskin, M.S.; Topham, D.K.W. Phytochemicals as Bioactive Agents; CRC press: Boca Raton, FL, USA, 2000; ISBN 1566767881. [Google Scholar]
- Meskin, M.S.; Davies, A.J.; Bidlack, W.R.; Omaye, S.T. Phytochemicals in Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2002; ISBN 1420031694. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. (Paris) 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddouchi, F.; Chaouche, T.M.; Ksouri, R.; Larbat, R. Leafy Stems of Phagnalon saxatile subsp. saxatile from Algeriaas a Source of Chlorogenic Acids and Flavonoids with Antioxidant Activity: Characterization and Quantification Using UPLC-DAD-ESI-MSn. Metabolites 2021, 11, 280. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [Green Version]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; Campos, R.A.D.S.; Borguini, M.G. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Casani-Cubel, J.; Benlloch, M.; Sanchis-Sanchis, C.E.; Marin, R.; Lajara-Romance, J.M.; de la Rubia Orti, J.E. The impact of microbiota on the pathogenesis of amyotrophic lateral sclerosis and the possible benefits of polyphenols. An overview. Metabolites 2021, 11, 120. [Google Scholar] [CrossRef]
- Keys, A. Coronary Heart Disease in Seven Countries. Nutrition 1997, 13, 249–253. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Mediterranean Diet-Intangible Heritage-Culture Sector-UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 12 May 2021).
- Sohaib, H.; Bryce, A.; Adrian, B. Wine and Cardiovascular Health. Circulation 2017, 136, 1434–1448. [Google Scholar]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Stockley, C.; Teissedre, P.-L.; Boban, M.; Di Lorenzo, C.; Restani, P. Bioavailability of wine-derived phenolic compounds in humans: A review. Food Funct. 2012, 3, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Suprun, A.R.; Dubrovina, A.S.; Tyunin, A.P.; Kiselev, K.V. Profile of Stilbenes and Other Phenolics in Fanagoria White and Red Russian Wines. Metabolites 2021, 11, 231. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical profile and antioxidant activity of aerial and underground parts of salvia bulleyana diels. Plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid. Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xu, X.; Tao, Z.; Wang, X.J.; Pan, Y. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kalliora, C.; Kyriazis, I.D.; Oka, S.I.; Lieu, M.J.; Yue, Y.; Area-Gomez, E.; Pol, C.J.; Tian, Y.; Mizushima, W.; Chin, A.; et al. Dual PPARα/γ activation inhibits SIRT1-PGC1α axis and causes cardiac dysfunction. JCI Insight 2019, 5, e129556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [Green Version]
