Impact of High-Pressure Processed Onion on Colonic Metabolism Using a Dynamic Gastrointestinal Digestion Simulator
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical and Chemical Characterization and Phenolic Compounds of Undigested HPP-Treated Onion Powder
2.2. Phenolic Compounds and Metabolites in the Colon Fermentation Products of HPP-Treated Onion Powder. Metabolomic Analysis by a Targeted Approach
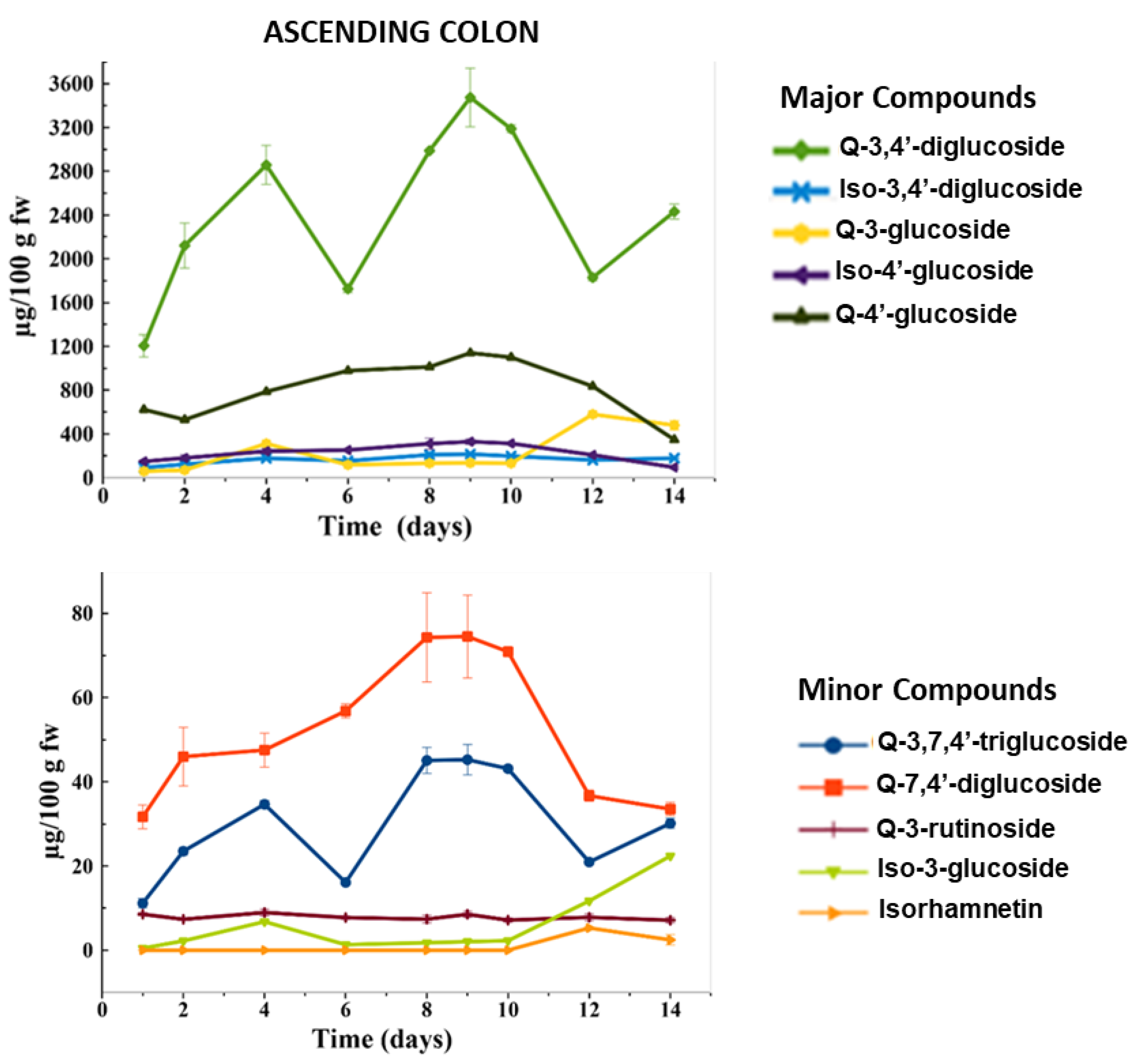
2.2.1. Evolution of Flavonol Glycosides during Colonic Fermentation
2.2.2. Evolution of Flavonol Glycosides during Colonic Fermentation
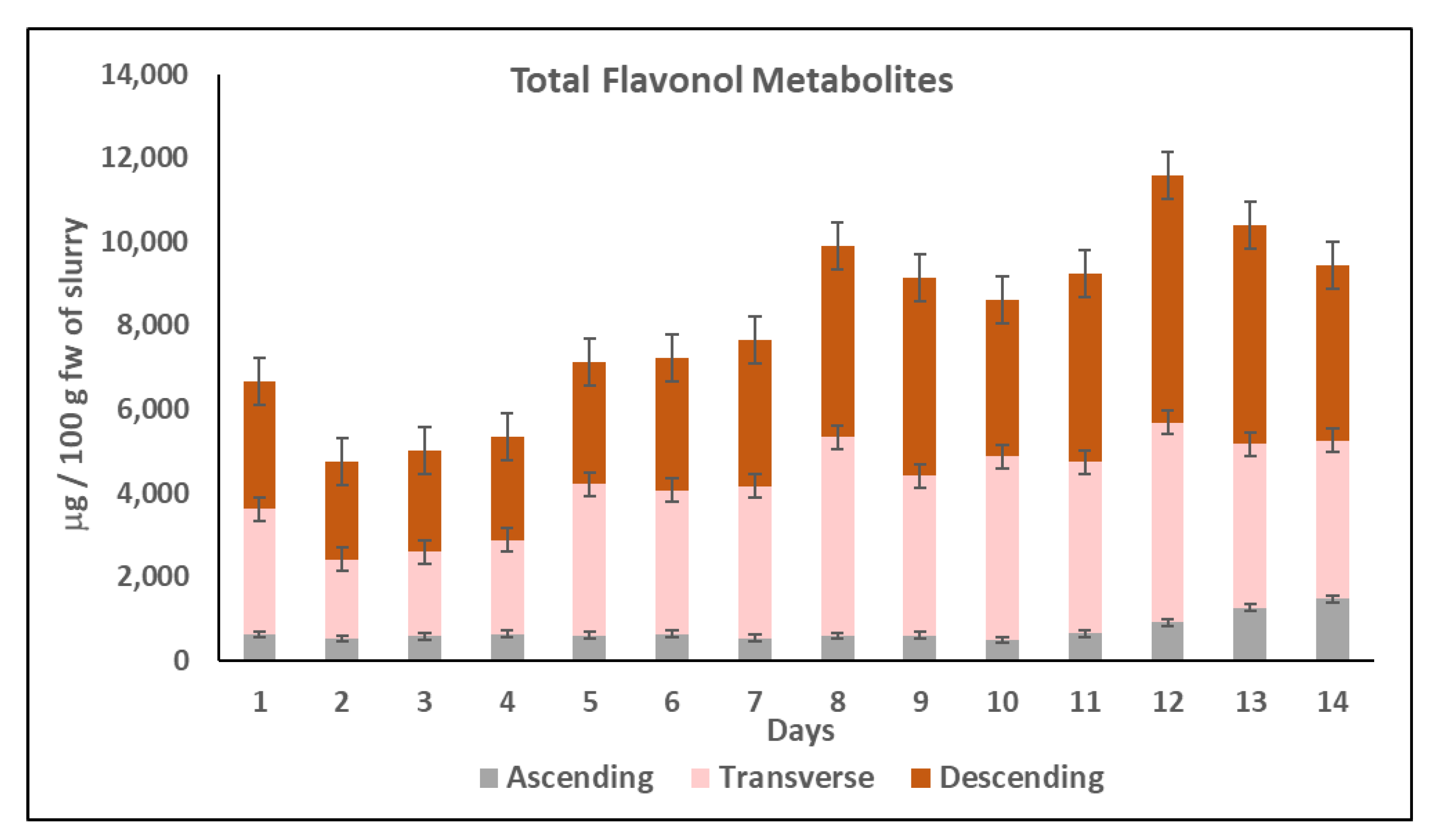
2.2.3. Evolution of Flavonol Metabolites during Colonic Fermentation
