Evaluating the Robustness of Biomarkers of Dairy Food Intake in a Free-Living Population Using Single- and Multi-Marker Approaches
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Validation Sub-Cohort
2.2. Intake Levels of Different Dairy Products
2.3. Assessment of Biomarkers for Milk Intake
2.4. Assessment of Biomarkers for Cheese Intake
2.5. Assessment of Biomarkers for Yoghurt Intake
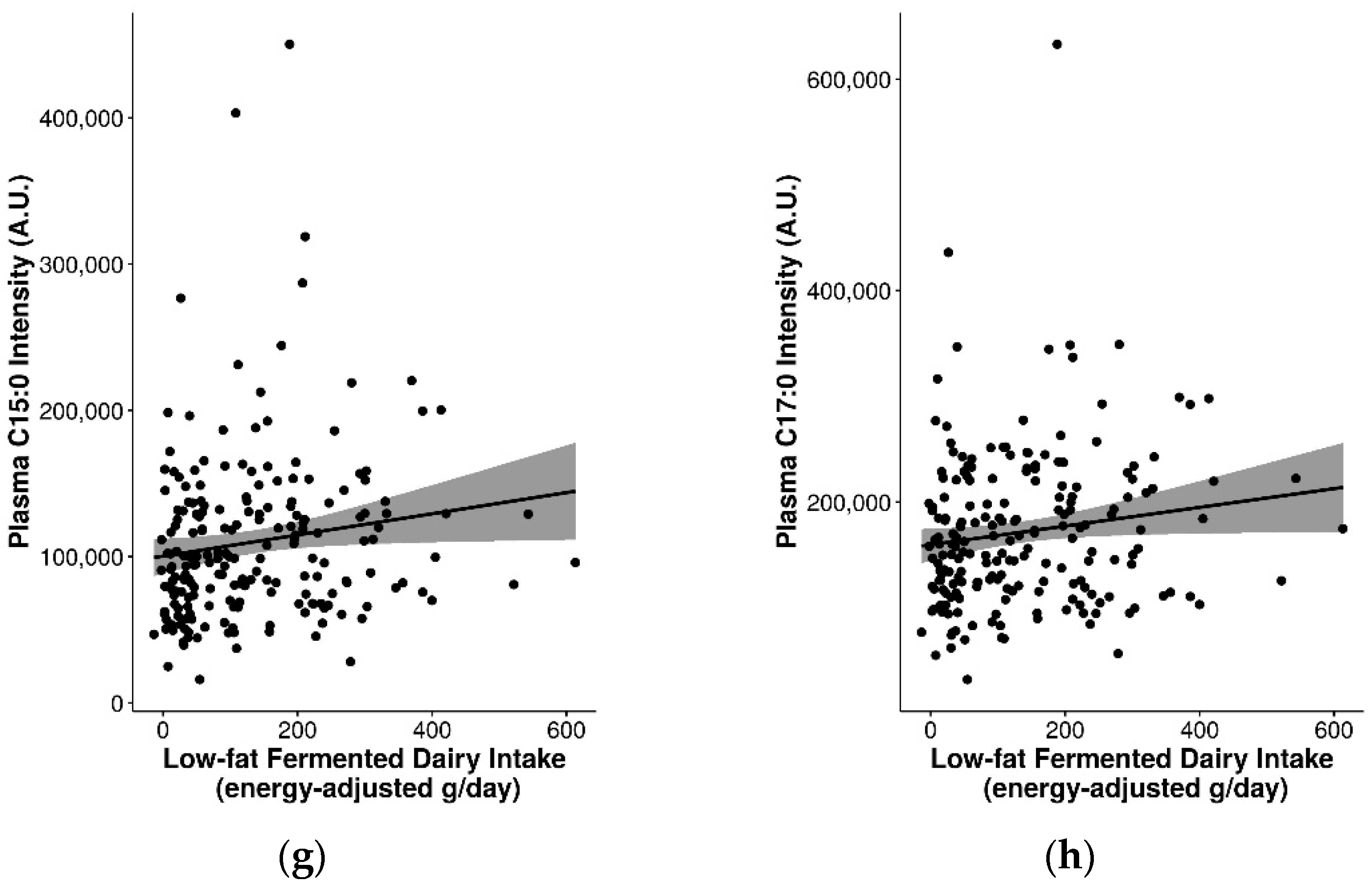
2.6. Assessment of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) as Biomarkers for General Dairy Intake
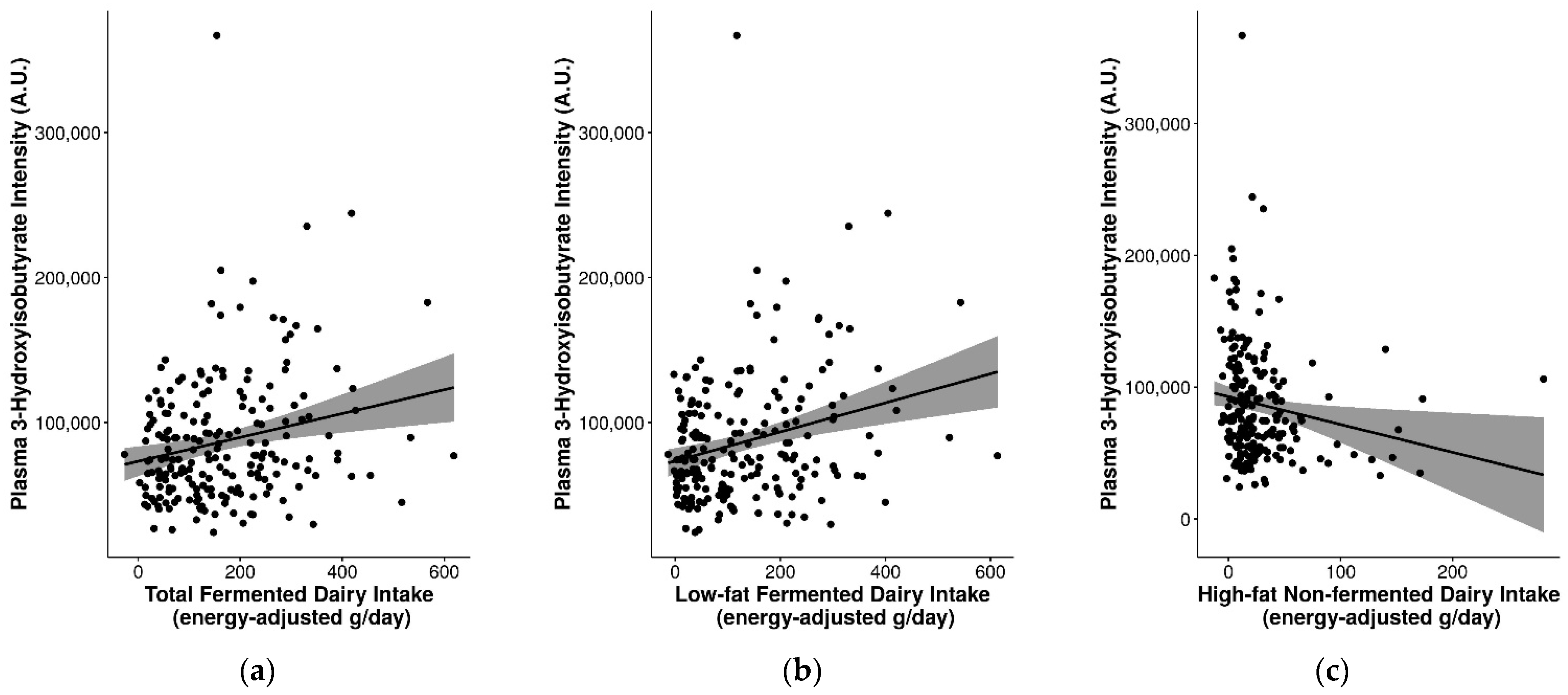
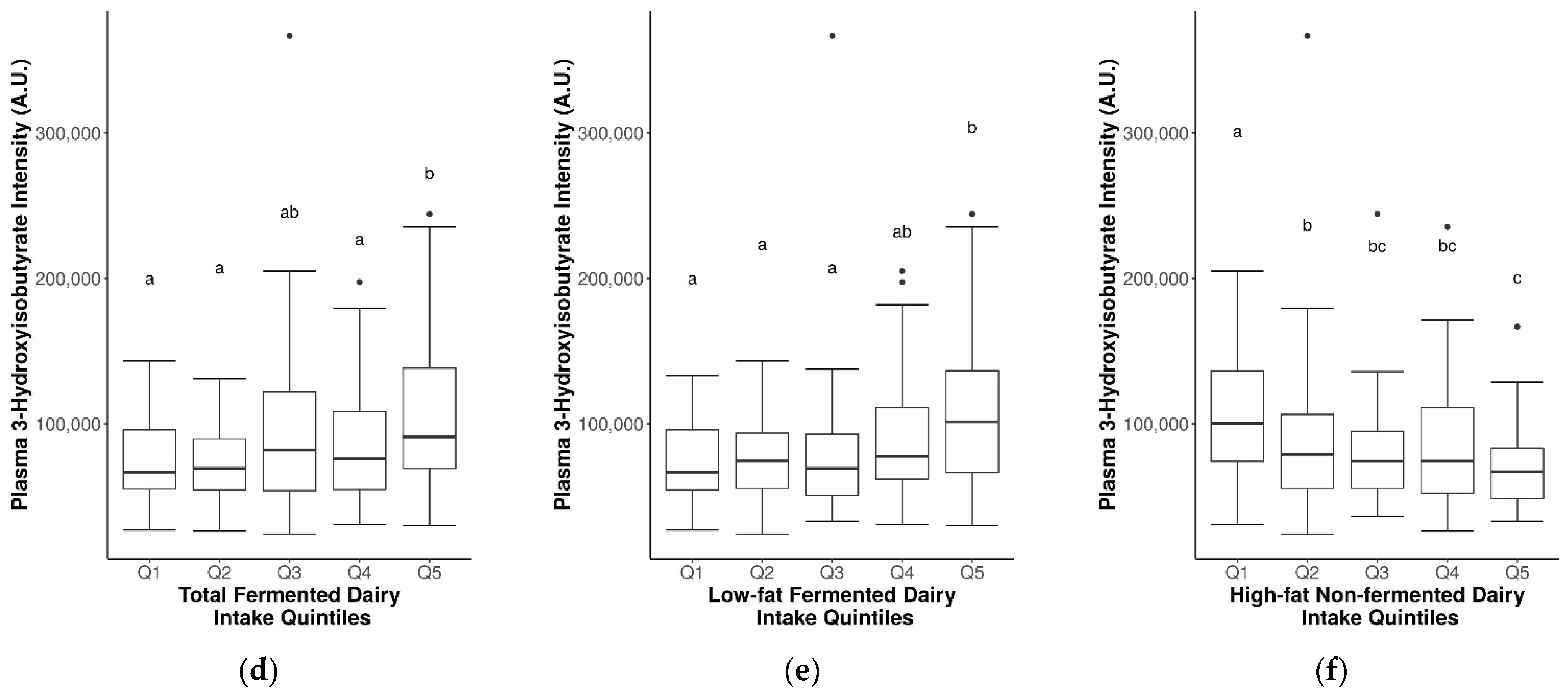
2.7. Suitability of Biomarkers for Discriminating between Fermented and Non-Fermented Dairy Intake
3. Discussion
3.1. Biomarkers for General Dairy Intake, Dairy Food Intake, and Their Specificity
3.2. Single- versus Multi-Marker Models for Evaluating the Robustness of FIBs
3.3. Evaluation of Other Facets of Validity
3.4. Influence of Fat Content and Fermentation on Dairy Biomarkers
3.5. Influence of Genetic Variants on Biomarkers of Milk Intake
3.6. Study Limitations
4. Materials and Methods
4.1. Study Population
4.2. Food Frequency Questionnaire and Levels of Dairy Food Consumption
4.3. LC-MS Sample Preparation and Analysis
4.4. GC-MS Sample Preparation and Analysis
4.5. Previously-Identified Candidate Biomarkers, Analytical Standards and Reagents
4.6. Determination of Lactase, FUT2, and FUT3 Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapaj, A.; Deci, E.; Watson, R.R. World milk production and socio-economic factors effecting its consumption A2. In Dairy in Human Health and Disease across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 107–115. [Google Scholar]
- Fontecha, J.; Calvo, M.V.; Juarez, M.; Gil, A.; Martínez-Vizcaino, V. Milk and Dairy Product Consumption and Cardiovascular Diseases: An Overview of Systematic Reviews and Meta-Analyses. Adv. Nutr. 2019, 10, S164–S189. [Google Scholar] [CrossRef]
- Yu, E.; Hu, F.B. Dairy Products, Dairy Fatty Acids, and the Prevention of Cardiometabolic Disease: A Review of Recent Evidence. Curr. Atheroscler. Rep. 2018, 20, 24. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and Cardiovascular Disease: Do We Really Need to Be Concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, T.T.; Virtanen, H.E.K.; Voutilainen, S.; Tuomainen, T.-P.; Mursu, J.; Virtanen, J.K. Intake of fermented and non-fermented dairy products and risk of incident CHD: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2018, 120, 1288–1297. [Google Scholar] [CrossRef]
- Buendia, J.R.; Li, Y.; Hu, F.B.; Cabral, H.J.; Bradlee, M.L.; Quatromoni, P.A.; Singer, M.R.; Curhan, G.C.; Moore, L.L. Regular Yogurt Intake and Risk of Cardiovascular Disease Among Hypertensive Adults. Am. J. Hypertens. 2018, 31, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part II: Bioactive peptide functions. Biotechnol. J. 2007, 2, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Burton, K.J.; Rosikiewicz, M.; Pimentel, G.; Bütikofer, U.; Von Ah, U.; Voirol, M.-J.; Croxatto, A.; Aeby, S.; Drai, J.; McTernan, P.G.; et al. Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. Br. J. Nutr. 2017, 117, 1312–1322. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Brennan, L.; Drevon, C.A.; Van Kranen, H.; Manach, C.; Dragsted, L.O.; Roche, H.; Andres-Lacueva, C.; Bakker, S.J.L.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the Food Biomarker Alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef]
- Risérus, U.; Marklund, M. Milk fat biomarkers and cardiometabolic disease. Curr. Opin. Lipidol. 2016, 28, 46–51. [Google Scholar] [CrossRef]
