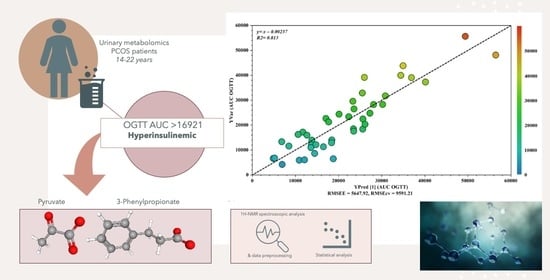
Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS)
Abstract
:1. Introduction
2. Results
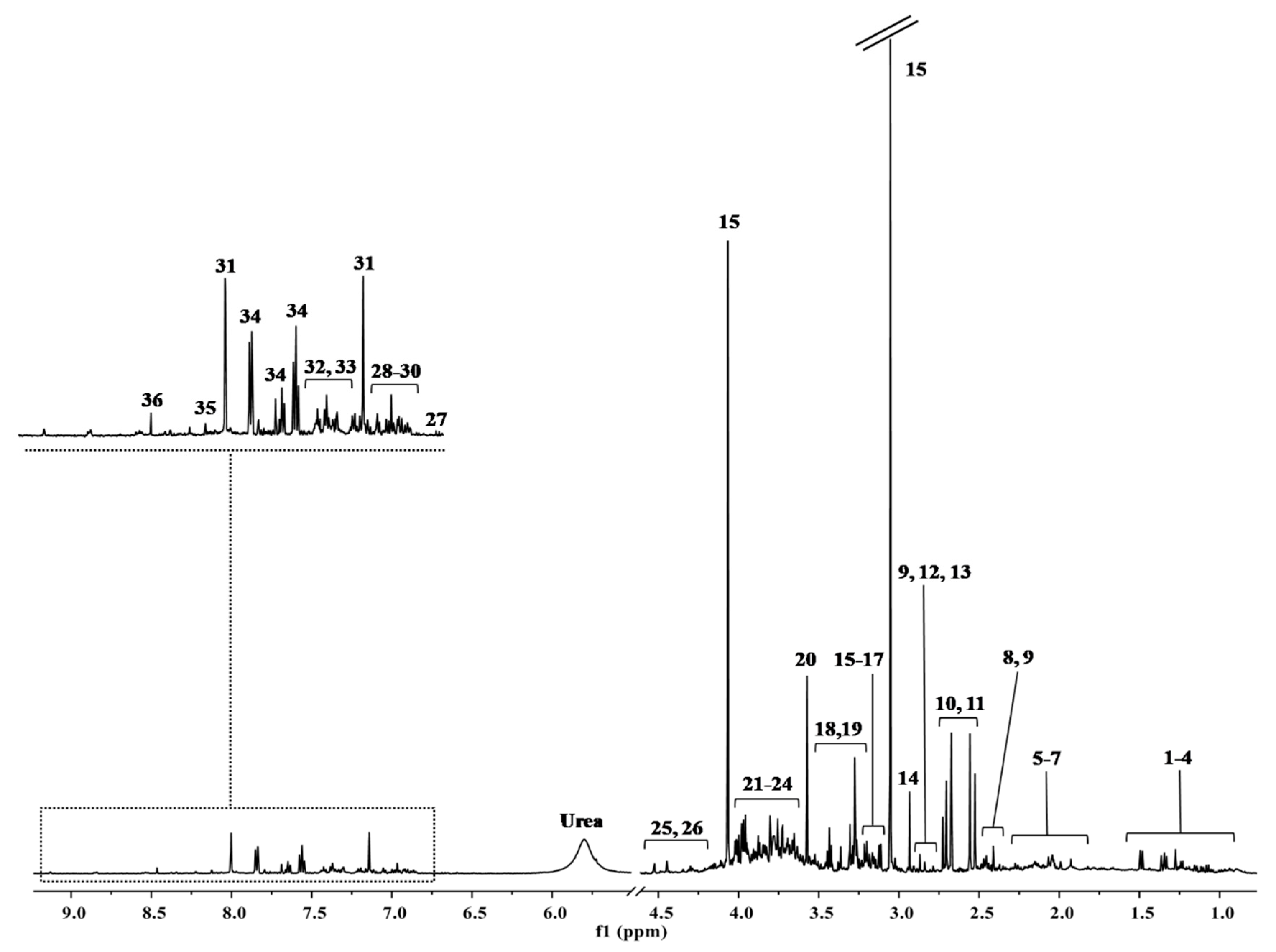
H-NMR Results
3. Discussion
4. Materials and Methods
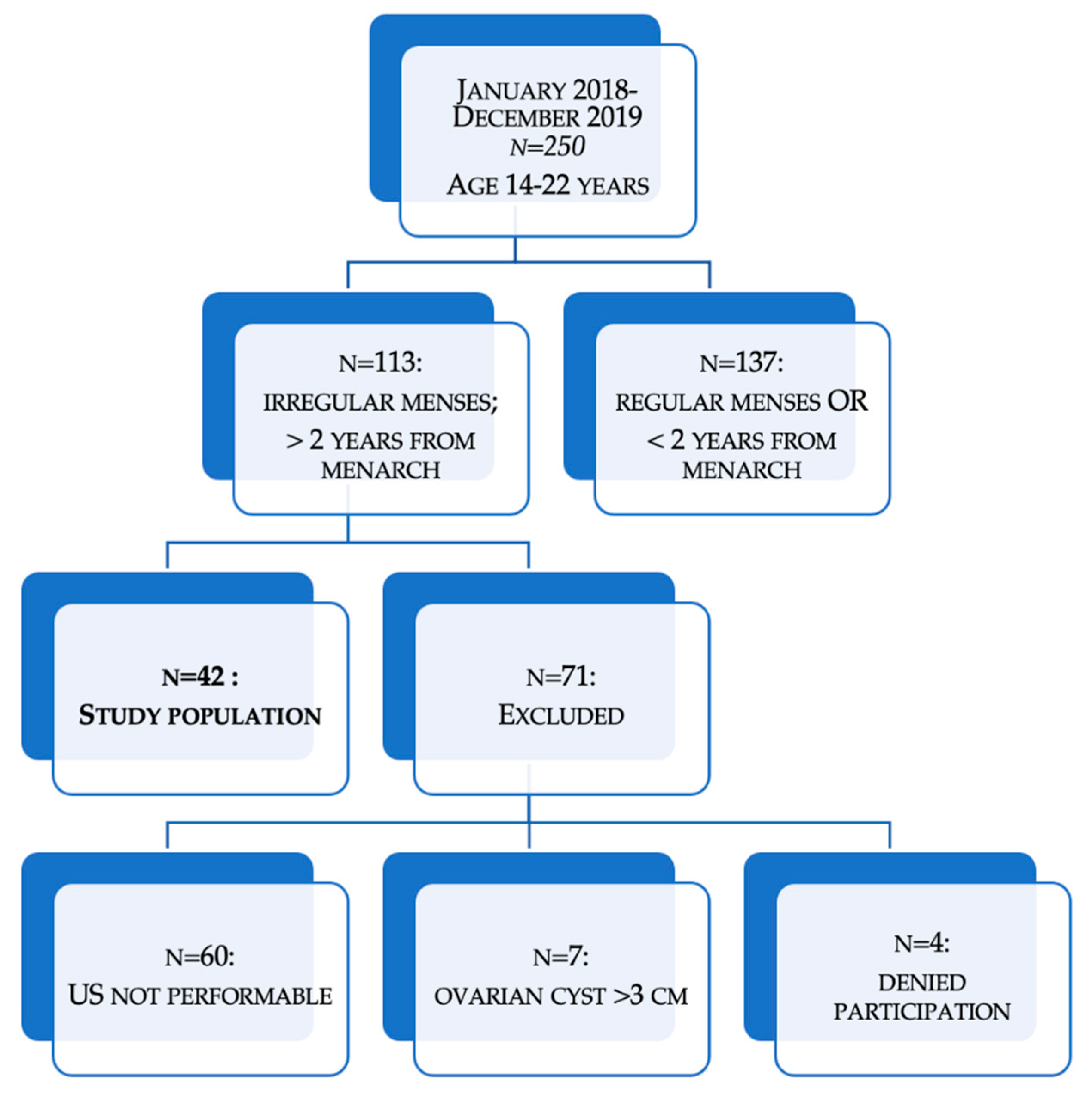
4.1. Study Population (Pilot Study)
4.2. Study Protocol
4.3. Assays
4.4. Urine Samples Preparation
4.5. 1H-NMR Spectroscopic Analysis
4.6. NMR Data Preprocessing
4.7. Data Analysis
4.8. Multivariate Statistical Analysis
4.9. Univariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norman, R.J.; Dewailly, D.; Legro, R.; Hickey, T. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kandarakis, H.; Legro, R.S. The Role of Genes and Environment in the Etiology of PCOS. Endocrine 2006, 30, 19–26. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.Y.; Lalia, A.Z.; Jenkins, G.D.; Dutta, T.; Carter, R.E.; Singh, R.J.; Nair, K.S. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism 2017, 71, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Huddleston, H.G.; Quinn, M.M.; Kao, C.-N.; Lenhart, N.; Rosen, M.P.; Cedars, M.I. Women with polycystic ovary syndrome demonstrate worsening markers of cardiovascular risk over the short-term despite declining hyperandrogenaemia: Results of a longitudinal study with community controls. Clin. Endocrinol. 2017, 87, 775–782. [Google Scholar] [CrossRef]
- Carmina, E.; Campagna, A.M.; Lobo, R.A. Emergence of ovulatory cycles with aging in women with polycystic ovary syndrome (PCOS) alters the trajectory of cardiovascular and metabolic risk factors. Hum. Reprod. 2013, 28, 2245–2252. [Google Scholar] [CrossRef] [Green Version]
- Fulghesu, A.M.; Angioni, S.; Portoghese, E.; Milano, F.; Batetta, B.; Paoletti, A.M.; Melis, G.B. Failure of the homeostatic model assessment calculation score for detecting metabolic deterioration in young patients with polycystic ovary syndrome. Fertil. Steril. 2006, 86, 398–404. [Google Scholar] [CrossRef]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef]
- Angioni, S.; Portoghese, E.; Milano, F.; Melis, G.B.; Fulghesu, A.M. Diagnosis of Metabolic Disorders in Women with Polycystic Ovary Syndrome. Obstet. Gynecol. Surv. 2008, 63, 796–802. [Google Scholar] [CrossRef]
- Fulghesu, A.; Magnini, R.; Portoghese, E.; Angioni, S.; Minerba, L.; Melis, G.B. Obesity-Related Lipid Profile and Altered Insulin Incretion in Adolescents with Polycystic Ovary Syndrome. J. Adolesc. Health 2010, 46, 474–481. [Google Scholar] [CrossRef]
- Burghen, G.A.; Givens, J.R.; Kitabchi, A.E. Correlation of Hyperandrogenism with Hyperinsulinism in Polycystic Ovarian Disease. J. Clin. Endocrinol. Metab. 1980, 50, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.; Andersen, M.; Azziz, R.; et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [Green Version]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef] [Green Version]
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound Peripheral Insulin Resistance, Independent of Obesity, in Polycystic Ovary Syndrome. Diabetes 1989, 38, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Molin, F.D.; Zamboni, F.; Saggiorato, E.; Salvagno, G.L.; Fiers, T.; Kaufman, J.-M.; Bonora, E.; Moghetti, P. Serum Androgens Are Independent Predictors of Insulin Clearance but Not of Insulin Secretion in Women With PCOS. J. Clin. Endocrinol. Metab. 2020, 105, e1981–e1989. [Google Scholar] [CrossRef] [PubMed]
- Arduc, A.; Sarıçam, O.; Dogan, B.A.; Tuna, M.M.; Tütüncü, Y.A.; Isik, S.; Berker, D.; Sennaroglu, E.; Guler, S. Should insulin resistance be screened in lean hirsute women? Gynecol. Endocrinol. 2014, 31, 291–295. [Google Scholar] [CrossRef]
- Toprak, S.; Yonem, A.; Çakır, B.; Güler, S.; Azal, Ö.; Özata, M.; Çorakçı, A. Insulin Resistance in Nonobese Patients with Polycystic Ovary Syndrome. Horm. Res. Paediatr. 2001, 55, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ciampelli, M.; Fulghesu, A.M.; Cucinelli, F.; Pavone, V.; Caruso, A.; Mancuso, S.; Lanzone, A. Heterogeneity in beta cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum. Reprod. 1997, 12, 1897–1901. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil. Steril. 2004, 82, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.P.; Baker, V.M.; DiMarino, P.; Gimpel, T.; Castracane, V. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: A comparison of two distinct populations. Am. J. Obstet. Gynecol. 2002, 187, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Yeckel, C.W.; Weiss, R.; Dziura, J.; Taksali, S.E.; Dufour, S.; Burgert, T.S.; Tamborlane, W.V.; Caprio, S. Validation of Insulin Sensitivity Indices from Oral Glucose Tolerance Test Parameters in Obese Children and Adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Angioni, S.; Sanna, S.; Magnini, R.; Melis, G.B.; Fulghesu, A.M. The quantitative insulin sensitivity check index is not able to detect early metabolic alterations in young patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2011, 27, 468–474. [Google Scholar] [CrossRef]
- Lanzone, A.; Fulghesu, A.M.; Andreani, C.L.; Apa, R.; Fortini, A.; Caruso, A.; Mancuso, S. Insulin secretion in polycystic ovarian disease: Effect of ovarian suppression by GnRH agonist. Hum. Reprod. 1990, 5, 143–149. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Santagni, S.; Ricchieri, F.; Campedelli, A.; Rattighieri, E.; Chierchia, E.; Marini, G.; Despini, G.; Prati, A.; Simoncini, T. Myo-inositol modulates insulin and luteinizing hormone secretion in normal weight patients with polycystic ovary syndrome: Myo-inositol modulates insulin and LH in lean PCOS. J. Obstet. Gynaecol. Res. 2014, 40, 1353–1360. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Xie, Y.; Hu, J.; Yang, S.; Lin, M. Prevalence and degree of insulin resistance in Chinese Han women with PCOS: Results from euglycemic-hyperinsulinemic clamps. Clin. Endocrinol. 2018, 90, 138–144. [Google Scholar] [CrossRef]
- Nicholson, J.; Lindon, J. Metabonomics. Nat. Cell Biol. 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Angioni, S.; D’Alterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2021, 28, 728–735. [Google Scholar] [CrossRef]
- Ezeh, U.; Chen, Y.I.; Azziz, R. Racial and ethnic differences in the metabolic response of polycystic ovary syndrome. Clin. Endocrinol. 2020, 93, 163–172. [Google Scholar] [CrossRef]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [Green Version]
- Haukka, J.K.; Sandholm, N.; Forsblom, C.; Cobb, J.E.; Groop, P.-H.; Ferrannini, E. Metabolomic Profile Predicts Development of Microalbuminuria in Individuals with Type 1 Diabetes. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [Green Version]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: Did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 483–490. [Google Scholar] [CrossRef]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clin. Med. 2015, 15 (Suppl. 6), s72–s76. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Arya, B.K.; Haq, A.U.; Chaudhury, K. Oocyte quality reflected by follicular fluid analysis in poly cystic ovary syndrome (PCOS): A hypothesis based on intermediates of energy metabolism. Med. Hypotheses 2012, 78, 475–478. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, Y.; Yin, A.; Liu, S.; Chen, M.; Lv, X.; Nie, X.; Tan, N.; Zhang, Z. Non-invasive urinary metabolomics reveals metabolic profiling of polycystic ovary syndrome and its subtypes. J. Pharm. Biomed. Anal. 2020, 185, 113262. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, Y.; Chen, M.; Tan, N.; Liu, S.; Nie, X.; Zhou, W. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yin, T.-L.; Yang, J.; Xiong, C.-L. Follicular metabolic changes and effects on oocyte quality in polycystic ovary syndrome patients. Oncotarget 2017, 8, 80472–80480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan-Goor, M.; Sasson, S.; Davarashvili, A.; Almagor, M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum. Reprod. 1997, 12, 2508–2510. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Hu, W.; Liu, Q.; Hao, Q.; Sun, B.; Zhang, Q.; Mao, S.; Qiao, J.; Yan, X. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 2012, 11, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, L.; Li, R.; Wang, L.-N.; Yang, Y.; Liu, N.-N.; Zhang, C.-M.; Wang, Y.; Liu, P.; Tu, B.-B.; et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferriman, D.D.; Gallwey, M.J.D. Clinical Assessment of Body Hair Growth in Women. J. Clin. Endocrinol. Metab. 1961, 21, 1440–1447. [Google Scholar] [CrossRef]
- Cremoncini, C.; Vignati, E.; Libroia, A. Treatment of hirsutism and acne in women with two combinations of cyproterone acetate and ethinylestradiol. Acta Eur. Fertil. 1976, 7, 299–314. [Google Scholar] [PubMed]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic review of endometriosis pain assessment: How to choose a scale? Hum. Reprod. Updat. 2015, 21, 136–152. [Google Scholar] [CrossRef]
- Carmina, E.; Oberfield, S.E.; Lobo, R.A. The diagnosis of polycystic ovary syndrome in adolescents. Am. J. Obstet. Gynecol. 2010, 203, 201.e1–201.e5. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric Acute-onset Neuropsychiatric Syndrome and Mycoplasma Pneumoniae Infection: A Case Report Analysis with a Metabolomics Approach. Curr. Pediatr. Rev. 2020, 16, 183–193. [Google Scholar] [CrossRef]
- Piras, C.; Arisci, N.; Poddighe, S.; Liggi, S.; Mariotti, S.; Atzori, L. Metabolomic profile in hyperthyroid patients before and after antithyroid drug treatment: Correlation with thyroid hormone and TSH concentration. Int. J. Biochem. Cell Biol. 2017, 93, 119–128. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A 2016, 1430, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Fulghesu, A.M.; Canu, E.; Casula, L.; Melis, F.; Gambineri, A. Polycystic Ovarian Morphology in Normocyclic Non-Hyperandrogenic Adolescents. J. Pediatr. Adolesc. Gynecol. 2021. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Ruiz, P.; García-Hermoso, A.; Cavero-Redondo, I.; Pozuelo-Carrascosa, D.P.; Martínez-Vizcaíno, V.; Solera-Martinez, M. Homeostasis Model Assessment cut-off points related to metabolic syndrome in children and adolescents: A systematic review and meta-analysis. Eur. J. Pediatr. 2019, 178, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.; Pibiri, M.; Leoni, V.P.; Balsamo, A.; Tronci, L.; Arisci, N.; Mariotti, S.; Atzori, L. Analysis of metabolomics profile in hypothyroid patients before and after thyroid hormone replacement. J. Endocrinol. Investig. 2021, 44, 1309–1319. [Google Scholar] [CrossRef]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted Profiling: Quantitative Analysis of 1H NMR Metabolomics Data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]

| BMI 16–24.99 | BMI 25–41 | p-Value | ||
|---|---|---|---|---|
| N. of patients | 42 | 20 | 22 | |
| Age | 18.85 ± 4.45 | 18.85 ± 4.78 | 18.86 ± 4.23 | NS |
| BMI (kg/m2) | 26.33 ± 5.94 | 21.42 ± 2.21 | 30.61 ± 4.67 | <0.001 |
| Ferriman & Gallwey score | 12.48 ± 5.36 | 12.15± 5.48 | 12.77 ± 5.36 | NS |
| Cremoncini score | 1.36 ± 1.39 | 1.55 ± 1.54 | 1.18 ± 1.26 | NS |
| Dysmenorrhea (VAS) | 2.74 ± 2.98 | 3.3 ± 3.45 | 2.23 ± 2.45 | NS |
| Familiarity with diabetes (%) | 59.52% | 40% | 77.27% | 0.01 |
| Glycaemia (mmol/L) | 4.55 ± 0.52 | 4.47 ± 0.68 | 4.62 ± 0.32 | NS |
| Insulin (pmol/L) | 119.13 ± 57.5 | 96.31 ± 43.97 | 140.04 ± 61.32 | 0.01 |
| HOMA | 3.49 ± 1.77 | 2.76 ± 1.34 | 4.16 ± 1.87 | 0.01 |
| AUC (pmol/L) | 149,019.81 ± 87,376.84 | 113,639.8 ± 63,511.19 | 181,388.27 ± 94,805.59 | 0.01 |
| Insulin 120′ (pmol/L) | 1003.76 ± 638.25 | 736.5 ± 462.49 | 1276.03 ± 675.18 | <0.01 |
| Insulin 180′ (pmol/L) | 564.12 ± 504.24 | 391.03 ± 376.81 | 723.02 ± 560.71 | 0.03 |
| Insulin—max. value (pmol/L) | 1289.52 ± 782.94 | 1051.18 ± 652.52 | 1507.97 ± 842.15 | NS |
| Glyc. 120′ (mmol/L) | 6.31 ± 1.33 | 5.92 ± 1.11 | 6.63 ± 1.43 | NS |
| Glyc 180′ (mmol/L) | 5.65 ± 1.8 | 5.47 ± 1.68 | 5.81 ± 1.93 | NS |
| Total cholesterol (nmol/L) | 4.36 ± 0.9 | 4.4 ± 1.02 | 4.33 ± 0.75 | NS |
| HDL (nmol/L) | 1.49 ± 0.46 | 1.54 ± 0.44 | 1.42 ± 0.48 | NS |
| LDL (nmol/L) | 2.51 ± 0.7 | 2.75 ± 0.68 | 2.2 ± 0.65 | NS |
| Triglyceride (nmol/L) | 0.82 ± 0.4 | 0.76 ± 0.26 | 0.9 ± 0.52 | NS |
| FSH (Ul/L) | 5.51 ± 1.86 | 5.66 ± 1.99 | 5.38 ± 1.78 | NS |
| LH (UI/L) | 6.84 ± 7.72 | 5.59 ± 3.54 | 7.92 ± 10 | NS |
| E2 (pmol/l) | 157.77 ± 114.23 | 133.99 ± 74.7 | 179.39 ± 139.29 | NS |
| T (nmol/l) | 1.54 ± 0.92 | 1.44 ± 0.83 | 1.63 ± 1.01 | NS |
| A (nmol/l) | 10.58 ± 5.15 | 9.77 ± 3.98 | 11.31 ± 6.02 | NS |
| PRL (mg/mL) | 13.98 ± 7.56 | 14.38 ± 8.99 | 13.59 ± 6.09 | NS |
| 17-OHP (nmol/L) | 2.75 ± 1.8 | 2.47 ± 1.61 | 3.03 ± 1.97 | NS |
| SHBG (nmol/L) | 54.44 ± 21.22 | 61.84 ± 18.99 | 44.39 ± 20.5 | 0.02 |
| AMH (pmol/L) | 60.34 ± 35.18 | 59.78 ± 30.97 | 61.13 ± 41.66 | NS |
| Ovarian volume (mm3) | 6084.16 ± 2753.42 | 5515.08 ± 2302.23 | 7080.04 ± 3334.58 | NS |
| N. of ovarian follicles | 11.42 ± 5.28 | 11.33 ± 4.5 | 11.54 ± 6.41 | NS |
| Insulin Sensibility | Insulin Secretion | |||||
|---|---|---|---|---|---|---|
| HOMA ≤ 3.6 | HOMA > 3.6 | p-Value | AUC ≤ 16,921 | AUC > 16,921 | p-Value | |
| N. of patients | 27 | 15 | 18 | 24 | ||
| Age | 19.19 ± 4.7 | 18.21 ± 4.02 | NS | 19.94 ± 4.52 | 18.08 ± 4.33 | NS |
| BMI (kg/m2) | 24.2 ± 4.22 | 30.16 ± 6.76 | <0.01 | 24.06 ± 3.29 | 28.03 ± 6.92 | 0.03 |
| Glycaemia (mmol/L) | 4.49 ± 0.6 | 4.65 ± 0.33 | NS | 4.45 ± 0.71 | 4.62 ± 0.31 | NS |
| Insulin (pmol/L) | 83.59 ± 18.18 | 183.09 ± 47.41 | <0.01 | 101.92 ± 52.01 | 132.03 ± 59.06 | NS |
| HOMA | 2.41 ± 0.63 | 5.45 ± 1.43 | <0.01 | 2.92 ± 1.6 | 3.92 ± 1.8 | NS |
| AUC (pmol/L) | 135,600.04 ± 85,392.28 | 173,175.38 ± 88,568.16 | NS | 73,130.77 ± 25,959.05 | 205,936.58 ± 72,277.93 | <0.01 |
| Insulin 120′ (pmol/L) | 907.18 ± 597.45 | 1218.54 ± 678.63 | NS | 467.36 ± 196.42 | 1431.64 ± 529.28 | <0.01 |
| Insulin 180′ (pmol/L) | 496.19 ± 488.56 | 686.38 ± 525.76 | NS | 283.47 ± 163.44 | 774.6 ± 570.64 | <0.01 |
| Insulin—max. value (pmol/L) | 1260.06 ± 873.65 | 1342.56 ± 610.75 | NS | 657.48 ± 295.72 | 1763.55 ± 693.54 | <0.01 |
| Glyc. 120′ (mmol/L) | 6 ± 1.12 | 6.87 ± 1.53 | NS | 5.77 ± 1.01 | 6.66 ± 1.41 | 0.05 |
| Glyc. 180′ (mmol/L) | 5.42 ± 1.9 | 6.11 ± 1.57 | NS | 5.69 ± 2.23 | 5.63 ± 1.51 | NS |
| Total cholesterol (nmol/L) | 4.34 ± 0.8 | 4.42 ± 1.14 | NS | 4.31 ± 0.85 | 4.41 ± 0.95 | NS |
| HDL (nmol/L) | 1.54 ± 0.47 | 1.37 ± 0.42 | NS | 1.47 ± 0.41 | 1.5 ± 0.5 | NS |
| LDL (nmol/L) | 2.63 ± 0.65 | 2.25 ± 0.79 | NS | 2.36 ± 0.41 | 2.6 ± 0.84 | NS |
| Triglyceride (nmol/L) | 0.8 ± 0.36 | 0.87 ± 0.51 | NS | 0.87 ± 0.48 | 0.78 ± 0.34 | NS |
| FSH (Ul/L) | 5.59 ± 1.76 | 5.36 ± 2.08 | NS | 5.46 ± 1.38 | 5.54 ± 2.17 | NS |
| LH (UI/L) | 6.16 ± 4.88 | 8.02 ± 11.19 | NS | 5.07 ± 3.54 | 8.1 ± 9.53 | NS |
| E2 (pmol/l) | 151.68 ± 93.86 | 168.73 ± 147.15 | NS | 126.78 ± 76 | 181.01 ± 132.96 | NS |
| T (nmol/l) | 1.42 ± 0.78 | 1.76 ± 1.14 | NS | 1.54 ± 0.87 | 1.54 ± 0.98 | NS |
| A (nmol/l) | 9.59 ± 3.54 | 12.58 ± 7.18 | NS | 9.69 ± 4.33 | 11.3 ± 5.73 | NS |
| PRL (mg/mL) | 14.57 ± 8.38 | 12.94 ± 5.99 | NS | 16.32 ± 9.19 | 12.14 ± 5.53 | NS |
| 17-OHP (nmol/L) | 2.69 ± 1.85 | 2.86 ± 1.76 | NS | 2.78 ± 1.74 | 2.72 ± 1.9 | NS |
| SHBG (nmol/L) | 55.58 ± 21.51 | 51.82 ± 21.44 | NS | 63.24 ± 18.96 | 47.11 ± 20.65 | 0.03 |
| AMH (pmol/L) | 63.35 ± 35.22 | 54.02 ± 36.11 | NS | 75.47 ± 38.08 | 50.79 ± 30.47 | NS |
| Ovarian volume (mm3) | 5715.4 ± 2300.42 | 7067.51 ± 3790.79 | NS | 7522.75 ± 3294.87 | 5262.1 ± 2097.55 | NS |
| N. of ovarian follicles | 9.95 ± 2.7 | 13.75 ± 7.39 | 0.05 | 11.83 ± 5.59 | 11.16 ± 5.22 | NS |
| OPLS Models | Permutation (500 Times) * | |||||
|---|---|---|---|---|---|---|
| Components a | R2Xcum b | R2Ycum c | Q2cum d | R2 Intercept | Q2 Intercept | |
| OPLS model | 1P + 1O | 0.216 | 0.684 | 0.500 | 0.362 | −0.516 |
| (a) | ||||
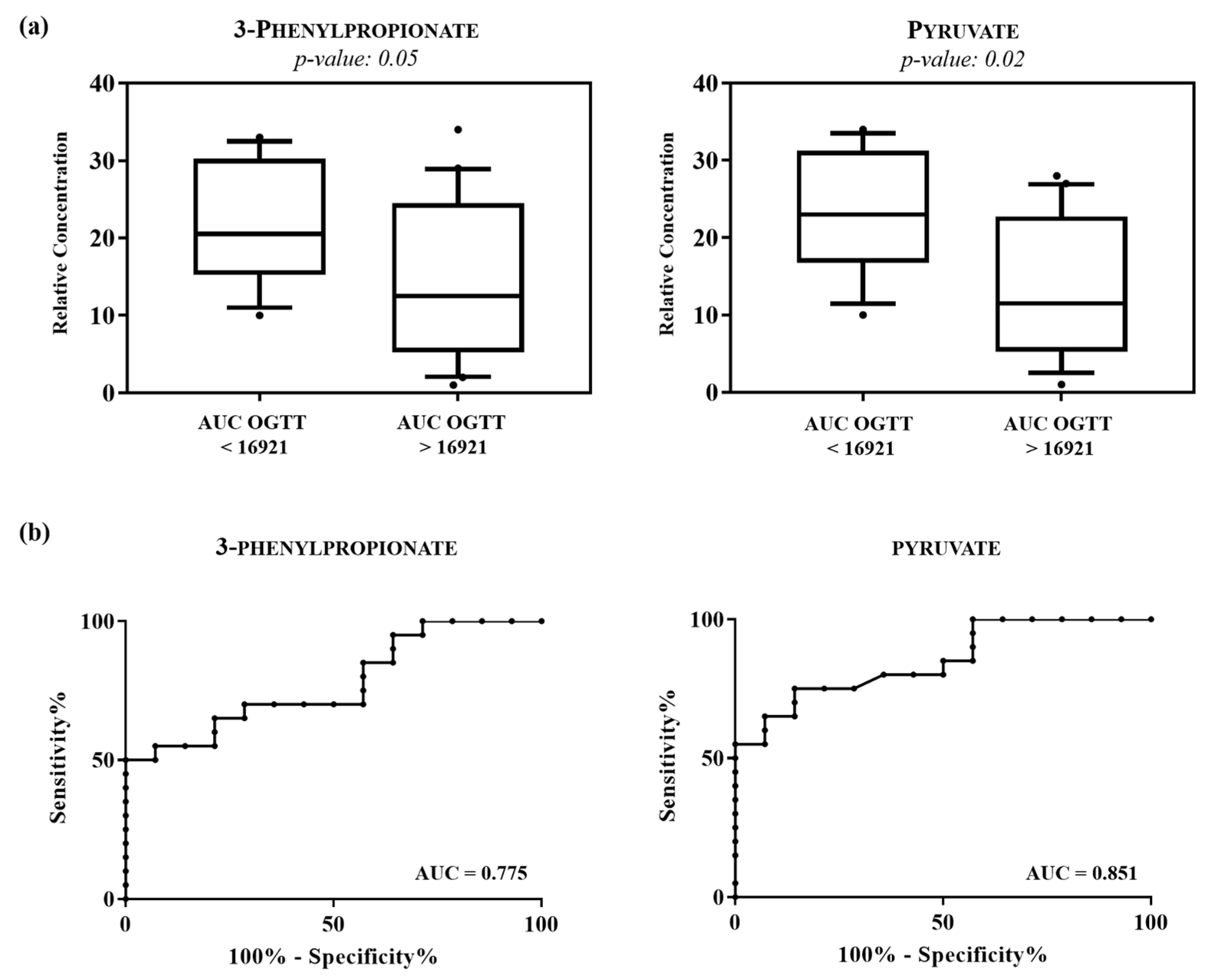
| Metabolites | Mean (SD) of Group (mM) a | p-Value b | ||
| AUC OGTT < 16,921 | AUC OGTT > 16,921 | |||
| 3-phenylpropionate | 1.945 ± 0.59 | 1.443 ± 0.71 | 0.05 | |
| Pyruvate | 3.489 ± 1.21 | 1.462 ± 0.57 | 0.02 | |
| (b) | ||||
| AUC | Standard Error | Confidence Interval 95% | p-Value | |
| 3-phenylpropionate | 0.775 | 0.07 | 0.619–0.930 | <0.01 |
| Pyruvate | 0.851 | 0.06 | 0.726–0.977 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulghesu, A.M.; Piras, C.; Dessì, A.; Succu, C.; Atzori, L.; Pintus, R.; Gentile, C.; Angioni, S.; Fanos, V. Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS). Metabolites 2021, 11, 437. https://doi.org/10.3390/metabo11070437
Fulghesu AM, Piras C, Dessì A, Succu C, Atzori L, Pintus R, Gentile C, Angioni S, Fanos V. Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS). Metabolites. 2021; 11(7):437. https://doi.org/10.3390/metabo11070437
Chicago/Turabian StyleFulghesu, Anna Maria, Cristina Piras, Angelica Dessì, Claudia Succu, Luigi Atzori, Roberta Pintus, Cecilia Gentile, Stefano Angioni, and Vassilios Fanos. 2021. "Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS)" Metabolites 11, no. 7: 437. https://doi.org/10.3390/metabo11070437
APA StyleFulghesu, A. M., Piras, C., Dessì, A., Succu, C., Atzori, L., Pintus, R., Gentile, C., Angioni, S., & Fanos, V. (2021). Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS). Metabolites, 11(7), 437. https://doi.org/10.3390/metabo11070437