2.2.4. Multivariate Data Analysis of the HPLC-DAD-ESI-QTOF-MS/MS Data
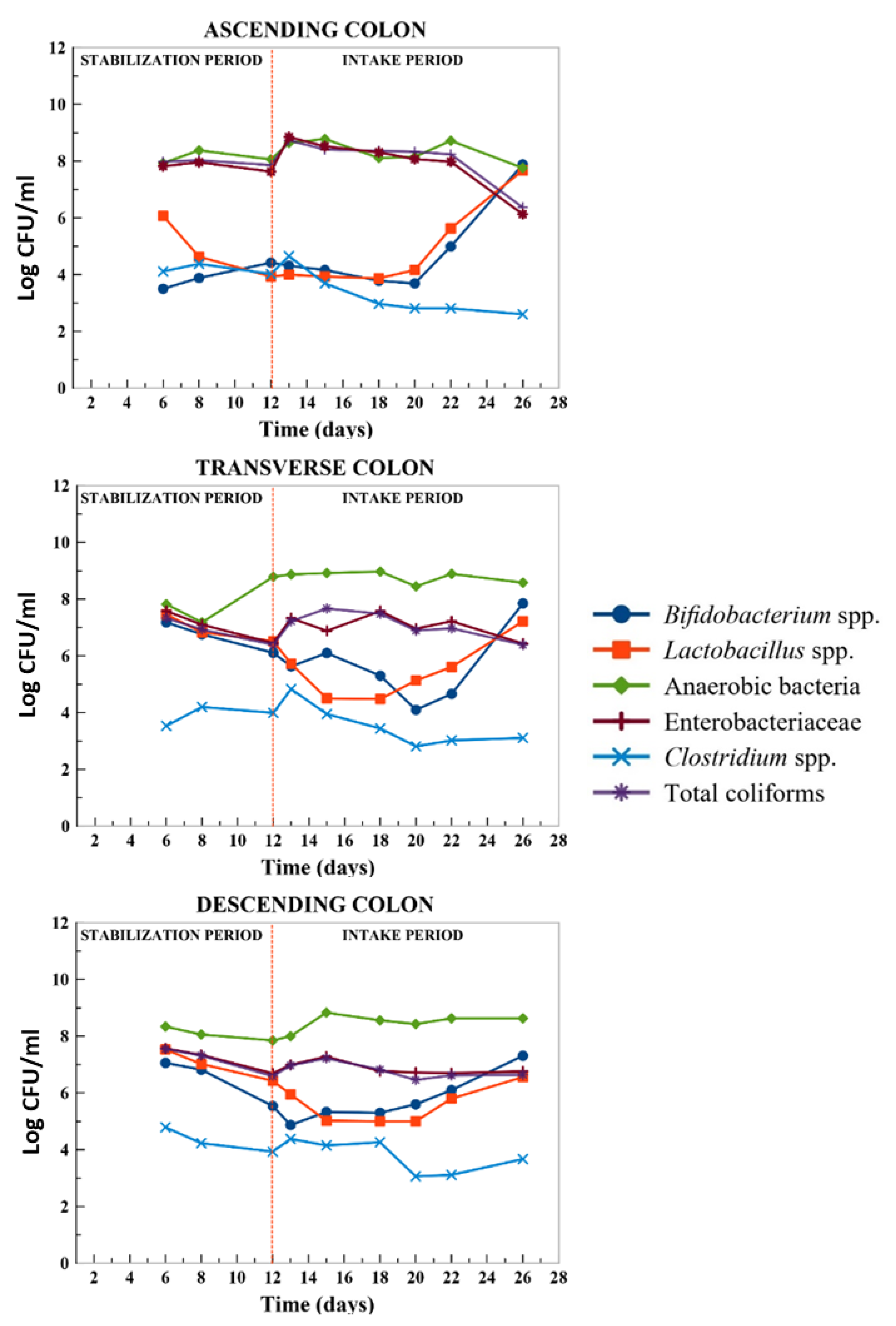
2.3. Modulation of Microbiota Population
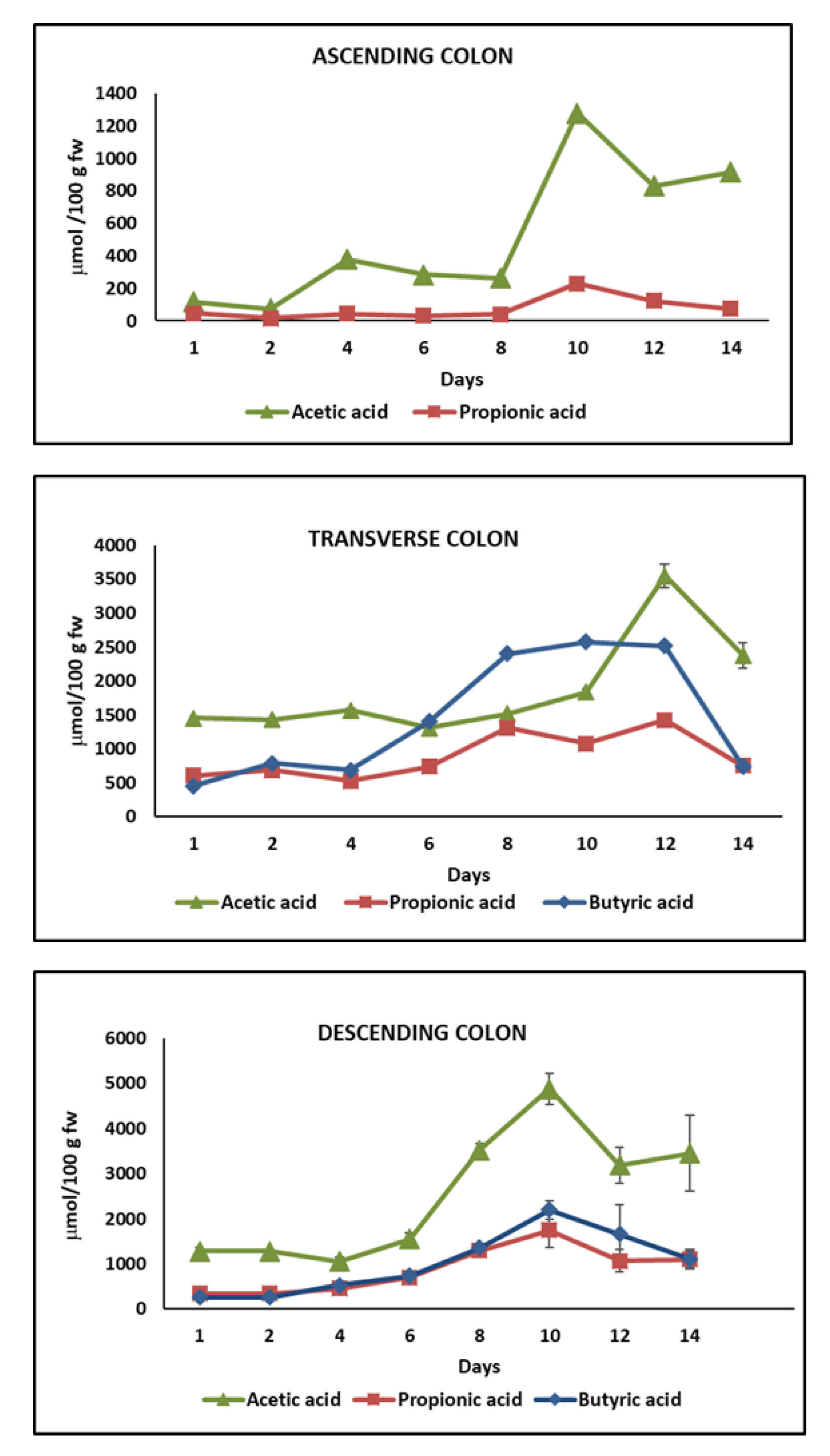
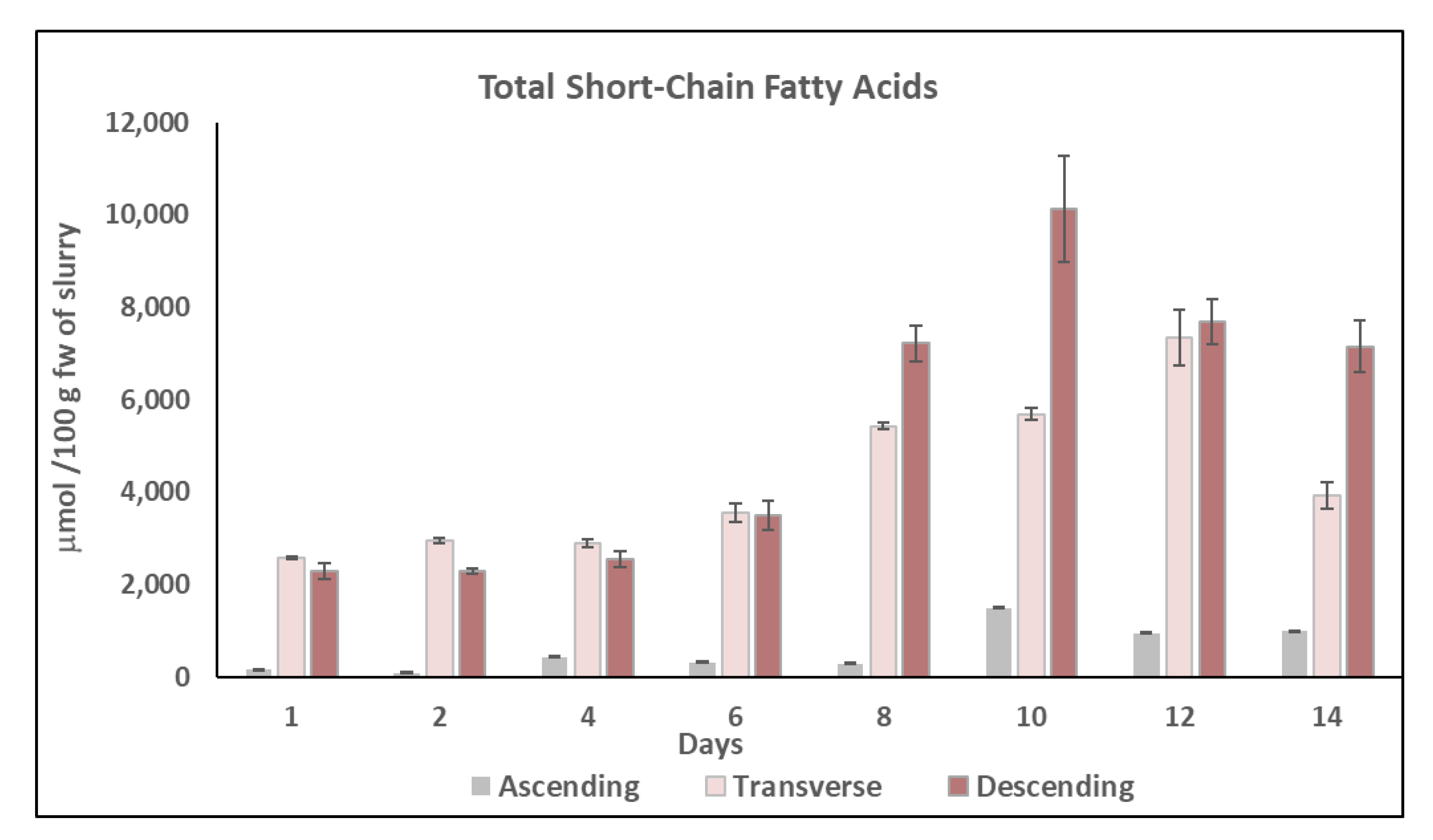
2.4. Short-Chain Fatty Acids (SCFAs) Content
3. Materials and Methods
3.1. Reagents
3.2. Raw Material
3.3. High-Pressure Processing (HPP)
3.4. Characterization of HPP-Treated Onion Powder
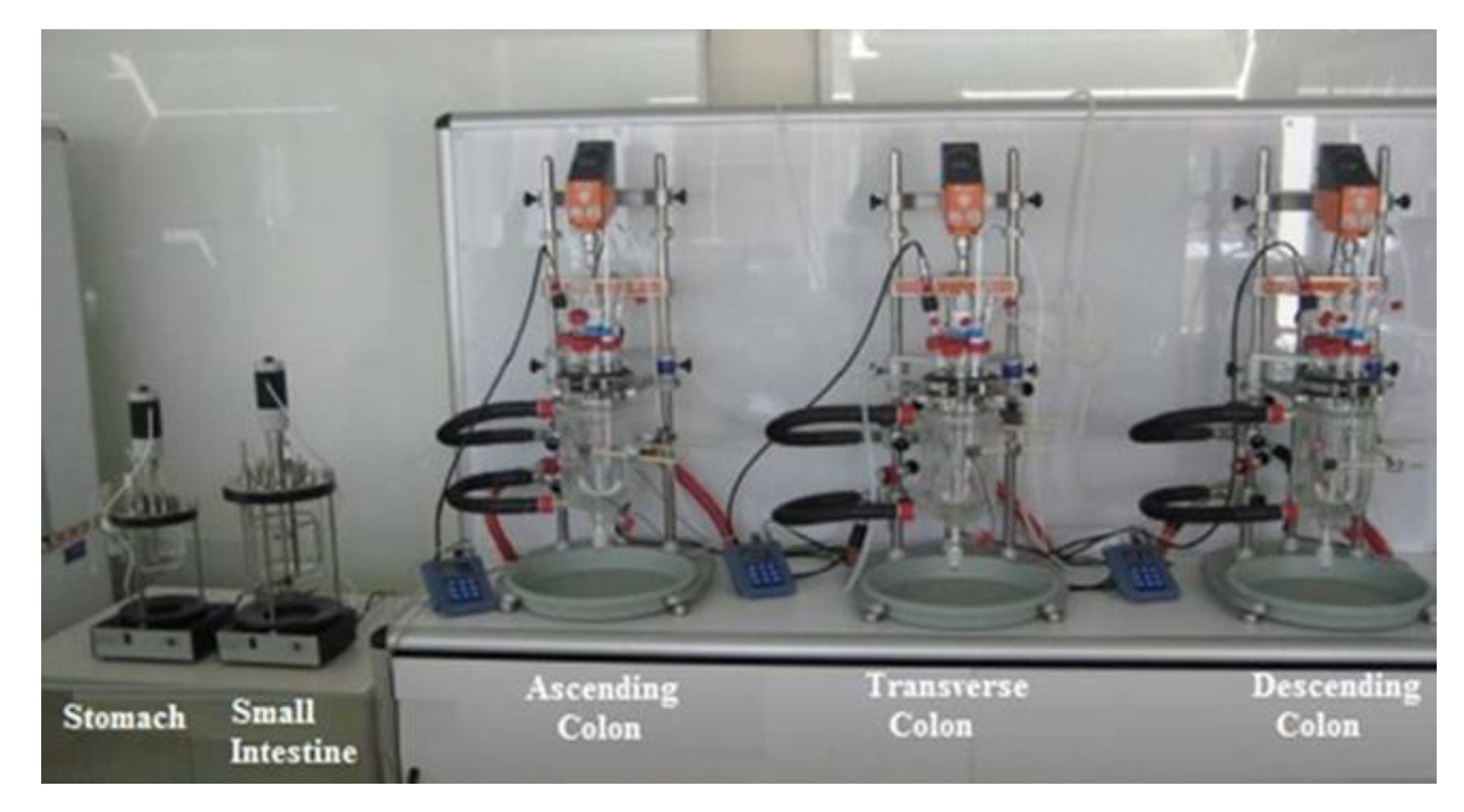
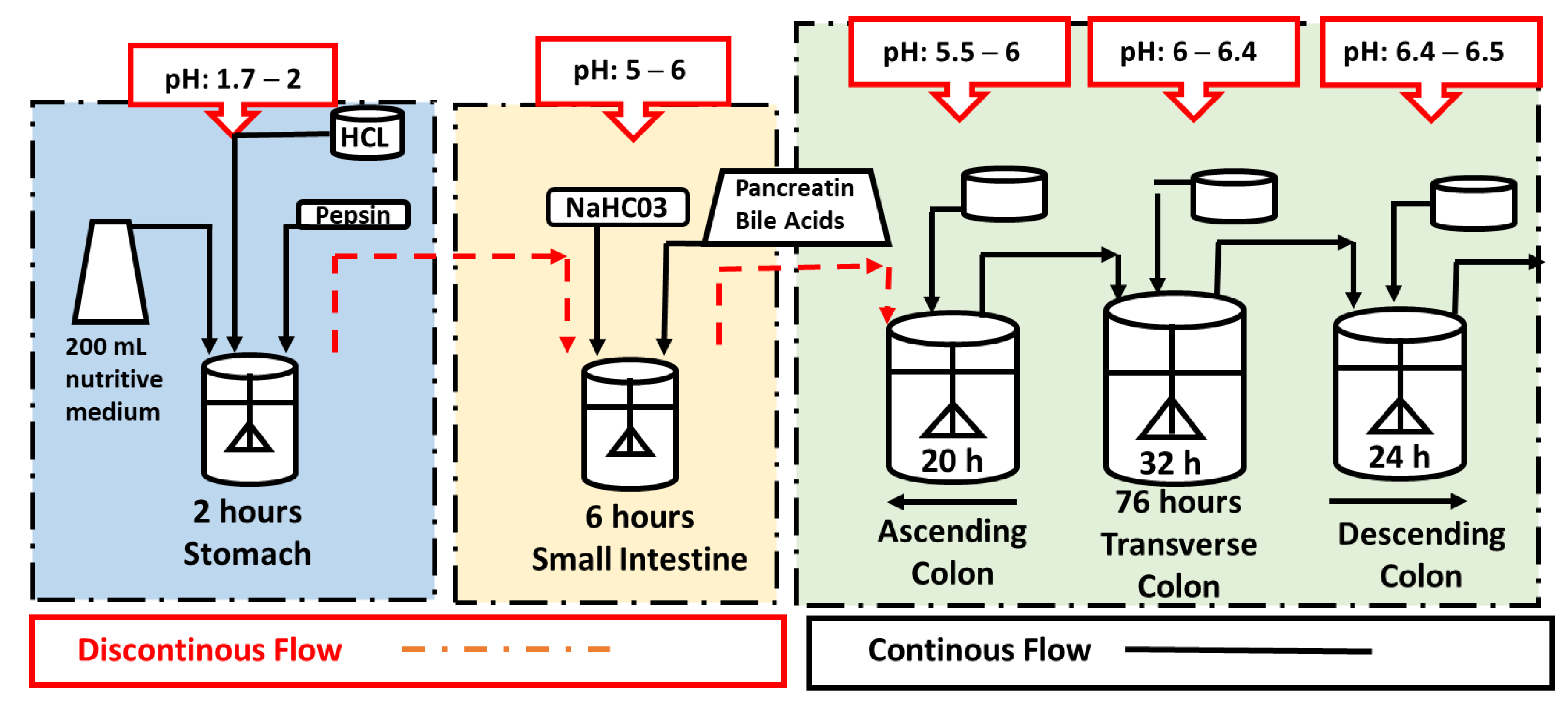
3.5. Dynamic In Vitro Gastrointestinal and Colonic Fermentation (DGID-CF) Model
3.6. Analysis of Phenolic Compounds and Metabolites by HPLC-DAD-ESI-QTOF-MS/MS
3.6.1. Phenolic Compounds in the HPP-Treated Onion Powder
3.6.2. Phenolic Compounds and Metabolites in the Colon Fermentation Products
3.6.3. HPLC-DAD-ESI-QTOF-MS/MS Analysis of Phenolic Compounds and Metabolites. Metabolomic Analysis by a Targeted Approach
3.7. Microbial Analysis
3.8. Analysis of Short-Chain Fatty Acids (SCFAs)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Crops. Onions, Dry. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 April 2020).
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef]
- Serra, A.; Macia, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vagen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Wang, W.Y.; Sun, C.X.; Mao, L.K.; Ma, P.H.; Liu, F.G.; Yang, J.; Gao, Y.X. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Ribas-Agusti, A.; Martin-Belloso, O.; Soliva-Fortuny, R.; Elez-Martinez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.B.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Ulbrich, K.; Reichardt, N.; Braune, A.; Kroh, L.W.; Blaut, M.; Rohn, S. The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res. Int. 2015, 67, 349–355. [Google Scholar] [CrossRef]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomas-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Surco-Laos, F.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Schloissnig, S.; Arumugam, M.; Sunagawa, S.; Mitreva, M.; Tap, J.; Zhu, A.; Waller, A.; Mende, D.R.; Kultima, J.R.; Martin, J.; et al. Genomic variation landscape of the human gut microbiome. Nature 2013, 493, 45–50. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.F.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.R.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.Y.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, 13. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martinez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food. Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Payne, A.N.; Zihler, A.; Chassard, C.; Lacroix, C. Advances and perspectives in in vitro human gut fermentation modeling. Trends Biotechnol. 2012, 30, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleivelan, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ekbatan, S.S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Sanchez-Patán, F.; Barroso, E.; van de Wiele, T.; Jiménez-Giron, A.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martin-Álvarez, P.J.; Pozo-Bayon, M.A.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- Colina-Coca, C.; González-Peña, D.; de Ancos, B.; Sanchez-Moreno, C. Dietary onion ameliorates antioxidant defence, inflammatory response, and cardiovascular risk biomarkers in hypercholesterolemic Wistar rats. J. Funct. Food. 2017, 36, 300–309. [Google Scholar] [CrossRef]
- González-Peña, D.; Dudzik, D.; García, A.; De Ancos, B.; Barbas, C.; Sánchez-Moreno, C. Metabolomic fingerprinting in the comprehensive study of liver changes associated with onion supplementation in hypercholesterolemic Wistar rats. Int. J. Mol. Sci. 2017, 2, 267. [Google Scholar] [CrossRef]
- Benitez, V.; Molla, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andreu, F.J.; Esteban, R.M. Onion (Allium cepa L.) by-products as source of dietary fiber: Physicochemical properties and effect on serum lipid levels in high-fat fed rats. Eur. Food Res. Technol. 2012, 234, 617–625. [Google Scholar] [CrossRef]
- Galdón, B.R.; Rodríguez, C.T.; Rodríguez, E.R.; Romero, C.D. Organic acid contents in onion cultivars (Allium cepa L.). J. Agric. Food Chem. 2008, 56, 6512–6519. [Google Scholar] [CrossRef]
- Liguori, L.; Califano, R.; Albanese, D.; Raimo, F.; Crescitelli, A.; Di Matteo, M. Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J. Food Qual. 2017, 2017, 6873651. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sanchez-Moreno, C.; De Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ’Golden Delicious’ from different geographical origin. Innov. Food Sci. Emerg. Technol. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sanchez-Moreno, C.; De Ancos, B. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion. J. Food Eng. 2017, 213, 60–68. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewieck, J.; Cvikrova, M.; Martincova, O.; Katrich, E.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Food. 2016, 27, 201–213. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberán, F.A.; Espín, J.C. Effect of food structure and processing on (poly)phenol-gut microbiota interactions and the effects on human health. In Annual Review of Food Science and Technology; Doyle, M.P., McClements, D.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2019; Volume 10, pp. 221–238. [Google Scholar]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts: Bioaccessible phenolic metabolites and impact on human gut microbiota. J. Food Compos. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Serra, A.; Macia, A.; Romero, M.P.; Angles, N.; Morello, J.R.; Motilva, M.J. Metabolic pathways of the colonic metabolism of procyanidins (monomers and dimers) and alkaloids. Food Chem. 2011, 126, 1127–1137. [Google Scholar] [CrossRef]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013, 4, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A Review from a historical perspective. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Romero, M.P.; Motilva, M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Gao, K.; Xu, A.L.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.T.; Lu, J.X.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef]
- Xue, H.T.; Xie, W.Y.; Jiang, Z.H.; Wang, M.; Wang, J.; Zhao, H.Q.; Zhang, X.Y. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Urpi-Sarda, M.; Vázquez-Agell, M.; Lamuela-Raventos, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.W.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Najmanova, I.; Pourova, J.; Voprsalova, M.; Pilarova, V.; Semecky, V.; Novakova, L.; Mladenka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.; Cueva, C.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Development of human colonic microbiota in the computer-controlled dynamic SIMulator of the GastroIntestinal tract SIMGI. LWT-Food Sci. Technol. 2015, 61, 283–289. [Google Scholar] [CrossRef]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Duque, A.; Monteiro, M.; Adorno, M.A.T.; Sakamoto, I.K.; Sivieri, K. An exploratory study on the influence of orange juice on gut microbiota using a dynamic colonic model. Food Res. Int. 2016, 84, 160–169. [Google Scholar] [CrossRef]
- Sanchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targeted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]
- Pascoal, G.B.; Filisetti, T.M.C.C.; Alvares, E.P.; Lajolo, F.M.; Menezes, E.W. Impact of onion (Allium cepa L) fructans fermentation on the cecum of rats and the use of in vitro biomarkers to assess in vivo effects. Bioact. Carbohydr. Diet. Fibre 2013, 1, 89–97. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Blanco, N.; Xu, J.; Ahrné, S.; Molin, G.; Sterner, O.; Nyman, M. Formation of short-chain fatty acids, excretion of anthocyanins, and microbial diversity in rats fed blackcurrants, blackberries, and raspberries. Int. J. Nutr. Metab. 2013, 2013, 202534. [Google Scholar] [CrossRef]
- González-Peña, D.; Colina-Coca, C.; Char, C.D.; Cano, M.P.; de Ancos, B.; Sanchez-Moreno, C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef]
- Colina-Coca, C.; De Ancos, B.; Sánchez-Moreno, C. Nutritional composition of processed onion: S-alk(en)yl-L-cysteine sulfoxides, organic acids, sugars, minerals, and vitamin C. Food Bioprocess. Technol. 2014, 7, 289–298. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Van de Wiele, T.R.; Oomen, A.G.; Wragg, J.; Cave, M.; Minekus, M.; Hack, A.; Cornelis, C.; Rompelberg, C.J.M.; De Zwart, L.L.; Klinck, B.; et al. Comparison of five in vitro digestion models to in vivo experimental results: Lead bioaccessibility in the human gastrointestinal tract. J. Environ. Sci. Health Part. A-Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1203–1211. [Google Scholar] [CrossRef]
- Marzorati, M.; Verhelst, A.; Luta, G.; Sinnott, R.; Verstraete, W.; Van de Wiele, T.; Possemiers, S. In vitro modulation of the human gastrointestinal microbial community by plant-derived polysaccharide-rich dietary supplements. Int. J. Food Microbiol. 2010, 139, 168–176. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food. An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Molly, K.; Vandewoestyne, M.; Desmet, I.; Verstraete, W. Validation of the simulator of the human intestinal microbial ecosystem (shime) reactor using microorganism-associated activities. Microb. Ecol. Health Dis. 1994, 7, 191–200. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Verstraete, W. Development of a 5-step multichamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kontula, P.; Jaskari, J.; Nollet, L.; De Smet, I.; von Wright, A.; Poutanen, K.; Mattila-Sandholm, T. The colonization of a simulator of the human intestinal microbial ecosystem by a probiotic strain fed on a fermented oat bran product: Effects on the gastrointestinal microbiota. Appl. Microbiol. Biotechnol. 1998, 50, 246–252. [Google Scholar] [CrossRef]
- Tannock, G.W.; Munro, K.; Harmsen, H.J.M.; Welling, G.W.; Smart, J.; Gopal, P.K. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 2000, 66, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Bunesova, V.; Killer, J.; Vlkova, E.; Musilova, S.; Tomaska, M.; Rada, V.; Kmet, V. Isolation and characterization of bifidobacteria from ovine cheese. Int. J. Food Microbiol. 2014, 188, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, F.; Fontecha, J.; Rodriguez-Alcalá, L.M. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta 2014, 128, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]


| Parameters | HPP-Treated Onion |
|---|---|
| Physicochemical characteristics | |
| pH | 5.50 ± 0.04 |
| Acidity (g citric acid/100 g dw) | 0.60 ± 0.08 |
| Chemical characteristics | |
| Ascorbic acid (mg/100 g dw) | 147.78 ± 3.80 |
| Vitamin C (mg/100 g dw) | 170.60 ± 2.57 |
| Proteins (g/100 g dw) | 3.41 ± 0.27 |
| Fibre (mg/100 g dw) | |
| Soluble | 4.57 ± 0.15 |
| Insoluble | 14.54 ± 0.05 |
| Total | 19.11 ± 0.50 |
| Sugars (g/100 g dw) | |
| Sacarose | 3.58 ± 0.21 |
| Glucose | 11.95 ± 0.75 |
| Fructose | 22.15 ± 1.25 |
| Organic acids | |
| Maleic acid (g/100 g dw) | 1.48 ± 0.18 |
| Citric acid (mg/100 g dw) | 239.52 ± 2.31 |
| Fumaric acid (mg/100 g dw) | 12.09 ± 0.92 |
| Acetic acid (mg/100 g dw) | 5.21 ± 1.32 |
| Pyruvic acid (mg/100 g dw) | 80.23 ± 2.58 |
| Flavonols (mg/100 g dw) | |
| Quercetin-3,7,4′-triglucoside | 26.74 ± 2.39 |
| Quercetin-7,4′-diglucoside | 13.31 ± 1.06 |
| Quercetin-3,4′-diglucoside | 5090.47 ± 104.64 |
| Quercetin-3-rutinoside | 2.02 ± 0.37 |
| Isorhamnetin-3,4′-diglucoside | 174.46 ± 32.23 |
| Quercetin-3-glucoside | 108.88 ± 15.56 |
| Quercetin-4′-glucoside | 1015.55 ± 156.38 |
| Isorhamnetin-3-glucoside | 2.04 ± 0.31 |
| Isorhamnetin-4′-glucoside | 322.77 ± 48.46 |
| Quercetin | 5.57 ± 0.57 |
| Isorhamnetin | 3.35 ± 0.3 |
| Phenolic acids (mg/100 g dw) | |
| Protocatechuic acid | 0.56 ± 0.08 |
| Gallic acid | 1.23 ± 0.25 |
| Salicylic acid | 1.87 ± 0.07 |
| Compounds | Formula | Mass | RT (min) | m/z (M − H) | Mayor ESI m/z [M − H]− and Fragments |
|---|---|---|---|---|---|
| Benzoic acid derivatives | |||||
| 3,4-Dihydroxybenzoic acid | C7H6O4 | 154.0243 | 1.91 | 153.0193 | 153.0199; 133.0260; 109.0291; 91.0226; 81.0286 |
| 3,4,5-Trihydroxybenzoic acid (gallic acid) | C7 H6 O5 | 170.0191 | 2.10 | 169.0151 | 169.0153; 125.0229; 107.0226; 96.9588; 97.0280; 89.027; 68.9951 |
| 2-Hydroxybenzoic acid (salicylic acid) | C7H6O3 | 138.0322 | 3.03 | 137.0244 | 137.0266; 129.0563; 119.0363; 112.9852; 93.0347; 89.0252; 68.9969 |
| 4-Hydroxybenzoic acid | C7H6O3 | 138.0322 | 3. 29 | 137.0257 | 137.0284; 124.0280; 112.9876; 108.1550; 98.957; 93.0345 |
| 3,5-Dihydroxybenzoic acid | C7H6O4 | 154.0267 | 5.25 | 153.0193 | 153.0267; 137.0336; 131.0709; 109.0295; 91.0038; 68.9951 |
| 3-Hydroxybenzoic acid | C7H6O3 | 138.0323 | 5.53 | 137.0255 | 137.0251; 123.0451; 121.0295; 93.0351; 91.0046 |
| 4-Hydroxy-3,5-dimethoxybenzoic acid (syringic acid) | C9H10O5 | 198.0528 | 6.20 | 197.0455 | 197.0458; 187.0215; 161.029; 153.0405; 151.0416; 137.0319 |
| Benzoic acid | C7H6O2 | 122.0364 | 12.53 | 121.0294 | 121.0646; 102.9488; 89.0262; 77.0457 |
| Phenylacetic acid derivatives | |||||
| 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 168.0425 | 2.78 | 167.0350 | 167.0343; 123.0447; 137.0271; 96.4594; 61.9887 |
| 3-Methoxy-4-hydroxyphenylacetic acid (homovanillic acid) | C9H10O4 | 182.0579 | 2.66 | 181.0506 | 181.0584; 167.0358; 137.0305; 123.0461; 122.0350 |
| 4-Hydroxyphenylacetic acid | C8H8O3 | 152.0474 | 4.61 | 151.0407 | 151.0534; 137,0281; 128.0355; 121.0295; 112.9862; 107.0497; 96.9503 |
| 3-Hydroxyphenylacetic acid | C8H8O3 | 152.0474 | 6.12 | 151.0407 | 151.0406; 107.0512; 112.9861; 108.0542; 119.0363; 65.0368 |
| Hydroxyphenylacetic acid | C8H8O3 | 152.0474 | 7.47 | 151.0407 | 151.0422; 137.0302; 119.036; 107.0513; 89.0253 |
| Phenylacetic acid | C8H8O2 | 136.0527 | 12.99 | 135.0451 | 135.0452; 128.0356; 117.2107; 105.0039; 91.0479; 65.0357 |
| Phenylpropionic acid derivatives | |||||
| 3,4-Dihydroxyphenylacetic acid) (dihydrocaffeic acid) | C9H10O4 | 182.0579 | 4.80 | 181.0471 | 181.0517; 137.0257; 121.0274; 119.0363; 112.9832; 109.0270 |
| 3-(3-Hydroxypheny)-propionic acid | C9H10O3 | 166.0630 | 9.31 | 165.0557 | 165.0417; 147.3718; 137.0349; 121.0909; 107.4270 |
| 3-(4-Hydroxypheny)-propionic acid | C9H10O3 | 166.0630 | 11.79 | 165.0557 | 165.0462; 148.3569; 121.0439; 107.0339; 92.8287 |
| Phenylpropionic acid | C9H10O2 | 150.0665 | 18.15 | 149.0607 | 149.0689; 105.2957; 133.4147; 88.1326, 74.0109 |
| Simple phenols | |||||
| Phloroglucinol | C6H6O3 | 126.0335 | 0.77 | 125.0249 | 125.0214; 108.3153; 121.3252; 83.0178 |
| Catechol | C6H6O2 | 110.0366 | 2.66 | 109.0298 | 109.0308; 108.0225; 96.9602; 91.0153; 89.0244 |
| Hydroxycinnamic derivatives | |||||
| Caffeic acid | C9H8O4 | 180.0446 | 6.20 | 179,0356 | 179.0367; 161.0325; 135.04595; 134.0401, 109.0283; 61.9901 |
| Isoferulic acid | C10H10O4 | 194.0579 | 16.72 | 193.0506 | 193.0515; 178.9886; 149.2177; 96.9549 |
| