The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance
Abstract
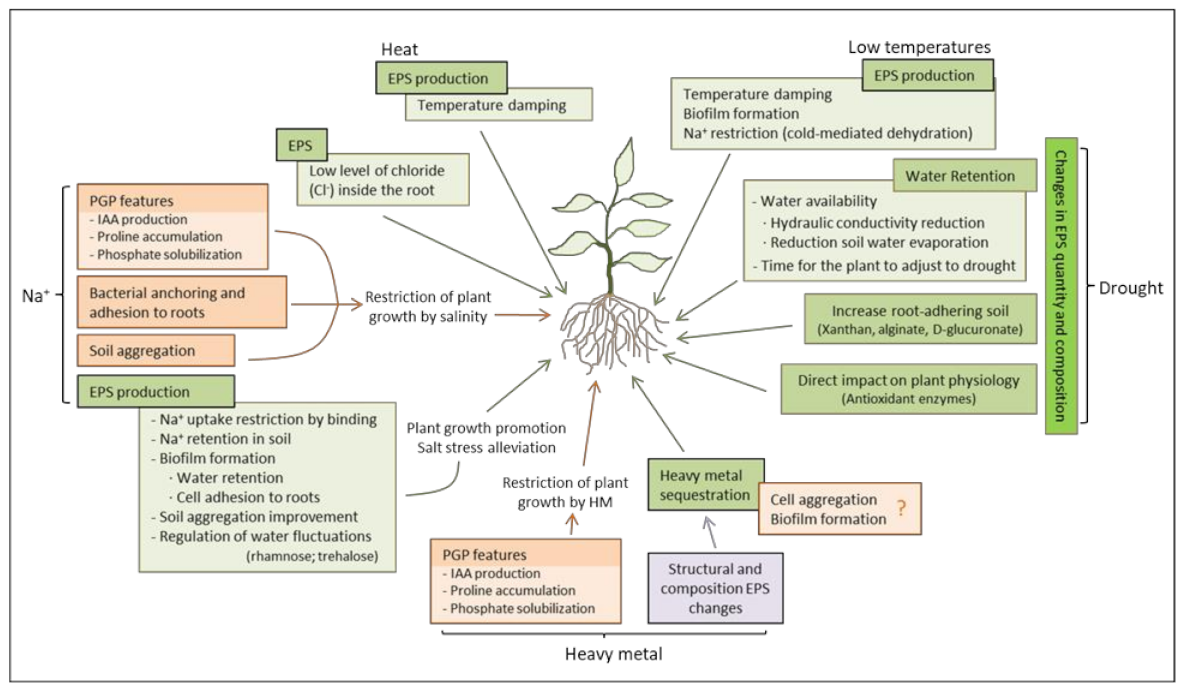
1. Introduction
2. Salinity
3. Drought
4. Heavy Metal
5. Temperature
6. Conclusions
7. Researched Literature
Author Contributions
Funding
Conflicts of Interest
References
- Iizumi, T.; Sakuma, H.; Yokozawa, M.; Luo, J.J.; Challinor, A.J.; Brown, M.E.; Sakurai, G.; Yamagata, T. Prediction of seasonal climate-induced variations in global food production. Nat. Clim. Chang. 2013, 3, 904–908. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bansal, K.C.; Aggarwal, P.K.; Datta, S.K.; Craufurd, P.Q. Agricultural biotechnology for crop improvement in a variable climate: Hope or hype? Trends Plant Sci. 2011, 16, 363–371. [Google Scholar] [CrossRef]
- Bansal, K.C.; Lenak, S.K.; Mondal, T.K. Genomic resources for breeding crops with enhanced abiotic stress tolerance. Plant Breed. 2013, 133, 1–11. [Google Scholar] [CrossRef]
- Henry, R.J. Genomics strategies for germplasm characterization and the development of climate resilient crops. Front. Plant Sci. 2014, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Vilma, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar]
- Timmusk, S.; Nicander, B.; Granhall, U.; Yillberg, E. Cytokinin production by Paenibacillus polymyxa. Soil. Biol. Biochem. 1999, 31, 1847–1852. [Google Scholar] [CrossRef]
- Belimov, A.; Dodd, I.; Safronova, V.; Davies, W. ACC deaminase-containing rhizobacteria improve vegetative development and yield of potato plants grown under water-limited conditions. Ann. Appl. Biol. 2009, 98, 163–169. [Google Scholar]
- Kudoyarova, G.R.; Arkhipova, T.N.; Melentev, A.I. Role of bacterial phytohormones in plant growth regulation and their development. In Bacterial Metabolites in Sustainable Agroecosystem. Sustainable Development and Biodiversity; Maheshwari, D.K., Ed.; Springer: Cham, Switzerland, 2015; Volume 12, pp. 69–86. [Google Scholar]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2014, 4, 701–712. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut Res. 2018, 25, 23236–23250. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Inter 2011, 6, 1–14. [Google Scholar]
- Vilchez, J.I.; Garcia-Fontana, C.; Román-Naranjo, D.; González-Lopéz, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Manzanera, M. Dealing with water stress and microbial preservation. Environ. Microbiol. 2021, in press. [Google Scholar]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Nandal, K.; Sehrawat, A.R.; Yadav, A.S.; Vashishat, R.K.; Boora, K.S. High temperature-induced changes in exopolysaccharides, lipopolysaccharides and protein profile of heat-resistant mutants of Rhizobium sp. (Cajanus). Microbiol. Res. 2005, 160, 367–373. [Google Scholar] [CrossRef]
- Pulsawat, W.; Leksawasdi, N.; Rogers, P.L.; Foster, L.J.R. Anions effects on biosorption of Mn(II) by extracellular polymeric substance (EPS) from Rhizobium etli. Biotechnol. Lett. 2003, 25, 1267–1270. [Google Scholar] [CrossRef]
- Aquastat. FAO’s Information System on Water and Agriculture. 2016. Available online: https://fao.org/aquastat (accessed on 20 April 2021).
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Manning, D.A.C. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 2010, 30, 281–294. [Google Scholar] [CrossRef]
- Buvaneshwari, S.; Riotte, J.; Sekhar, M.; Sharma, A.K.; Helliwell, R.; Mohan Kumar, M.S.; Braun, J.J.; Ruiz, L. Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020, 10, 3691. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D. Quantifying Vulnerability to Climate Change: Implications for Adaptation Assistance; Center for Global Development Working Paper No. 240; Center for Global Development: Washington, DC, USA, 2011. [Google Scholar]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.J.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Abadia, A.; Gomez-Aparisi, J.; Abadia, J. Chlorophyll fluorescence as a possible tool for salinity tolerance screening in barley (Hordeum vulgare L.). Plant Physiol. 1994, 104, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dubey, R.S. Changes in chlorophyll a and b contents and activities of photosystems 1 and 2 in rice seedlings induced by NaCl. Photosynthetica 1995, 31, 489–499. [Google Scholar]
- Mishra, S.K.; Subrahmanyam, D.; Singhal, G.S. Inter-relationship between salt and light stress on the primary processes of photosynthesis. J. Plant Physiol. 1991, 138, 92–96. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II. BBA Bioenergy 2009, 1787, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F. Cell permeability under salt stress. In Strategies for Improving Salt Tolerance in Higher Plants; Jaiwal, P.K., Singh, R.P., Gulati, A., Eds.; Science Publishing: Enfield, UK, 1997; pp. 87–110. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ. Exp. Bot. 2009, 65, 270–281. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity of growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Egamberdieva, D. The role of phytohormone producing bacteria in alleviating salt stress in crop plants. In Biotechnological Techniques of Stress Tolerance in Plants; Miransari, M., Ed.; Stadium Press LLC: Cedar City, UT, USA, 2013; pp. 21–39. [Google Scholar]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Int. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef]
- Kohler, J.; Hernandez, J.A.; Caravaca, F.; Roldan, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2008, 65, 245–252. [Google Scholar] [CrossRef]
- Armada, E.; Roldan, A.; Azcon, R. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2014, 67, 410–420. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Khodair, T.A.; Galal, G.F.; El-Tayeb, T.S. Effect of inoculating wheat seedlings with exopolysaccharide-producing bacteria in saline soil. J. Appl. Sci. Res. 2008, 4, 2065–2070. [Google Scholar]
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr. Sci. 2016, 12, 1961–1967. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Wolf, O. External potassium is not required for root growth in saline conditions: Experiments with Ricinus communis L. growth in a reciprocal split-root system. J. Exp. Bot. 1988, 39, 1149–1167. [Google Scholar] [CrossRef]
- Geddie, J.L.; Sutherland, I.W. Uptake of metals by bacterial polysaccharides. J. Appl. Bacteriol. 1993, 74, 467–472. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Physiological responses of soybean-inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 2005, 1, 216–221. [Google Scholar]
- Han, H.S.; Lee, K.D. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Arora, M.; Kaushik, A.; Rani, N.; Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J. Environ. Biol. 2010, 31, 701–704. [Google Scholar]
- Qurashi, A.W.; Sabri, A.N. Osmoadaptation and plant growth promotion by salt tolerant bacteria under salt stress. Afr. J. Microbiol. Res. 2011, 5, 3546–3554. [Google Scholar]
- Qurashi, A.W.; Sabri, A.N. Biofilm formation in moderately halophilic bacteria is influenced by varying salinity levels. J. Basic Microbiol. 2012, 52, 566–572. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N. Talc based exopolysaccharides formulation enhancing growth and production of Hellianthus annuus under saline conditions. Cell Mol. Biol. 2014, 60, 73–81. [Google Scholar] [PubMed]
- Nunkaew, T.; Kantachotea, D.; Nitodac, T.; Kanzakic, H.; Ritchied, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.W.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Platarum. 2006, 158, 34–44. [Google Scholar] [CrossRef]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef]
- Mohammed, A.F. Effectiveness of exopolysaccharides and biofilm forming plant growth promoting rhizobacteria on salinity tolerance of faba bean (Vicia faba L.). Afr. J. Microbiol. Res. 2018, 12, 399–404. [Google Scholar]
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of Salinity Stress on Some Growth Parameters of Wheat by Exopolysaccharide-Producing Bacteria. Iran. J. Sci. Technol. Trans. Sci. 2019, 43, 2725–2733. [Google Scholar] [CrossRef]
- Watanabe, M.; Kawahara, K.; Sasaki, K.; Noparatnaraporn, N. Biosorption of cadmium ions using a photosynthetic bacterium, Rhodobacter sphaeroides and a marine photosynthetic bacterium, Rhodovulum sp. and their biosorption kinetics. J. Biosci. Bioeng. 2003, 95, 374–378. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Yue, Z.B. Factors influencing the production of extra-cellular polymeric substances by Rhodopseudomonas acidophila. Int. Biodeterior. Biodegrad. 2006, 58, 89–93. [Google Scholar] [CrossRef]
- Moshabaki Isfahani, F.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Characterizing the new bacterial isolates of high yielding exopolysaccharides under hypersaline conditions. J. Clean Prod. 2018, 185, 922–928. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.K.Z.; Minakshi, G.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Jofré, J.; Fischer, S.; Rivarola, V.; Balegno, H.; Mori, G. Saline stress affects the attachment of Azospirillum brasilense Cd to maize and wheat roots. Can. J. Microbiol. 1998, 44, 416–422. [Google Scholar] [CrossRef]
- Parida, S.K.; Das, A.B. Salt tolerance and salinity effects on plants. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; McCully, M.E.; Jeffree, C.E. Plant and bacterial mucilages of maize rhizosphere: Comparison of their soil binding properties and histochemistry in a model system. Plant Soil 1993, 151, 151–165. [Google Scholar] [CrossRef]
- Bezzate, S.; Aymerich, S.; Chambert, R.; Czarnes, S.; Berge, O.; Heulin, T. Disruption of the Paenibacillus polymyxa levansucrase gene impairs its ability to aggregate soil in the wheat rhizosphere. Environ. Microbiol. 2000, 2, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Vanhaverbeke, C.; Heyraud, A.; Mazeau, K. Conformational analysis of the exopolysaccharide from Burkholderia caribensis strain MWAP71: Impact on the interaction with soils. Biopolymers 2003, 69, 480–497. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3e22. [Google Scholar] [CrossRef]
- Uad, I.; Silva-Castro, G.A.; Abrusci, C.; Catalina, F.; González-López, J.; Manzanera, M.; Calvo, C. Production index: A new index to evaluate EPSs as surfactants and bioemulsifiers applied to Halomonas variabilis strain W10 for hydrocarbon bioremediation. Ecotoxicol. Environ. Saf. 2019, 175, 66–73. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Ishii, S.; Koki, J.; Unno, H.; Hori, K. Two Morphological Types of Cell Appendages on a Strongly Adhesive Bacterium, Acinetobacter sp. Strain Tol 5. Appl. Environ. Microbiol. 2004, 70, 5026–5029. [Google Scholar] [CrossRef]
- Fujishige, N.A.; Kapadia, N.N.; Hirsch, A.M. A feeling for the micro-organism: Structure on a small scale. Biofilms on plant roots. Bot. J. Linn. Soc. 2006, 150, 79–88. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 44, 967–988. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M.; Shahzad, S.M. Variation in growth and ion uptake of maize due to inoculation with plant growth promoting rhizobacteria under salt stress. Soil Environ. 2006, 25, 78–84. [Google Scholar]
- Karlidag, H.; Esitken, A.; Yildirim, E.; Donmez, M.F.; Turan, M. Effects of plant growth promoting bacteria on yield, growth, leaf water content, membrane permeability, and ionic composition of strawberry under saline conditions. J. Plant Nutr. 2010, 34, 34–45. [Google Scholar] [CrossRef]
- Abd El-Azeem, S.A.M.; Elwan, M.W.M.; Sung, J.K.; Ok, Y.S. Alleviation of Salt Stress in Eggplant (Solanum melongena L.) by Plant-Growth-Promoting Rhizobacteria. Commun. Soil Sci. Plant Anal. 2012, 43, 1303–1315. [Google Scholar] [CrossRef]
- Abd El-Ghany, M.F.; Attia, M. Effect of Exopolysaccharide-producing bacteria and melatonin on Faba Bean production in saline and non-saline Soil. Agronomy 2020, 10, 316. [Google Scholar] [CrossRef]
- Guibaud, G.; Tixier, N.; Bouju, A.; Baudu, M. Relation between extracellular polymers’ composition and its ability to complex Cd Cu and Pb. Chemosphere 2003, 52, 1701–1710. [Google Scholar] [CrossRef]
- Fukushi, K. Phytoextraction of cadmium from contaminated soil assisted by microbial biopolymers. Agrotechnology 2012, 2, 110. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism, and remediationstrategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Ding, P.; Song, W.; Yang, Z.; Jian, J. Influence of Zn(II) stressinduction on component variation and sorption performance of extracellular polymeric substances (EPS) from Bacillus vallismortis. Bioprocess. Biosyst Eng. 2018, 41, 781–791. [Google Scholar] [CrossRef]
- Marschner, H. Mineral. Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1999. [Google Scholar]
- FAO. FAO Water Reports; FAO: Quebec City, QC, Canada, 2008; ISBN 978-92-5-107304-9. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water- deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 4, 178–182. [Google Scholar]
- Glick, B.R. Bacterial ACC deaminase and the alleviation of plant stress. Adv. Appl. Microbiol. 2004, 56, 291–312. [Google Scholar] [PubMed]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botanique 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin producing, plant growth promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Mantelin, S.; Touraine, B. Plant growth-promoting rhizobacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Expt. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z. Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol. Fert. Soil 2009, 45, 561–573. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Hui, L.J.; Kim, S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, S.F.; Yue, L.; Zhao, X.; Zhang, Y.B.; Xie, Z.K.; Wang, R.Y. Epsc Involved in the Encoding of Exopolysaccharides Produced by Bacillus amyloliquefaciens FZB42 Act to Boost the Drought Tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 219, 3795. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Sang-Mo, K.; Radhakrishnan, R.; Khan, A.L.; Min-Ji, K.; Jae-Man, P.; Bo-Ra, K.; Dong-Hyun, S.; In-Jung, L. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Pierik, R.; Sasidharan, R.; Voesenek, L.A.C.J. Growth control by ethylene: Adjusting phenotypes to the environment. J. Plant Growth Regul. 2007, 26, 188–200. [Google Scholar] [CrossRef]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water stress: Evolution of osmolyte system. Science 1982, 217, 122–1214. [Google Scholar] [CrossRef]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signalling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2006, 62, 2130. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi. Soci. Agri. Sci. 2011, 11, 57–61. [Google Scholar] [CrossRef]
- Moreno-Galván, A.; Romero-Perdomo, F.A.; Estrada-Bonilla, G.; Meneses, C.H.S.G.; Bonilla, R.R. Dry-caribbean Bacillus spp. strains ameliorate drought stress in maize by a strain-specific antioxidant response modulation. Microorganisms 2020, 8, 823. [Google Scholar] [CrossRef]
- Hartel, P.G.; Alexander, M. Role of extracellular polysaccharide production and clays in the desiccation tolerance of cowpea Bradyrhizobia. Soil Sci. Soc. Am. J. 1986, 50, 1193–1198. [Google Scholar] [CrossRef]
- Roberson, E.B.; Firestone, M.K. Relationship between desiccation and exopolysaccharide production in soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Konnova, S.A.; Brykova, O.S.; Sachkova, O.A.; Egorenkova, I.V.; Ignatov, V.V. Protective role of the polysaccharide containing capsular components of Azospirillum brasilense. Microbiology 2001, 70, 436–440. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in P. seudomonas and B. urkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Amellal, N.; Burtin, G.; Bartoli, F.; Heulin, T. Colonization of wheat rhizosphere by EPS producing Pantoea agglomerans and its effect on soil aggregation. Appl. Environ. Microbiol. 1998, 64, 3740–3747. [Google Scholar] [CrossRef]
- Ben-Hur, M.; Letey, J. Effect of exopolysaccharides, clay dispersion, and impact energy on water infiltration. Soil Sci. Soc. Am. J. 1989, 53, 233–238. [Google Scholar] [CrossRef]
- Chenu, C. Clay- or sand-polysaccharide associations as models for the interface between micro-organisms and soil: Water related properties and microstructure. Geoderma 1993, 56, 143–156. [Google Scholar] [CrossRef]
- Fetsiukh, A.; Conrad, J.; Bergquist, J.; Ayele, F.; Timmusk, S. Silica particles trigger the production of exopolysaccharides in harsh environment plant growth-promoting rhizobacteria and increase their ability to enhance drought tolerance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Or, D.; Phutane, S.; Dechesne, A. Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils. Vadose Zone J. 2007, 6, 298–305. [Google Scholar] [CrossRef]
- Chaney, K.; Swift, R.S. Studies on aggregate stability. I. Reformation of soil aggregates. J. Soil Sci. 1986, 37, 329–335. [Google Scholar]
- Chenu, C.; Guérif, J. Mechanical Strength of Clay Minerals as Influenced by an Adsorbed Polysaccharide. Soil Sci. Soc. Am. J. 1991, 55, 1076–1080. [Google Scholar] [CrossRef]
- Kaci, Y.; Heyraud, A.; Barakat, M.; Heulin, T. Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): Characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res. Microbiol. 2005, 156, 522–531. [Google Scholar] [CrossRef]
- Carminati, A.; Schneider, C.L.; Moradi, A.B.; Zarebanadkouki, M.; Vetterlein, D.; Vogel, H.J.; Hildebrandt, A.; Weller, U.; Schüler, L.; Oswald, S.E. How the rhizosphere may favor water availability to roots. Vadose Zone J. 2011, 10, 988–998. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, S.; LaManna, J.; Bais, H.; Jacobson, D.; Jin, Y. Plant Growth-Promoting Rhizobacteria (PGPR) Reduce Evaporation and Increase Soil Water Retention. Water Resour. Res. 2018, 54, 3673–3687. [Google Scholar] [CrossRef]
- Deng, J.; Orner, E.P.; Chau, J.F.; Anderson, E.M.; Kadilak, A.L.; Rubinstein, R.L.; Bouchillon, G.M.; Goodwin, R.A.; Gage, D.J.; Shor, L.M. Synergistic effects of soil microstructure and bacterial EPS on drying rate in emulated soil micromodels. Soil Biol. Biochem. 2015, 83, 116–124. [Google Scholar] [CrossRef]
- Putrie, R.F.W.; Wahyudi, A.T.; Nawangsih, A.A.; Husen, E. Screening of Rhizobacteria for Plant Growth Promotion and Their Tolerance to Drought Stress. Microbiol. Indones. 2013, 7, 2. [Google Scholar]
- Sivapriya, S.L.; Priya, P.R. Selection of Hyper Exopolysaccharide Producing and Cyst Forming Azotobacter Isolates for Better Survival under Stress Conditions. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2310–2320. [Google Scholar] [CrossRef]
- Yadav, S.N.; Singh, A.K.; Peter, J.K.; Masih, H.; Benjamin, J.C.; Singh, D.K.; Chaudhary, S.; Ramteke, P.W.; Ojha, S.K. Study of Exopolysaccharide Containing PGPRs on Growth of Okra Plant under Water Stress Conditions. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3337–3374. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef]
- Manara, A. Plant responses to heavy metal toxicity. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 27–53. [Google Scholar]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef]
- Rouch, D.A.; Lee, T.O.B.; Morby, A.P. Understanding cellular responses to toxic agents: A model for mechanisms-choice in bacterial resistance. J. Ind. Microbiol. 1995, 14, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Qurat ul ain; Khan, A.A.; Ali, J.; et al. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Desale, P.; Patel, B.; Singh, S.; Malhotra, A.; Nawani, N. Plant growth promoting properties of Halobacillus sp. and Halomonas sp. in presence of salinity and heavy metals. J. Basic. Microbiol. 2014, 54, 781–791. [Google Scholar]
- Dimkpa, C.O.; Svatos, A.; Dabrowska, P.; Schmidt, A.; Boland, W.; Kothe, E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 2008, 74, 19–25. [Google Scholar] [CrossRef]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas Rhizobacteria of Avicennia marina of Indian Sundarbans Promote Rice Growth Under Saline and Heavy Metal Stresses Through Exopolysaccharide Production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Kowalkowski, T.; Krakowska, A.; Złoch, M.; Hrynkiewicz, K.; Buszewski, B. Cadmium-affected synthesis of exopolysaccharides by rhizosphere bacteria. J. Appl. Microbiol. 2019, 127, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of Extracellular Polysaccharides and Biofilm Formation in Heavy Metal Resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pre-treatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Adam, S.; Murthy, S.D.S. Effect of Cold Stress on Photosynthesis of Plants and Possible Protection Mechanisms. In Approaches to Plant Stress and Their Management; Gaur, R., Sharma, P., Eds.; Springer: New Delhi, India, 2014. [Google Scholar]
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plant 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Rodríguez, M.; Canales, E.; Borrás-Hidalgo, O. Molecular aspects of abiotic stress in plants. Biotechnol. Appl. 2005, 22, 1–10. [Google Scholar]
- Savitch, L.V.; Leonardos, E.D.; Krol, M.; Jansson, S.; Grodzinski, B.; Huner, N.P.A.; Oquist, G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 2002, 25, 761–771. [Google Scholar] [CrossRef]
- Ensminger, I.; Bosch, F.; Huner, N.P.A. Photo stasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Janska, A.; Marsık, P.; Zelenkova, S.; Ovesna, J. Cold stress and acclimation–What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Julca, I.; Alaminos, M.; González-López, J.; Manzanera, M. Xeroprotectants for the stabilization of biomaterials. Biotechnol. Adv. 2012, 30, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J. Responses of Plants to Environmental Stress, Volume 1: Chilling, Freezing, and High Temperature Stresses; Academic Press: New York, NY, USA; London, UK; Toronto, ON, Canada; Sydney, Australia; San Francisco, CA, USA, 1980. [Google Scholar]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Inter. 2011, 4, 239–246. [Google Scholar]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of plant growth-promoting rhizobacteria from rhizosphere soil of heat-stressed and unstressed wheat and their use as bio-inoculant. Plant Biol. 2019, 21, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Chakraborty, B.; Chakraborty, U. Plant Growth Promoting Rhizobacteria Protect Wheat Plants Against Temperature Stress Through Antioxidant Signalling and Reducing Chloroplast and Membrane Injury. J. Plant Growth Regul. 2018, 37, 1396–1412. [Google Scholar] [CrossRef]
- Mukhtar, T.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Ali, S.; Chaudhary, H.J.; Solieman, T.H.; Ibrahim, A.A.; Saad, M.A. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Ogden, A.J.; McAleer, J.M.; Kahn, M.L. Characterization of the Sinorhizobium meliloti HslUV and ClpXP Protease Systems in Free-Living and Symbiotic States. J. Bacteriol. 2019, 201, e00498-18. [Google Scholar] [CrossRef]
- Nishihata, S.; Kondo, T.; Tanaka, K.; Ishikawa, S.; Takenaka, S.; Kang, C.H.; Yoshida, K. Bradyrhizobium diazoefficiens USDA110 PhaR functions for pleiotropic regulation of cellular processes besides PHB accumulation. BMC Microbiol. 2018, 18, 156. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, M.; Linga, V.R.; Bandi, V. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Mishra, P.K.; Bisht, S.C.; Ruwari, P.; Selvakumar, G.; Joshi, G.K.; Bisht, J.K.; Bhatt, J.C.; Gupta, H.S. Alleviation of cold stress in inoculated wheat (Triticum aestivum L.) seedlings with psychrotolerant Pseudomonads from NW Himalayas. Arch. Microbiol. 2011, 193, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and Expression Analysis of Psychrophilic Bacillus spp. Reveal Their Potential to Alleviate Cold Stress and Modulate Phytohormones in Wheat. Microorganisms 2109, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Marvasi, M.; Visscher, P.T.; Martinez, L.C. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbial. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Bacteria | EPS Mechanism | Crop | Reference |
|---|---|---|---|
| Aeromonas hydrophila/caviae MAS765 Bacillus insolitus MAS17 Bacillus sp. MAS617 Bacillus sp. MAS620 Bacillus sp. MAS820 | Restricted Na+ uptake by a reduced passive flow of Na+ into the stele due to the higher proportion of root zones covered with soil sheaths in inoculated plants | Wheat | Ashraf et al. [52] |
| Bacillus circulans UBF 26 | |||
| Bacillus polymyxa UBF 15 | |||
| Bacillus subtilis Serratia proteamaculans | Restricted Na+ influx due to free soil Na+ binding by EPS | Soybean | Han and Lee [58] |
| Consortium of Cyanobacteria | Removes Na+ from aqueous solution by Na+ adsorption | Wheat; Maize; Rice | Arora et al. [59] |
| Bacillus licheniformis SKU3 | Restricted Na+ uptake in saline and non-saline soil by EPS | Wheat | Upadhyay et al. [17] |
| Bacillus pumilus SKU4 | |||
| Bacillus sp. SKU5 | |||
| Burkholderia cepacia SKU6 | |||
| Enterobacter sp. SKU7 | |||
| Enterobacter sp. SKU8 | |||
| Microbacterium sp. SKU9 | |||
| Paenibacillus macerans SKU10 | |||
| Paenibacillus sp. SKU11 | |||
| Bacillus coagulans SKU12 | |||
| Bacillus insolitus SKU13 | |||
| Oceanobacillus profundus Pmt2 Staphylococcus saprophyticus ST1 | Na+ chelation. Increased mass of roots adhering soil (RAS) and biofilm stability | Lens esculenta | Qurashi and Sabri [60] |
| Halomonas variabilis HT1 Planococcus rifietoensis RT4 | Enhances soil aggregation and biofilm formation | Chickpea | Qurashi and Sabri [61] |
| Pseudomonas aeruginosa PF07 | Enhances root-adhering soil to root tissue ratio (RAS/RT) | Sunflower | Tewari and Arora [62] |
| Rhodopseudomonas palustris TN114 Rhodopseudomonas palustris PP803 | Reduces Na+ in aqueous solution by EPS binding of Na+ (Na+ binding by galacturonic acid) | Nunkaew et al. [63] | |
| Bacillus amyloliquefaciens SQR9 | Improves Na+ homeostasis | Maize | Chen et al. [64] |
| Bacillus amyloliquefaciens HM6 | Root protection by enhancing biofilm stability | Barley | Kasim et al. [65] |
| Pseudomonas sp. AK-1 | Restricted Na+ influx due to free soil Na+ binding by EPS | Soybean | Kasotia et al. [54] |
| Pseudomonas anguilliseptica SAW24 | Root protection by enhancing biofilm stability | Faba bean | Mohammed [66] |
| Bacillus subtilis TP7 Marinobacter lipolyticus SM19 | Restricted Na+ uptake | Wheat | Atouei et al. [67] |
| Bacteria | EPS Mechanism | Crop | Reference |
|---|---|---|---|
| Bacillus polymyxa CF43 | Increases the mass of soil adhering to the roots | Wheat | Bezzate et al. [75] |
| Pantoea agglomerans NAS206 | Augments the root-adhering soil (RAS) in both excessive or deficit water | Wheat | Amellal et al. [129] |
| Rhizobium sp. YAS34 | Increases root-adhering soil (RAS) per root dry mass and soil macropore volume | Sunflower | Alami et al. [109] |
| Rhizobium sp. KYGT207 | Enhances root-adhering soil (RAS) dry mass (dm) per root dm (RAS/RT) and RAS aggregate water stability | Wheat | Kaci et al. [136] |
| Pseudomonas putida GAP-P45 | Improves soil aggregates stability | Sunflower | Sandhya et al. [71] |
| Pseudomonas entomophila BV-P13 | Influence higher soil aggregates stability and mean weight diameter of root-adhering soil (RAS) | Maize | Sandhya et al. [120] |
| Pseudomonas stutzei GRFHAP-P14 | |||
| Pseudomonas putida GAP-P45 | |||
| Pseudomonas syringae GRFHYTP52 | |||
| Pseudomonasmonteilli WAPP53 | |||
| Bacillus sp. HYD-B17 | Increase root-adhering soil (RAS) dry mass (dm) per root dm (RAS/RT) and weight diameter of soil aggregates | Maize | Sandhya et al. [14] |
| Bacillus sp. HYTAPB18 | |||
| Bacillus sp. HYDGRFB19 | |||
| Bacillus sp. BKB30 | |||
| Bacillus sp. RMPB44 | |||
| Pseudomonas aeruginosa B2 | Augment soil aggregates stability | Maize | Putrie et al. [141] |
| Brevibacillus brevis B33 | |||
| Proteus penneri Pp1 | Enhance moisture and water content of soil | Maize | Nassem and Bano [138] |
| Pseudomonas aeruginosa Pa2 | |||
| Alcaligenes faecalis AF3 | |||
| Azotobacter sp. AztRMD2 | Augment soil aggregates stability | Rice | Sivapriya et al. [142] |
| Bacillus amyloliquefaciens FZB42 | Plant protection by enhancing biofilm stability | Arabidopsis thaliana | Lu et al. [110] |
| Pseudomonas aeruginosa MCCB0035 | Increase moisture and water content of soil | Okra Plant | Yadav et al. [143] |
| Bacillus coagulans MCCB0059 | |||
| Planomicrobium chinese P1 | Maintain high moisture content in soil | Wheat | Khan and Bano [47] |
| Bacillus cereus P2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcillo, R.J.L.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. https://doi.org/10.3390/metabo11060337
Morcillo RJL, Manzanera M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites. 2021; 11(6):337. https://doi.org/10.3390/metabo11060337
Chicago/Turabian StyleMorcillo, Rafael J. L., and Maximino Manzanera. 2021. "The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance" Metabolites 11, no. 6: 337. https://doi.org/10.3390/metabo11060337
APA StyleMorcillo, R. J. L., & Manzanera, M. (2021). The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites, 11(6), 337. https://doi.org/10.3390/metabo11060337