- Biagi, M.; Bertelli, A.A.E. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef]
- Djoussé, L.; Ellison, R.C.; Beiser, A.; Scaramucci, A.; D’Agostino, R.B.; Wolf, P.A. Alcohol consumption and risk of ischemic stroke: The Framingham Study. Stroke 2002, 33, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Lucas, D.L.; Brown, R.A.; Wassef, M.; Giles, T.D. Alcohol and the cardiovascular system: Research challenges and opportunities. J. Am. Coll. Cardiol. 2005, 45, 1916–1924. [Google Scholar] [CrossRef] [Green Version]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 2006, 11, 217–225. [Google Scholar]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vislocky, L.M.; Fernandez, M.L. Biomedical effects of grape products. Nutr. Rev. 2010, 68, 656–670. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Kyriazis, I.; Skaperda, Z.; Tekos, F.; Makri, S.; Vardakas, P.; Vassi, E.; Patouna, A.; Terizi, K.; Angelakis, C.; Kouretas, D. Methodology for the biofunctional assessment of honey (Review). Int. J. Funct. Nutr. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Jaitz, L.; Siegl, K.; Eder, R.; Rak, G.; Abranko, L.; Koellensperger, G.; Hann, S. LC-MS/MS analysis of phenols for classification of red wine according to geographic origin, grape variety and vintage. Food Chem. 2010, 122, 366–372. [Google Scholar] [CrossRef]
- La Torre, G.L.; Saitta, M.; Vilasi, F.; Pellicanò, T.; Dugo, G. Direct determination of phenolic compounds in Sicilian wines by liquid chromatography with PDA and MS detection. Food Chem. 2006, 94, 640–650. [Google Scholar] [CrossRef]
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Tzanova, M.; Atanassova, S.; Atanasov, V.; Grozeva, N. Content of Polyphenolic Compounds and Antioxidant Potential of Some Bulgarian Red Grape Varieties and Red Wines, Determined by HPLC, UV, and NIR Spectroscopy. Agriculture 2020, 10, 193. [Google Scholar] [CrossRef]
- Soleas, G.J.; Grass, L.; Josephy, P.D.; Goldberg, D.M.; Diamandis, E.P. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin. Biochem. 2002, 35, 119–124. [Google Scholar] [CrossRef]
- He, S.; Sun, C.; Pan, Y. Red wine polyphenols for cancer prevention. Int. J. Mol. Sci. 2008, 9, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Quero, J.; Jiménez-Moreno, N.; Esparza, I.; Osada, J.; Cerrada, E.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Grape stem extracts with potential anticancer and antioxidant properties. Antioxidants 2021, 10, 243. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D. Antioxidative properties of methanolic extracts from peanut hulls. J. Am. Oil Chem. Soc. 1993, 70, 383–386. [Google Scholar] [CrossRef]
- Noda, Y.; Anzai, K.; Mori, A.; Kohno, M.; Shinmei, M.; Packer, L. Hydroxyl, end superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem. Mol. Biol. Int. 1997, 42, 35–44. [Google Scholar]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rosé Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Arvanitoyannis, I.S.; Kefalas, P.; El-Zajouli, A.; Soufleros, E.; Psarra, E. Instrumental and sensory analysis of Greek wines; Implementation of principal component analysis (PCA) for classification according to geographical origin. Food Chem. 2001, 73, 501–514. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Tourtoglou, C.; Nenadis, N.; Paraskevopoulou, A. Phenolic composition and radical scavenging activity of commercial Greek white wines from Vitis vinifera L. cv. Malagousia. J. Food Compos. Anal. 2014, 33, 166–174. [Google Scholar] [CrossRef]
- Gerogiannaki-Christopoulou, M.; Athanasopoulos, P.; Kyriakidis, N.; Gerogiannaki, I.A.; Spanos, M. trans-Resveratrol in wines from the major Greek red and white grape varieties. Food Control 2006, 17, 700–706. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Karumanchiri, A.; Ng, E.; Yan, J.; Diamandis, E.P.; Soleas, G.J. Direct Gas Chromatographic-Mass Spectrometric Method To Assay cis-Resveratrol in Wines: Preliminary Survey of Its Concentration in Commercial Wines. J. Agric. Food Chem. 1995, 43, 1245–1250. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Ng, E.; Karumanchiri, A.; Yan, J.; Diamandis, E.P.; Soleas, G.J. Assay of resveratrol glucosides and isomers in wine by direct-injection high-performance liquid chromatography. J. Chromatogr. A 1995, 708, 89–98. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Ng, E.; Yan, J.; Karumanchiri, A.; Soleas, G.J.; Diamandis, E.P. Regional differences in resveratrol isomer concentrations of wines from various cultivars. Int. J. Phytoremediation 1996, 21, 13–24. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Sbagli, M.; Meunier, P.; Trollart, P. Resveratrol content of wine of different ages: Relationship with fungal disease pressure in the vineyard. Am. J. Enol. Vitic. 1995, 46, 1–4. [Google Scholar]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Sbaghi, M. Analysis of resveratrol in Burgundy wines. J. Wine Res. 1993, 4, 79–85. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. H NMR-based metabonomics for the classification of Greek wines according to variety, region, and vintage. Comparison with HPLC data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Cassino, C.; Gianotti, V.; Bonello, F.; Tsolakis, C.; Cravero, M.C.; Osella, D. Antioxidant Composition of a Selection of Italian Red Wines and Their Corresponding Free-Radical Scavenging Ability. J. Chem. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Stratil, P.; Kubáň, V.; Fojtová, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef] [Green Version]
- Roussis, I.G.; Lambropoulos, I.; Soulti, K. Scavenging Capacities of Some Wines and Wine Phenolic Extracts. Food Technol. Biotechnol. 2005, 43, 351–358. [Google Scholar]
- Skaperda, Z.; Tekos, F.; Makri, S.; Angelakis, C.; Vassi, E.; Vardakas, P.; Patouna, A.; Terizi, K.; Kyriazi, D.; Kouretas, D. A novel combined bioactivity / chemoactivity holistic approach for the evaluation of dietary supplements. Food Chem. Toxicol. 2021, 152. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Tsatsakis, A.; Skaperda, Z.; Kouretas, D.; Shahraki, J. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel Feed Including Olive Oil Mill Wastewater Bioactive Compounds Enhanced the Redox Status of Lambs. In Vivo 2018, 32, 291–302. [Google Scholar]
- Kouka, P.; Tekos, F.; Valta, K.; Mavros, P.; Veskoukis, A.S.; Angelis, A.; Skaltsounis, A.-L.; Kouretas, D. Οlive tree blossom polyphenolic extracts exert antioxidant and antimutagenic activities in vitro and in various cell lines. Oncol. Rep. 2019, 42, 2814–2825. [Google Scholar] [CrossRef]
- Priftis, A.; Stagos, D.; Konstantinopoulos, K.; Tsitsimpikou, C.; Spandidos, D.A.; Tsatsakis, A.M.; Tzatzarakis, M.N.; Kouretas, D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 2015, 12, 7293–7302. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, A. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem. Anal. 1998, 9, 196–202. [Google Scholar] [CrossRef]
- Spanidis, Y.; Stagos, D.; Papanikolaou, C.; Karatza, K.; Theodosi, A.; Veskoukis, A.S.; Deli, C.K.; Poulios, A.; Koulocheri, S.D.; Jamurtas, A.Z.; et al. Resistance-Trained Individuals Are Less Susceptible to Oxidative Damage after Eccentric Exercise. Oxid. Med. Cell. Longev. 2018, 2018, 6857190. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-F.; Hsu, Y.-W.; Ting, H.-C.; Huang, C.-F.; Yen, C.-C. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae). Food Chem. 2013, 136, 1337–1344. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Grundler, F.; Mesnage, R.; Goutzourelas, N.; Tekos, F.; Makri, S.; Brack, M.; Kouretas, D.; Wilhelmi de Toledo, F. Interplay between oxidative damage, the redox status, and metabolic biomarkers during long-term fasting. Food Chem. Toxicol. 2020, 145, 111701. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-K.; Osawa, T.; Kawakishi, S. Hydroxyl Radical-scavenging Effects of Spices and Scavengers from Brown Mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 1997, 61, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Veskoukis, A.S.; Vassi, E.; Poulas, K.; Kokkinakis, M.; Asprodini, E.; Haroutounian, S.; Kouretas, D. Grape Stem Extracts From Three Native Greek Vine Varieties Exhibit Strong Antioxidant and Antimutagenic Properties. Anticancer Res. 2020, 40, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.-L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Kouka, P.; Tsakiri, G.; Tzortzi, D.; Dimopoulou, S.; Sarikaki, G.; Stathopoulos, P.; Veskoukis, A.S.; Halabalaki, M.; Skaltsounis, A.-L.; Kouretas, D. The polyphenolic composition of extracts derived from 2 different Greek extra virgin olive oils is correlated with their 3 antioxidant potency. Oxid. Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
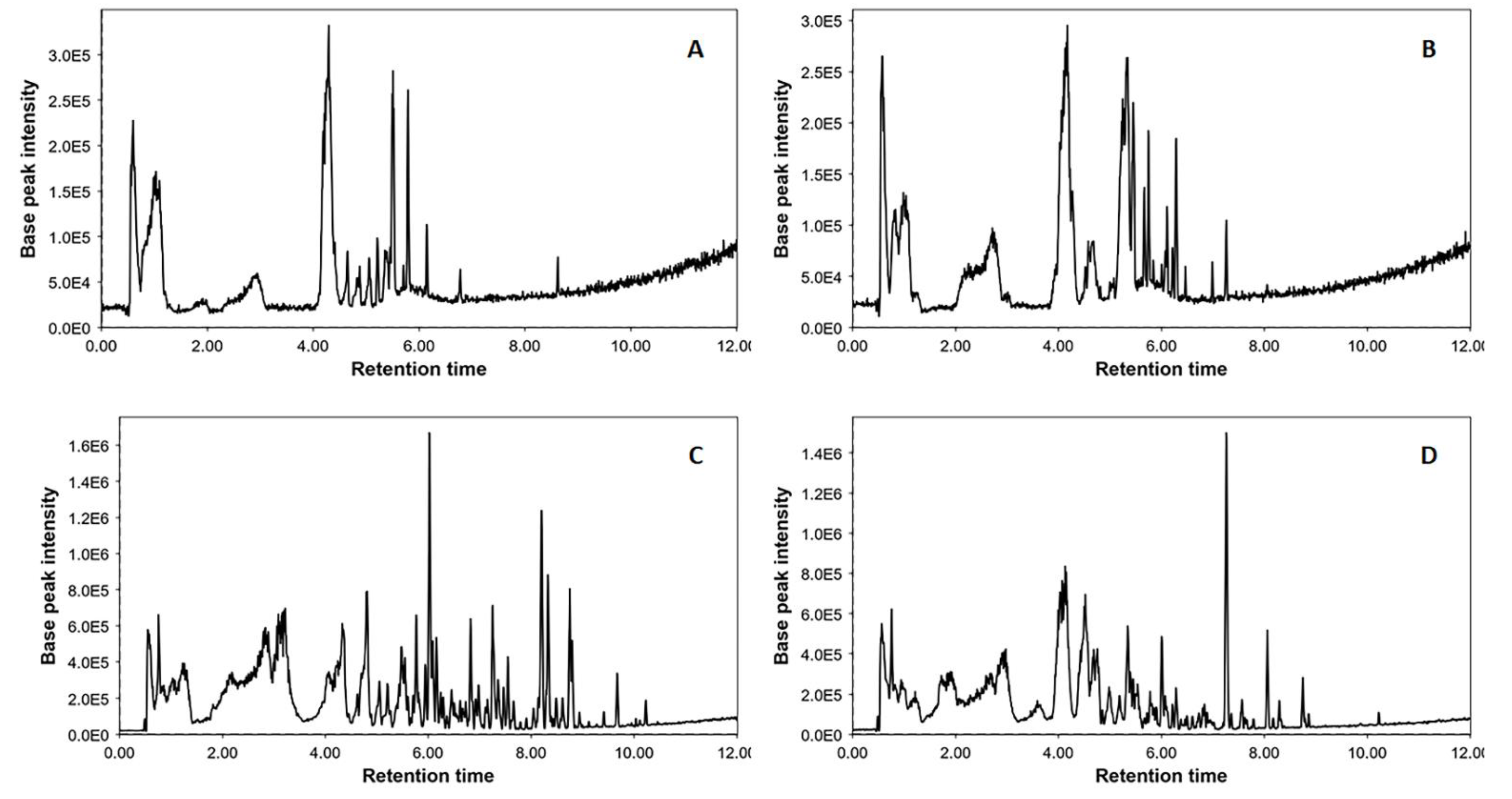
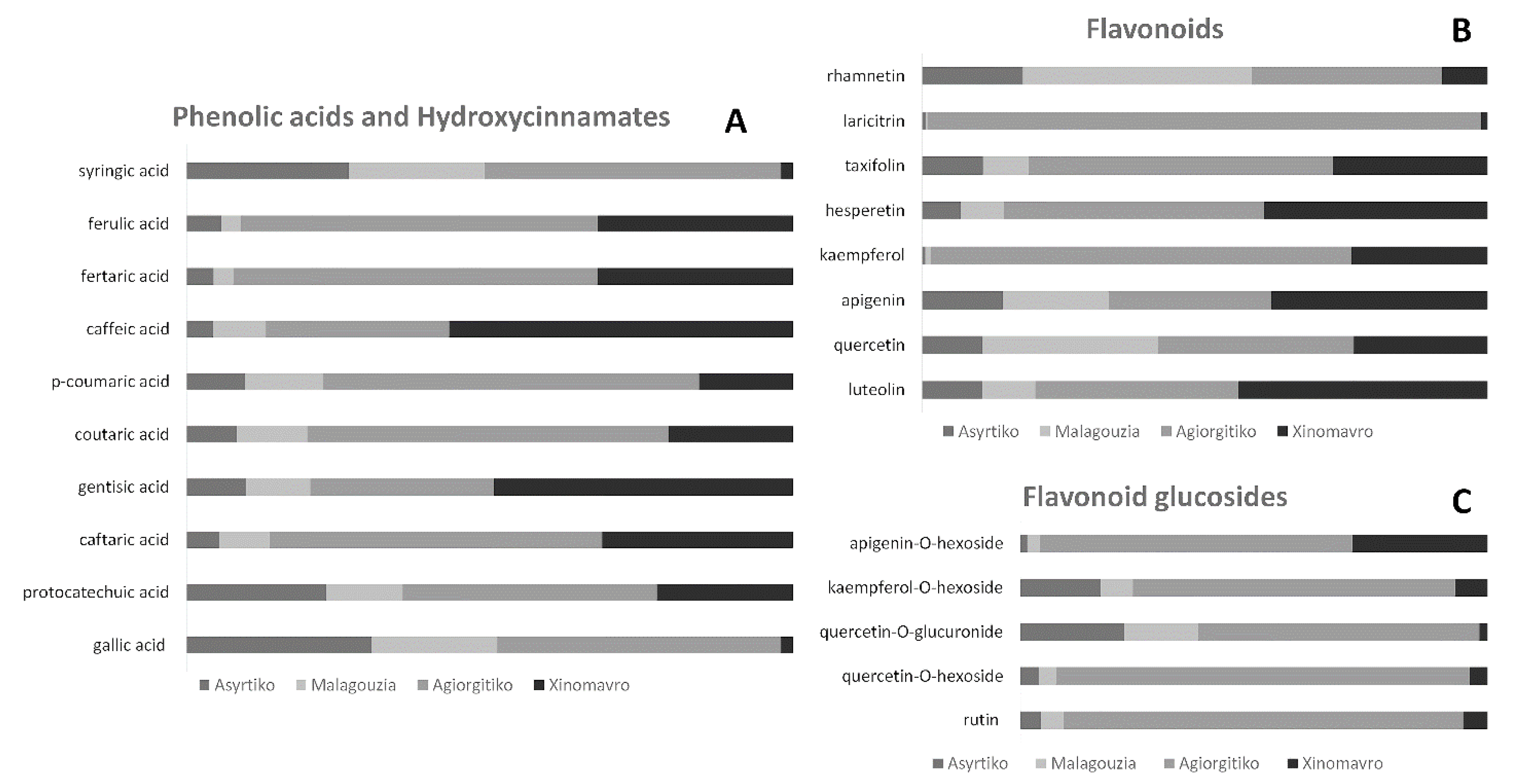
| Component Name | Retention Time (min) | Pseudomolecular Ion [M-H]− | Mass Error (ppm) | Molecular Formula |
|---|---|---|---|---|
| gallic acid | 1.02 | 169.014 | 1.053 | C7H5O5 |
| protocatechuic acid | 1.93 | 153.019 | 0.749 | C7H5O4 |
| hydroxytyrosol | 2.3 | 153.056 | 0.968 | C8H9O3 |
| caftaric acid | 2.86. | 311.041 | 0.848 | C13H11O9 |
| gentisic acid | 3.49 | 153.019 | 0.526 | C7H5O4 |
| coutaric acid | 4.39 | 295.046 | 0.998 | C13H11O8 |
| p-coumaric acid | 4.39 | 163.04 | −0.144 | C9H7O3 |
| caffeic acid | 4.76 | 179.035 | 1.149 | C9H7O4 |
| fertaric acid | 4.82 | 325.057 | 2.367 | C14H13O9 |
| ferulic acid | 4.83 | 193.051 | 2.154 | C10H9O4 |
| tyrosol | 5.45 | 137.061 | 1.194 | C8H9O2 |
| syringic acid | 5.5 | 197.046 | 2.384 | C9H9O5 |
| rutin | 5.69 | 609.146 | 0.796 | C27H29O16 |
| ellagic acid | 5.71 | 300.999 | 1.724 | C14H5O8 |
| quercetin-O-hexoside | 5.76 | 463.088 | 0.819 | C21H19O12 |
| quercetin-O-glucuronide | 5.79 | 477.067 | 0.706 | C21H17O13 |
| kaempferol-O-hexoside | 5.84 | 447.093 | 0.270 | C21H19O11 |
| piceid | 6.25 | 389.124 | −0.036 | C20H21O8 |
| taxifolin | 6.45 | 303.051 | 0.203 | C15H11O7 |
| apigenin-O-hexoside | 6.5 | 431.098 | 1.841 | C21H19O10 |
| astringin | 6.59 | 405.119 | 0.785 | C20H21O9 |
| chlorogenic acid | 6.65 | 353.088 | 1.944 | C16H17O9 |
| luteolin | 7.01 | 285.04 | 3.032 | C15H9O6 |
| quercetin | 7.03 | 301.035 | 2.187 | C15H9O7 |
| apigenin | 7.56 | 269.046 | 0.020 | C15H9O5 |
| kaempferol | 7.66 | 285.04 | 1.626 | C15H9O6 |
| hesperetin | 7.78 | 301.072 | 1.188 | C16H13O6 |
| laricitrin | 7.81 | 331.046 | 0.803 | C16H11O8 |
| rhamnetin | 7.81 | 315.051 | 0.242 | C16H11O7 |
| Sample Name | TPC (mg GA/g Extract) | |
|---|---|---|
| Red wines | Xinomavro | 267.1 |
| Agiorgitiko | 265.4 | |
| White wines | Assyrtiko | 155.7 |
| Malagouzia | 81.1 |
| Plasmid Relaxation Assay | |||
|---|---|---|---|
| Red Wines IC50 (μg/mL) | White Wines IC20 (μg/mL) | ||
| Xinomavro | Agiorgitiko | Assyrtiko | Malagouzia |
| 260.5 ± 27.4 a | 116.1 ± 19.4 b | 220.3 ± 14.1 | 150.1 ± 15.0 |
| Ames Test | ||
|---|---|---|
| Sample Name | IC50 (μg/mL) | |
| Red wines | Xinomavro | 8.0 ± 0.02 a |
| Agiorgitiko | 7.5 ± 0.37 a | |
| White wines | Assyrtiko | 16.9 ± 0.24 b |
| Malagouzia | 25.9 ± 0.30 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Terizi, K.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. https://doi.org/10.3390/metabo11070436
Tekos F, Makri S, Skaperda Z-V, Patouna A, Terizi K, Kyriazis ID, Kotseridis Y, Mikropoulou EV, Papaefstathiou G, Halabalaki M, et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites. 2021; 11(7):436. https://doi.org/10.3390/metabo11070436
Chicago/Turabian StyleTekos, Fotios, Sotiria Makri, Zoi-Vasiliki Skaperda, Anastasia Patouna, Kallirroi Terizi, Ioannis D. Kyriazis, Yorgos Kotseridis, Eleni Vaskani Mikropoulou, Georgios Papaefstathiou, Maria Halabalaki, and et al. 2021. "Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro" Metabolites 11, no. 7: 436. https://doi.org/10.3390/metabo11070436
APA StyleTekos, F., Makri, S., Skaperda, Z.-V., Patouna, A., Terizi, K., Kyriazis, I. D., Kotseridis, Y., Mikropoulou, E. V., Papaefstathiou, G., Halabalaki, M., & Demetrios, K. (2021). Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites, 11(7), 436. https://doi.org/10.3390/metabo11070436