- Lankinen, M.; Schwab, U. Biomarkers of dairy fat. Am. J. Clin. Nutr. 2015, 101, 1101–1102. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Rabassa, M.; Casas-Agustench, P.; Liberona, N.H.; Llorach, R.; Lacueva, M.C.A. Novel strategies for improving dietary exposure assessment: Multiple-data fusion is a more accurate measure than the traditional single-biomarker approach. Trends Food Sci. Technol. 2017, 69, 220–229. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Khymenets, O.; Bulló, M.; Corella, D.; Fitó, M.; Martínez-González, M.A.; Estruch, R.; Andres-Lacueva, C. An NMR metabolomics approach reveals a combined-biomarkers model in a wine interventional trial with validation in free-living individuals of the PREDIMED study. Metabolomics 2014, 11, 797–806. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Jáuregui, O.; Corella, D.; Ruiz-Canela, M.; Salas-Salvadó, J.; Fitó, M.; Ros, E.; Estruch, R.; et al. A metabolomics-driven approach to predict cocoa product consumption by designing a multimetabolite biomarker model in free-living subjects from the PREDIMED study. Mol. Nutr. Food Res. 2014, 59, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Fretts, A.; Marklund, M.; Korat, A.V.A.; Yang, W.-S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake: Critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Münger, L.H.; Trimigno, A.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Burton, K.J.; Pralong, F.P.; Vionnet, N.; Capozzi, F.; Badertscher, R.; et al. Identification of Urinary Food Intake Biomarkers for Milk, Cheese, and Soy-Based Drink by Untargeted GC-MS and NMR in Healthy Humans. J. Proteome Res. 2017, 16, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.; Burnand, D.; Münger, L.H.; Pralong, F.P.; Vionnet, N.; Portmann, R.; Vergères, G. Identification of Milk and Cheese Intake Biomarkers in Healthy Adults Reveals High Interindividual Variability of Lewis System–Related Oligosaccharides. J. Nutr. 2020, 150, 1058–1067. [Google Scholar] [CrossRef]
- Pimentel, G.; Burton, K.J.; Von Ah, U.; Bütikofer, U.; Pralong, F.P.; Vionnet, N.; Portmann, R.; Vergères, G. Metabolic Footprinting of Fermented Milk Consumption in Serum of Healthy Men. J. Nutr. 2018, 148, 851–860. [Google Scholar] [CrossRef]
- Trimigno, A.; Münger, L.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Vionnet, N.; Pralong, F.; Capozzi, F.; Badertscher, R.; Vergères, G. GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans. Metabolites 2018, 8, 26. [Google Scholar] [CrossRef]
- Bhinderwala, F.; Wase, N.; DiRusso, C.; Powers, R. Combining Mass Spectrometry and NMR Improves Metabolite Detection and Annotation. J. Proteome Res. 2018, 17, 4017–4022. [Google Scholar] [CrossRef]
- Vionnet, N.; Münger, L.H.; Freiburghaus, C.; Burton, K.J.; Pimentel, G.; Pralong, F.P.; Badertscher, R.; Vergères, G. Assessment of lactase activity in humans by measurement of galactitol and galactonate in serum and urine after milk intake. Am. J. Clin. Nutr. 2019, 109, 470–477. [Google Scholar] [CrossRef]
- Jenkins, B.; Aoun, M.; Feillet-Coudray, C.; Coudray, C.; Ronis, M.; Koulman, A. The Dietary Total-Fat Content Affects the In Vivo Circulating C15:0 and C17:0 Fatty Acid Levels Independently. Nutrients 2018, 10, 1646. [Google Scholar] [CrossRef]
- Münger, L.H.; Garcia-Aloy, M.; Vázquez-Fresno, R.; Gille, D.; Rosana, A.R.R.; Passerini, A.; Soria-Florido, M.-T.; Pimentel, G.; Sajed, T.; Wishart, D.S.; et al. Biomarker of food intake for assessing the consumption of dairy and egg products. Genes Nutr. 2018, 13, 26. [Google Scholar] [CrossRef]
- Santaren, I.D.; Watkins, S.M.; Liese, A.D.; Wagenknecht, L.E.; Rewers, M.J.; Haffner, S.M.; Lorenzo, C.; Hanley, A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Hu, F.B. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am. J. Clin. Nutr. 2007, 86, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.A.B.; Assunção, D.C.B.; Penaforte, F.R.D.O.; Japur, C.C. Lactose em alimentos industrializados: Avaliação da disponibilidade da informação de quantidade. Ciên. Saúde Colet. 2018, 23, 4119–4128. [Google Scholar] [CrossRef]
- Heuer, T.; Krems, C.; Moon, K.; Brombach, C.; Hoffmann, I. Food consumption of adults in Germany: Results of the German National Nutrition Survey II based on diet history interviews. Br. J. Nutr. 2015, 113, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef]
- Drake, S.; Whetstine, M.C.; Drake, M.; Courtney, P.; Fligner, K.; Jenkins, J.; Pruitt, C. Sources of Umami Taste in Cheddar and Swiss Cheeses. J. Food Sci. 2007, 72, S360–S366. [Google Scholar] [CrossRef]
- Heck, J.M.L.; van Valenberg, H.; Dijkstra, J.; Van Hooijdonk, A.C.M. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Amores, G.; Virto, M. Total and Free Fatty Acids Analysis in Milk and Dairy Fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef]
- Arab, L. Biomarkers of Fat and Fatty Acid Intake. J. Nutr. 2003, 133, 925S–932S. [Google Scholar] [CrossRef]
- Baylin, A.; Campos, H. The use of fatty acid biomarkers to reflect dietary intake. Curr. Opin. Lipidol. 2006, 17, 22–27. [Google Scholar] [CrossRef]
- Andersen, L.F.; Solvoll, K.; Johansson, L.R.K.; Salminen, I.; Aro, A.; Drevon, C.A. Evaluation of a Food Frequency Questionnaire with Weighed Records, Fatty Acids, and Alpha-Tocopherol in Adipose Tissue and Serum. Am. J. Epidemiol. 1999, 150, 75–87. [Google Scholar] [CrossRef]
- Furse, S.; Torres, A.G.; Koulman, A. Fermentation of Milk into Yoghurt and Cheese Leads to Contrasting Lipid and Glyceride Profiles. Nutrients 2019, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Walton, J.; Kelton, D.; Leblanc, S.; Leslie, K.; Duffield, T. The Effect of Subclinical Ketosis in Early Lactation on Reproductive Performance of Postpartum Dairy Cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-persistence and Lactose Intolerance. Curr. Gastroenterol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef]
- Mottram, L.; Wiklund, G.; Larson, G.; Qadri, F.; Svennerholm, A.-M. FUT2 non-secretor status is associated with altered susceptibility to symptomatic enterotoxigenic Escherichia coli infection in Bangladeshis. Sci. Rep. 2017, 7, 10649. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.S.; Alakulppi, N.; Paavola-Sakki, P.; Kurppa, K.; Halme, L.; Färkkilä, M.; Turunen, U.; Lappalainen, M.; Kontula, K.; Kaukinen, K.; et al. Association study ofFUT2(rs601338) with celiac disease and inflammatory bowel disease in the Finnish population. Tissue Antigens 2012, 80, 488–493. [Google Scholar] [CrossRef]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- McGovern, D.P.; Jones, M.R.; Taylor, K.D.; Marciante, K.; Yan, X.; Dubinsky, M.; Ippoliti, A.; Vasiliauskas, E.; Berel, D.; Derkowski, C.; et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 2010, 19, 3468–3476. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Streppel, M.T.; Van Lee, L.; Geelen, A.; Sluik, D.; Van De Wiel, A.M.; De Vries, J.H.M.; Veer, P.V.; Feskens, E.J.M. A National Dietary Assessment Reference Database (NDARD) for the Dutch Population: Rationale behind the Design. Nutrients 2017, 9, 1136. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Van Lee, L.; Streppel, M.T.; Sluik, D.; Van De Wiel, A.M.; De Vries, J.H.M.; Geelen, A.; Feskens, E.J.M. Nutrition Questionnaires plus (NQplus) study, a prospective study on dietary determinants and cardiometabolic health in Dutch adults. BMJ Open 2018, 8, e020228. [Google Scholar] [CrossRef] [PubMed]
- Dutch National Institute for Public Health and the Environment. Nevo-Tabel. Nederlands Voedingsstoffenbestand. Voedingscentrum: Den Haag, The Netherlands, 2011. Available online: https://nevo-online.rivm.nl/ (accessed on 8 December 2020).
- Streppel, M.T.; De Vries, J.H.M.; Meijboom, S.; Beekman, M.; De Craen, A.J.M.; Slagboom, P.E.; Feskens, E.J.M. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; De Vries, J.H.M. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton, K.J.; Vergères, G.; Feskens, E.J.M. Prevalence of fermented foods in the Dutch adult diet and validation of a food frequency questionnaire for estimating their intake in the NQplus cohort. BMC Nutr. 2020, 6, 69. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Allison, P.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Enattah, N.S.; Jensen, T.G.; Nielsen, M.; Lewinski, R.; Kuokkanen, M.; Rasinpera, H.; El-Shanti, H.; Seo, J.K.; Alifrangis, M.; Khalil, I.F.; et al. Independent Introduction of Two Lactase-Persistence Alleles into Human Populations Reflects Different History of Adaptation to Milk Culture. Am. J. Hum. Genet. 2008, 82, 57–72. [Google Scholar] [CrossRef]
- Ingram, C.J.E.; Elamin, M.F.; Mulcare, C.A.; Weale, M.; Tarekegn, A.; Raga, T.O.; Bekele, E.; Elamin, F.M.; Thomas, M.G.; Bradman, N.; et al. A novel polymorphism associated with lactose tolerance in Africa: Multiple causes for lactase persistence? Qual. Life Res. 2006, 120, 779–788. [Google Scholar] [CrossRef]
- Ingram, C.J.E.; Raga, T.O.; Tarekegn, A.; Browning, S.L.; Elamin, M.F.; Bekele, E.; Thomas, M.G.; Weale, M.; Bradman, N.; Swallow, D.M. Multiple Rare Variants as a Cause of a Common Phenotype: Several Different Lactase Persistence Associated Alleles in a Single Ethnic Group. J. Mol. Evol. 2009, 69, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- Liebert, A.; López, S.; Jones, B.L.; Montalva, N.; Gerbault, P.; Lau, W.; Thomas, M.G.; Bradman, N.; Maniatis, N.; Swallow, D.M. World-wide distributions of lactase persistence alleles and the complex effects of recombination and selection. Qual. Life Res. 2017, 136, 1445–1453. [Google Scholar] [CrossRef]
- Liebert, A.; Jones, B.L.; Danielsen, E.T.; Olsen, A.K.; Swallow, D.M.; Troelsen, J.T. In VitroFunctional Analyses of Infrequent Nucleotide Variants in the Lactase Enhancer Reveal Different Molecular Routes to Increased Lactase Promoter Activity and Lactase Persistence. Ann. Hum. Genet. 2016, 80, 307–318. [Google Scholar] [CrossRef]
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef]
- Vissers, L.E.; Soedamah-Muthu, S.S.; Van Der Schouw, Y.T.; Zuithoff, N.P.; Geleijnse, J.M.; Sluijs, I. Consumption of a diet high in dairy leads to higher 15:0 in cholesteryl esters of healthy people when compared to diets high in meat and grain. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef] [PubMed]
- Aitchison, J. The Statistical Analysis of Compositional Data. J. R. Stat. Soc. Ser. B 1982, 44, 139–160. [Google Scholar] [CrossRef]
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 2.0-0, 2020. Available online: https://CRAN.R-project.org/package=compositions (accessed on 26 March 2021).
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.9.0, 2013. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 25 January 2014).
- Yan, Y. MLmetrics: Machine Learning Evaluation Metrics. R Package Version 1.1.1, 2016. Available online: https://CRAN.R-project.org/package=MLmetrics (accessed on 26 March 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]


| All (n = 246) | Men (n = 165) | Women (n = 81) | p-Value | |
|---|---|---|---|---|
| Age, years | 54.4 ± 12.5 | 55.9 ± 11.6 | 51.2 ± 13.6 | 0.01 ** |
| BMI, kg/m2 | 25.9 ± 3.9 | 26.1 ± 3.6 | 25.4 ± 4.4 | 0.18 |
| BMI-category, n (%) | 0.010 ** | |||
| <25 kg/m2 | 105 (42.7) | 61 (37.0) | 44 (54.3) | |
| ≥25 kg/m2 | 141 (57.3) | 104 (63.0) | 37 (45.7) | |
| Waist circumference, cm | 92.5 ± 11.6 | 95.8 ± 10.5 | 85.6 ± 10.7 | <0.001 *** |
| Education, n (%) | 0.38 | |||
| Low | 19 (7.7) | 12 (7.3) | 7 (8.8) | |
| Intermediate | 77 (31.3) | 49 (29.7) | 28 (35.0) | |
| High | 149 (60.6) | 104 (63.0) | 45 (56.2) | |
| Smoking status, n (%) | 0.09 | |||
| Never | 119 (48.4) | 71 (46.4) | 48 (63.2) | |
| Former | 85 (34.6) | 65 (42.5) | 20 (26.3) | |
| Current | 25 (10.2) | 17 (1.1) | 8 (10.5) | |
| Disease history, n (%) | ||||
| Cancer | 11 (4.5) | 5 (3.0) | 6 (7.4) | 0.12 |
| Diabetes | 6 (2.4) | 5 (3.0) | 1 (1.2) | 0.39 |
| Heart attack | 7 (2.8) | 6 (3.6) | 1 (1.2) | 0.29 |
| Hypertension | 60 (24.4) | 44 (26.7) | 16 (19.8) | 0.47 |
| High cholesterol | 52 (21.1) | 38 (23.0) | 14 (17.3) | 0.58 |
| Stroke | 2 (0.8) | 1 (0.6) | 1 (1.2) | 0.61 |
| Diet during past month, n (%) | <0.001 *** | |||
| No | 228 (92.7) | 159 (96.4) | 69 (85.2) | |
| Yes, always | 9 (3.7) | 1 (0.6) | 8 (9.9) | |
| Yes, sometimes | 9 (3.7) | 5 (3.0) | 4 (4.9) | |
| Lactase status, n (%) | 1.00 | |||
| Persistent | 104 (94.5) | 81 (94.2) | 23 (95.8) | |
| Non-persistent | 6 (5.5) | 5 (5.8) | 1 (4.2) | |
| FUT2/FUT3 status, n (%) | 0.41 | |||
| Secretor (Le a−b+) | 87 (79.1) | 69 (80.2) | 18 (75.0) | |
| Non-secretor (Le a+b−) | 19 (17.3) | 13 (15.1) | 6 (25.0) | |
| Lewis negative (Le a−b−) | 4 (3.6) | 4 (4.7) | 0 (0) |
| Food Group | Median Energy-Adjusted Intakes in g/d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nc | Q1 (n = 50) | nc | Q2 (n = 49) | nc | Q3 (n = 49) | nc | Q4 (n = 49) | nc | Q5 (n = 49) | |
| Total dairy | 50 | 98 (71, 129) | 49 | 214 (197, 235) | 49 | 304 (279, 323) | 49 | 372 (355, 394) | 49 | 527 (469, 616) |
| High-fat dairy | 47 | 10 (6, 15) | 49 | 24 (21, 28) | 49 | 42 (35, 48) | 49 | 73 (64, 81) | 49 | 135 (109, 163) |
| Low-fat dairy | 49 | 43 (25, 59) | 49 | 148 (119, 173) | 49 | 242 (224, 257) | 49 | 317 (304, 340) | 49 | 480 (404, 590) |
| Total fermented dairy | 49 | 41 (24, 49) | 49 | 90 (69, 109) | 49 | 143 (134, 161) | 49 | 224 (204, 237) | 49 | 334 (291, 393) |
| High-fat fermented dairy | 9 | 3 (−1, 4) | 49 | 9 (7, 10) | 49 | 17 (14, 19) | 49 | 37 (30, 45) | 49 | 82 (65, 117) |
| Low-fat fermented dairy | 48 | 15 (7, 23) | 49 | 50 (40, 62) | 49 | 108 (99, 124) | 49 | 195 (158, 210) | 49 | 304 (269, 370) |
| Total non-fermented dairy | 48 | 12 (4, 22) | 49 | 54 (44, 63) | 49 | 103 (91, 124) | 49 | 179 (160, 207) | 49 | 322 (282, 340) |
| High-fat non-fermented dairy | 41 | 3 (1, 5) | 49 | 10 (9, 12) | 49 | 18 (16, 20) | 49 | 31 (25, 35) | 49 | 60 (48, 89) |
| Low-fat non-fermented dairy | 0 | −4 (−9, 5) | 34 | 22 (14, 32) | 49 | 69 (55, 89) | 49 | 146 (127, 173) | 49 | 293 (263, 373) |
| Cheese | 46 | 8 (4, 12) | 49 | 19 (17, 21) | 49 | 27 (24, 29) | 49 | 43 (39, 47) | 49 | 67 (58, 90) |
| Yoghurt | 16 | 0 (0, 5) | 49 | 38 (22, 53) | 49 | 83 (72, 96) | 49 | 126 (105, 139) | 49 | 193 (150, 212) |
| Milk | 13 | 4 (−8, 14) | 49 | 40 (29, 48) | 49 | 87 (72, 108) | 49 | 162 (144, 191) | 49 | 303 (272, 371) |
| Biomarker | Analytical Platform (Biosample) a | Spearman’s Correlation Coefficient (rs) | Unadjusted GLM b | Adjusted GLM b,c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p-Value | rap | R2 | MAE | Coefficient | SE | p-Value | rap | R2 | MAE | ||||
| C15:0 | GC-MS (P) | 0.03 | M: 0.05 | (Int: 5.05) 0.04 | (0.06) 0.12 | (0.00 ***) 0.76 | 0.13 | 0.02 | 88.5 | (Int: 5.24) 0.08 | (0.12) 0.12 | (0.00 ***) 0.49 | 0.09 | 0.01 | 89.0 |
| W: 0.00 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.02 | M: 0.06 | (Int: 5.05) 0.03 | (0.06) 0.14 | (0.00 ***) 0.82 | −0.12 | 0.01 | 88.5 | (Int: 5.24) 0.08 | (0.12) 0.14 | (0.00 ***) 0.55 | 0.12 | 0.01 | 88.8 |
| W: 0.00 | |||||||||||||||
| Phenylalanine | LC-MS (P) | 0.11 | M: 0.03 | (Int: 5.06) 0.08 | (0.05) 0.05 | (0.00 ***) 0.10 | 0.25 | 0.06 | 104.0 | (Int: 5.29) 0.09 | (0.11) 0.05 | (0.00 ***) 0.07 | 0.47 | 0.22 | 99.8 |
| W: 0.32 ** | |||||||||||||||
| Tyrosine | LC-MS (P) | 0.08 | M: 0.01 | (Int: 5.06) 0.08 | (0.05) 0.05 | (0.00 ***) 0.12 | 0.15 | 0.02 | 104.5 | (Int: 5.29) 0.09 | (0.11) 0.05 | (0.00 ***) 0.06 | 0.53 | 0.28 | 100.5 |
| W: 0.25 * | |||||||||||||||
| Tryptophan | LC-MS (P) | 0.12 | M: 0.06 | (Int: 5.06) 0.11 | (0.05) 0.06 | (0.00 ***) 0.10 | 0.16 | 0.03 | 105.6 | (Int: 5.28) 0.11 | (0.11) 0.06 | (0.00 ***) 0.09 | 0.38 | 0.14 | 101.7 |
| W: 0.25 * | |||||||||||||||
| Indole-3-propionic acid | LC-MS (P) | 0.04 | M: −0.03 | (Int: 5.07) 0.02 | (0.05) 0.06 | (0.00 ***) 0.68 | 0.05 | 0.00 | 106.4 | (Int: 5.27) 0.02 | (0.11) 0.06 | (0.00 ***) 0.75 | 0.40 | 0.16 | 102.7 |
| W: 0.16 | |||||||||||||||
| Indole-3-acetic acid | LC-MS (P) | 0.10 | M: −0.01 | (Int: 5.06) 0.09 | (0.05) 0.07 | (0.00 ***) 0.18 | −0.08 | 0.01 | 106.3 | (Int: 5.26) 0.08 | (0.11) 0.07 | (0.00 ***) 0.22 | 0.25 | 0.07 | 103.7 |
| W: 0.29 * | |||||||||||||||
| Lactose | GC-MS (U) | 0.16 * | M: 0.23 ** | (Int: 5.12) 0.12 | (0.05) 0.06 | (0.00 ***) 0.05 | 0.16 | 0.03 | 91.8 | (Int: 5.30) 0.13 | (0.11) 0.06 | (0.00 ***) 0.03 * | 0.20 | 0.04 | 92.7 |
| W: 0.08 | |||||||||||||||
| GC-MS (P) | −0.01 | M: −0.05 | (Int: 5.05) 0.06 | (0.06) 0.09 | (0.00 ***) 0.55 | 0.09 | 0.01 | 88.3 | (Int: 5.23) 0.05 | (0.12) 0.09 | (0.00 ***) 0.59 | 0.10 | 0.01 | 88.8 | |
| W: 0.11 | |||||||||||||||
| Galactose | GC-MS (U) | 0.04 | M: 0.11 | (5.12) 0.04 | (0.05) 0.03 | (0.00 ***) 0.20 | 0.22 | 0.05 | 94.3 | (Int: 5.33) 0.07 | (0.11) 0.03 | (0.00 ***) 0.04 | 0.21 | 0.04 | 93.0 |
| W: 0.10 | |||||||||||||||
| GC-MS (P) | −0.02 | M: −0.02 | (5.05) −0.11 | (0.06) 0.27 | (0.00 ***) 0.68 | −0.08 | 0.01 | 89.4 | (Int: 5.24) −0.12 | (0.12) 0.27 | (0.00 ***) 0.65 | 0.08 | 0.01 | 88.5 | |
| W: −0.02 | |||||||||||||||
| Galactitol | GC-MS (U) | 0.20 ** | M: 0.23 ** | (Int: 5.12) 0.21 | (0.05) 0.10 | (0.00 ***) 0.03 | 0.17 | 0.03 | 93.6 | (Int: 5.28) 0.20 | (0.11) 0.10 | (0.00 ***) 0.04 | 0.17 | 0.03 | 93.3 |
| W: 0.07 | |||||||||||||||
| GC-MS (P) | 0.00 | M: −0.02 | (Int: 5.05) 0.01 | (0.06) 0.12 | (0.00 ***) 0.94 | −0.13 | 0.02 | 88.6 | (Int: 5.24) 0.06 | (0.12) 0.12 | (0.00 ***) 0.60 | 0.14 | 0.02 | 88.7 | |
| W: 0.05 | |||||||||||||||
| Galactonate | LC-MS (U) | 0.14 | M: 0.01 | (Int: 5.12) 0.04 | (0.05) 0.05 | (0.00 ***) 0.36 | 0.12 | 0.01 | 96.7 | (Int: 5.29) 0.05 | (0.11) 0.05 | (0.00 ***) 0.30 | 0.15 | 0.02 | 96.7 |
| W: 0.22 | |||||||||||||||
| GC-MS (U) | 0.04 | M: 0.08 | (Int: 5.12) 0.02 | (0.05) 0.07 | (0.00 ***) 0.72 | 0.19 | 0.04 | 95.6 | (Int: 5.30) 0.05 | (0.11) 0.07 | (0.00 ***) 0.43 | 0.17 | 0.03 | 96.0 | |
| W: 0.03 | |||||||||||||||
| GC-MS (P) | 0.02 | M: 0.04 | (Int: 5.05) 0.07 | (0.06) 0.08 | (0.00 ***) 0.36 | 0.13 | 0.02 | 87.8 | (Int: 5.22) 0.09 | (0.12) 0.08 | (0.00 ***) 0.27 | 0.13 | 0.02 | 87.7 | |
| W: 0.02 | |||||||||||||||
| Blood group H disaccharide | LC-MS (P) | −0.07 | M: −0.10 | (Int: 5.07) −0.02 | (0.05) 0.05 | (0.00 ***) 0.62 | 0.09 | 0.01 | 106.0 | (Int: 5.27) −0.02 | (0.11) 0.05 | (0.00 ***) 0.63 | 0.38 | 0.15 | 102.3 |
| W: −0.06 | |||||||||||||||
| LC-MS (U) | −0.05 | M: 0.05 | (Int: 5.12) 0.00 | (0.05) 0.05 | (0.00 ***) 0.94 | 0.04 | 0.00 | 97.1 | (Int: 5.30) 0.03 | (0.11) 0.05 | (0.00 ***) 0.60 | 0.12 | 0.02 | 97.0 | |
| W: −0.10 | |||||||||||||||
| Lewis A trisaccharide | LC-MS (P) | 0.07 | M: −0.01 | (Int: 5.07) 0.00 | (0.05) 0.04 | (0.00 ***) 0.97 | −0.01 | 0.00 | 106.4 | (Int: 5.27) 0.00 | (0.11) 0.04 | (0.00 ***) 0.93 | 0.30 | 0.09 | 102.5 |
| W: 0.26 * | |||||||||||||||
| Hippurate | GC-MS (U) | −0.10 | M: −0.02 | (Int: 5.12) −0.04 | (0.05) 0.11 | (0.00 ***) 0.73 | −0.15 | 0.02 | 95.7 | (Int: 5.30) 0.01 | (0.11) 0.11 | (0.00 ***) 0.91 | 0.12 | 0.02 | 95.2 |
| W: −0.08 | |||||||||||||||
| Methionine | GC-MS (P) | 0.01 | M: 0.03 | (Int: 5.05) 0.08 | (0.06) 0.13 | (0.00 ***) 0.53 | 0.00 | 0.00 | 88.1 | (Int: 5.25) 0.13 | (0.12) 0.13 | (0.00 ***) 0.32 | 0.10 | 0.01 | 87.5 |
| W: 0.05 | |||||||||||||||
| Biomarker | Analytical Platform (Biosample) a | Spearman’s Correlation Coefficient (rs) | Unadjusted GLM b | Adjusted GLM b,c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p-Value | rap | R2 | MAE | Coefficient | SE | p-Value | rap | R2 | MAE | ||||
| C15:0 | GC-MS (P) | 0.12 | M: 0.15 | (Int: 3.91) 0.12 | (0.03) 0.07 | (0.00 ***) 0.10 | 0.21 | 0.04 | 15.5 | (Int: 4.00) 0.12 | (0.07) 0.07 | (0.00 ***) 0.11 | 0.10 | 0.01 | 16.0 |
| W: 0.07 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.08 | M: 0.12 | (Int: 3.91) 0.10 | (0.03) 0.08 | (0.00 ***) 0.23 | 0.19 | 0.04 | 15.8 | (Int: 4.00) 0.10 | (0.07) 0.08 | (0.00 ***) 0.23 | 0.03 | 0.00 | 16.3 |
| W: 0.04 | |||||||||||||||
| 3-Phenyllactic acid | GC-MS (U) | −0.11 | M: 0.08 | (Int: 3.97) 0.01 | (0.03) 0.04 | (0.00 ***) 0.78 | −0.17 | 0.03 | 18.9 | (Int: 4.099) 0.00 | (0.07) 0.04 | (0.00 ***) 0.98 | 0.16 | 0.02 | 18.5 |
| W: 0.08 | |||||||||||||||
| GC-MS (P) | −0.05 | M: 0.07 | (Int: 3.91) −0.06 | (0.03) 0.08 | (0.00 ***) 0.45 | −0.07 | 0.01 | 16.4 | (Int: 3.99) −0.05 | (0.07) 0.08 | (0.00 ***) 0.58 | −0.02 | 0.00 | 16.7 | |
| W: −0.17 | |||||||||||||||
| 3-Hydroxy-isobutyrate | GC-MS (P) | −0.04 | M: −0.04 | (Int: 3.91) 0.00 | (0.03) 0.07 | (0.00 ***) 0.99 | −0.04 | 0.00 | 16.2 | (Int: 3.99) 0.00 | (0.07) 0.08 | (0.00 ***) 0.95 | 0.03 | 0.00 | 16.6 |
| W: 0.01 | |||||||||||||||
| Phenylalanyl-proline | LC-MS (P) | 0.05 | M: 0.01 | (Int: 3.88) 0.04 | (0.04) 0.03 | (0.00 ***) 0.17 | −0.11 | 0.01 | 20.6 | (Int: 4.06)0.05 | (0.08) 0.03 | (0.00 ***) 0.09 | 0.07 | 0.01 | 20.3 |
| W: 0.18 | |||||||||||||||
| LC-MS (U) | −0.07 | M: −0.08 | (Int: 3.97) 0.00 | (0.03) 0.08 | (0.00 ***) 0.97 | −0.12 | 0.01 | 22.5 | (Int: 4.11) 0.04 | (0.07) 0.08 | (0.00 ***) 0.68 | −0.06 | 0.00 | 22.7 | |
| W: 0.01 | |||||||||||||||
| Indole-3-lactic acid | LC-MS (P) | 0.06 | M: 0.02 | (Int: 3.89) 0.05 | (0.04) 0.04 | (0.00 ***) 0.25 | −0.07 | 0.01 | 20.8 | (Int: 4.05) 0.05 | (0.08) 0.04 | (0.00 ***) 0.24 | 0.07 | 0.01 | 20.5 |
| W: 0.18 | |||||||||||||||
| LC-MS (U) | 0.13 | M: 0.20 * | (Int: 3.97) 0.18 | (0.03) 0.11 | (0.00 ***) 0.11 | 0.11 | 0.01 | 22.2 | (Int: 4.08) 0.10 | (0.08) 0.12 | (0.00 ***) 0.40 | 0.04 | 0.00 | 22.6 | |
| W: 0.13 | |||||||||||||||
| Proline | LC-MS (U) | −0.05 | M: 0.03 | (Int: 3.97) 0.06 | (0.03) 0.07 | (0.00 ***) 0.40 | 0.24 | 0.06 | 22.3 | (Int: 4.10) 0.07 | (0.07) 0.07 | (0.00 ***) 0.32 | 0.04 | 0.00 | 22.6 |
| W: 0.05 | |||||||||||||||
| GC-MS (P) | −0.16 * | M: −0.15 | (Int: 3.9) −0.07 | (0.03) 0.04 | (0.00 ***) 0.07 | 0.05 | 0.00 | 17.1 | (Int: 3.98) −0.07 | (0.07) 0.04 | (0.00 ***) 0.10 | 0.01 | 0.00 | 17.7 | |
| W: −0.11 | |||||||||||||||
| Alanine | GC-MS (U) | 0.12 | M: 0.04 | (Int: 3.97) 0.00 | (0.03) 0.05 | (0.00 ***) 0.96 | −0.22 | 0.05 | 18.9 | (Int: 4.10) 0.02 | (0.07) 0.05 | (0.00 ***) 0.73 | 0.07 | 0.00 | 18.5 |
| W: −0.14 | |||||||||||||||
| Pyroglutamate | GC-MS (U) | −0.01 | M: −0.06 | (Int: 3.97) −0.12 | (0.03) 0.10 | (0.00 ***) 0.24 | 0.11 | 0.01 | 18.4 | (Int: 4.09) −0.08 | (0.07) 0.10 | (0.00 ***) 0.45 | 0.26 | 0.07 | 18.2 |
| W: −0.09 | |||||||||||||||
| Methionine | GC-MS (P) | −0.14 | M: −0.10 | (Int: 3.91) −0.13 | (0.03) 0.08 | (0.00 ***) 0.08 | −0.02 | 0.00 | 17.3 | (Int: 3.98) −0.13 | (0.07) 0.08 | (0.00 ***) 0.10 | −0.08 | 0.01 | 17.8 |
| W: −0.16 | |||||||||||||||
| Leucine | GC-MS (P) | −0.11 | M: −0.03 | (Int: 3.91) −0.14 | (0.03) 0.10 | (0.00 ***) 0.15 | −0.09 | 0.01 | 16.7 | (Int: 3.98) −0.14 | (0.07) 0.11 | (0.00 ***) 0.19 | −0.04 | 0.00 | 17.1 |
| W: −0.19 | |||||||||||||||
| Glutamic acid | GC-MS (P) | −0.04 | M: 0.00 | (Int: 3.91) −0.02 | (0.03) 0.05 | (0.00 ***) 0.77 | −0.01 | 0.00 | 16.3 | (Int: 3.99) −0.01 | (0.07) 0.05 | (0.00 ***) 0.81 | −0.02 | 0.00 | 16.8 |
| W: −0.05 | |||||||||||||||
| Valine | GC-MS (P) | −0.12 | M: −0.08 | (Int: 3.91) −0.13 | (0.03) 0.08 | (0.00 ***) 0.13 | −0.05 | 0.00 | 17.0 | (Int: 3.98) −0.12 | (0.07) 0.09 | (0.00 ***) 0.16 | −0.07 | 0.01 | 17.5 |
| W: −0.13 | |||||||||||||||
| Isoleucine | GC-MS (P) | −0.12 | M: −0.06 | (Int: 3.91) −0.14 | (0.03) 0.08 | (0.00 ***) 0.08 | −0.10 | 0.01 | 17.2 | (Int: 3.97) −0.13 | (0.07) 0.08 | (0.00 ***) 0.12 | −0.07 | 0.00 | 17.5 |
| W: −0.20 | |||||||||||||||
| Biomarker | Analytical Platform (Biosample) a | Spearman’s Correlation Coefficient (rs) | Unadjusted GLM b | Adjusted GLM b,c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p-Value | rap | R2 | MAE | Coefficient | SE | p-Value | rap | R2 | MAE | ||||
| Proline | LC-MS (P) | 0.01 | M: 0.01 | (Int: 4.53) −0.01 | (0.06) 0.06 | (0.00 ***) 0.89 | 0.13 | 0.02 | 68.0 | (Int: 4.69) 0.01 | (0.13) 0.06 | (0.00 ***) 0.92 | −0.12 | 0.02 | 68.5 |
| W: 0.17 | |||||||||||||||
| Indole-3-lactic acid | LC-MS (P) | 0.03 | M: 0.01 | (Int: 4.53) 0.02 | (0.06) 0.08 | (0.00 ***) 0.80 | −0.05 | 0.00 | 67.8 | (Int: 4.68) 0.03 | (0.13) 0.08 | (0.00 ***) 0.73 | −0.15 | 0.02 | 68.7 |
| W: 0.14 | |||||||||||||||
| Lysine | LC-MS (P) | 0.02 | M: −0.02 | (Int: 4.53) 0.01 | (0.06) 0.07 | (0.00 ***) 0.89 | 0.08 | 0.01 | 67.8 | (Int: 4.69) 0.02 | (0.13) 0.07 | (0.00 ***) 0.81 | −0.16 | 0.03 | 68.5 |
| W: 0.20 | |||||||||||||||
| Threonine | LC-MS (P) | 0.04 | M: −0.01 | (Int: 4.53) −0.01 | (0.06) 0.06 | (0.00 ***) 0.92 | 0.02 | 0.00 | 67.9 | (Int: 4.68) −0.00 | (0.13) 0.06 | (0.00 ***) 0.97 | −0.13 | 0.02 | 68.5 |
| W: 0.20 | |||||||||||||||
| Phenylalanine | LC-MS (P) | 0.08 | M: 0.07 | (Int: 4.53) 0.03 | (0.06) 0.06 | (0.00 ***) 0.64 | 0.01 | 0.00 | 67.6 | (Int: 4.69) 0.04 | (0.13) 0.06 | (0.00 ***) 0.53 | −0.12 | 0.01 | 68.3 |
| W: 0.17 | |||||||||||||||
| Tyrosine | LC-MS (P) | 0.12 | M: 0.10 | (Int: 4.52) 0.06 | (0.06) 0.06 | (0.00 ***) 0.29 | −0.09 | 0.01 | 67.4 | (Int: 4.70) 0.07 | (0.13) 0.06 | (0.00 ***) 0.21 | −0.15 | 0.02 | 68.1 |
| W: 0.21 | |||||||||||||||
| Tryptophan | LC-MS (P) | 0.03 | M: 0.02 | (Int: 4.53) 0.02 | (0.06) 0.08 | (0.00 ***) 0.83 | −0.15 | 0.02 | 68.0 | (Int: 4.69) 0.02 | (0.13) 0.07 | (0.00 ***) 0.75 | −0.17 | 0.03 | 69.0 |
| W: 0.10 | |||||||||||||||
| Indole-3-acetaldehyde | LC-MS (P) | 0.03 | M: 0.00 | (Int: 4.53) 0.03 | (0.06) 0.09 | (0.00 ***) 0.70 | −0.17 | 0.03 | 67.7 | (Int: 4.69) 0.05 | (0.13) 0.09 | (0.00 ***) 0.59 | −0.15 | 0.02 | 68.4 |
| W: 0.15 | |||||||||||||||
| Biomarker | Analytical Platform (Biosample) | Spearman’s Correlation Coefficient (rs) | Unadjusted GLM a | Adjusted GLM a,b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p-Value | rap | R2 | MAE | Coefficient | SE | p-Value | rap | R2 | MAE | ||||
| Total Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.17 * | M: 0.17 | (Int: 5.89) 0.16 | (0.03) 0.07 | (0.00 ***) 0.02 * | 0.06 | 0.00 | 130.9 | (Int: 6.08) 0.17 | (0.07) 0.07 | (0.00 ***) 0.02 * | 0.31 | 0.10 | 125.4 |
| W: 0.13 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.12 | M: 0.14 | (Int: 5.89) 0.15 | (0.03) 0.08 | (0.00 ***) 0.07 | −0.01 | 0.00 | 128.1 | (Int: 6.08) 0.16 | (0.07) 0.08 | (0.00 ***) 0.05 | 0.37 | 0.14 | 122.7 |
| W: 0.12 | |||||||||||||||
| High-Fat Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | −0.01 | M: −0.04 | (Int: 4.22) 0.15 | (0.06) 0.12 | (0.00 ***) 0.21 | 0.03 | 0.00 | 56.2 | (Int: 4.30) 0.16 | (0.12) 0.12 | (0.00 ***) 0.20 | 0.10 | 0.01 | 56.2 |
| W: 0.09 | |||||||||||||||
| C17:0 | GC-MS (P) | −0.06 | M: −0.08 | (Int: 4.22) 0.10 | (0.06) 0.14 | (0.00 ***) 0.49 | −0.03 | 0.00 | 56.0 | (Int: 4.30) 0.10 | (0.12) 0.14 | (0.00 ***) 0.50 | −0.01 | 0.00 | 56.1 |
| W: 0.00 | |||||||||||||||
| Low-Fat Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.16 * | M: 0.19 * | (Int: 5.68) 0.16 | (0.04) 0.09 | (0.00 ***) 0.06 | 0.07 | 0.01 | 139.5 | (Int: 5.89) 0.17 | (0.08) 0.09 | (0.00 ***) 0.05 * | 0.26 | 0.07 | 136.6 |
| W: 0.07 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.13 | M: 0.17* | (Int: 5.68) 0.16 | (0.04) 0.10 | (0.00 ***) 0.11 | 0.04 | 0.00 | 137.9 | (Int: 5.89) 0.17 | (0.08) 0.10 | (0.00 ***) 0.09 | 0.32 | 0.10 | 133.8 |
| W: 0.07 | |||||||||||||||
| Total Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.24 *** | M: 0.24 ** | (Int: 5.25) 0.27 | (0.04) 0.09 | (0.00 ***) 0.00 * | 0.01 | 0.00 | 107.3 | (Int: 5.43) 0.25 | (0.09) 0.09 | (0.00 ***) 0.01 ** | 0.09 | 0.01 | 106.7 |
| W: 0.21 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.19 ** | M: 0.20 * | (Int: 5.25) 0.26 | (0.04) 0.11 | (0.00 ***) 0.01 * | −0.04 | 0.00 | 105.3 | (Int: 5.43) 0.23 | (0.09) 0.11 | (0.00 ***) 0.03 * | 0.06 | 0.00 | 103.7 |
| W: 0.18 | |||||||||||||||
| High-fat Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.05 | M: 0.07 | (Int: 3.80) 0.20 | (0.06) 0.13 | (0.00 ***) 0.11 | 0.18 | 0.03 | 35.7 | (Int: 3.85) 0.24 | (0.13) 0.13 | (0.00 ***) 0.06 | 0.14 | 0.02 | 35.9 |
| W: 0.04 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.01 | M: 0.06 | (Int: 3.80) 0.14 | (0.06) 0.15 | (0.00 ***) 0.34 | 0.10 | 0.01 | 36.2 | (Int: 3.85) 0.17 | (0.13) 0.15 | (0.00 ***) 0.26 | 0.02 | 0.00 | 36.4 |
| W: −0.07 | |||||||||||||||
| Low-Fat Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.19 ** | M: 0.19 * | (Int: 4.98) 0.29 | (0.06) 0.12 | (0.00 ***) 0.01 * | −0.03 | 0.00 | 101.1 | (Int: 5.20) 0.25 | (0.11) 0.12 | (0.00 ***) 0.03 * | 0.04 | 0.00 | 103.6 |
| W: 0.19 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.16 * | M: 0.15 | (Int: 4.99) 0.30 | (0.06) 0.14 | (0.00 ***) 0.03 * | −0.05 | 0.00 | 97.7 | (Int: 5.20) 0.25 | (0.11) 0.13 | (0.00 ***) 0.06 | 0.04 | 0.00 | 99.0 |
| W: 0.19 | |||||||||||||||
| Total Non-Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.03 | M: 0.06 | (Int: 5.14) 0.04 | (0.05) 0.11 | (0.00 ***) 0.74 | 0.12 | 0.01 | 87.3 | (Int: 5.33) 0.08 | (0.11) 0.11 | (0.00 ***) 0.48 | 0.07 | 0.01 | 88.3 |
| W: 0.02 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.02 | M: 0.06 | (Int: 5.14) 0.03 | (0.05) 0.13 | (0.00 ***) 0.84 | −0.11 | 0.01 | 87.2 | (Int: 5.33) 0.07 | (0.11) 0.13 | (0.00 ***) 0.57 | 0.09 | 0.01 | 88.0 |
| W: 0.01 | |||||||||||||||
| High-Fat Non-Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | −0.09 | M: −0.12 | (Int: 3.71) 0.03 | (0.06) 0.13 | (0.00 ***) 0.83 | −0.13 | 0.02 | 29.5 | (Int: 3.79) 0.00 | (0.13) 0.13 | (0.00 ***) 1.00 | −0.20 | 0.04 | 29.9 |
| W: −0.01 | |||||||||||||||
| C17:0 | GC-MS (P) | −0.12 | M: −0.19 * | (Int: 3.71) 0.01 | (0.06) 0.15 | (0.00 ***) 0.96 | −0.09 | 0.01 | 29.4 | (Int: 3.79) −0.02 | (0.13) 0.15 | (0.000 ***) 0.88 | −0.18 | 0.03 | 29.7 |
| W: 0.00 | |||||||||||||||
| Low-Fat Non-Fermented Dairy | |||||||||||||||
| C15:0 | GC-MS (P) | 0.03 | M: 0.10 | (Int: 4.99) 0.03 | (0.06) 0.13 | (0.00 ***) 0.79 | 0.15 | 0.02 | 97.1 | (Int: 5.19) 0.09 | (0.12) 0.13 | (0.00 ***) 0.48 | 0.19 | 0.04 | 95.0 |
| W: −0.05 | |||||||||||||||
| C17:0 | GC-MS (P) | 0.03 | M: 0.12 | (Int: 4.99) 0.03 | (0.06) 0.15 | (0.00 ***) 0.85 | −0.14 | 0.02 | 97.1 | (Int: 5.19) 0.09 | (0.12) 0.15 | (0.00 ***) 0.55 | 0.21 | 0.04 | 95.0 |
| W: −0.07 | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.J.; Burton-Pimentel, K.J.; Brouwer-Brolsma, E.M.; Feskens, E.J.M.; Blaser, C.; Badertscher, R.; Portmann, R.; Vergères, G. Evaluating the Robustness of Biomarkers of Dairy Food Intake in a Free-Living Population Using Single- and Multi-Marker Approaches. Metabolites 2021, 11, 395. https://doi.org/10.3390/metabo11060395
Li KJ, Burton-Pimentel KJ, Brouwer-Brolsma EM, Feskens EJM, Blaser C, Badertscher R, Portmann R, Vergères G. Evaluating the Robustness of Biomarkers of Dairy Food Intake in a Free-Living Population Using Single- and Multi-Marker Approaches. Metabolites. 2021; 11(6):395. https://doi.org/10.3390/metabo11060395
Chicago/Turabian StyleLi, Katherine J., Kathryn J. Burton-Pimentel, Elske M. Brouwer-Brolsma, Edith J. M. Feskens, Carola Blaser, René Badertscher, Reto Portmann, and Guy Vergères. 2021. "Evaluating the Robustness of Biomarkers of Dairy Food Intake in a Free-Living Population Using Single- and Multi-Marker Approaches" Metabolites 11, no. 6: 395. https://doi.org/10.3390/metabo11060395
APA StyleLi, K. J., Burton-Pimentel, K. J., Brouwer-Brolsma, E. M., Feskens, E. J. M., Blaser, C., Badertscher, R., Portmann, R., & Vergères, G. (2021). Evaluating the Robustness of Biomarkers of Dairy Food Intake in a Free-Living Population Using Single- and Multi-Marker Approaches. Metabolites, 11(6), 395. https://doi.org/10.3390/metabo11060395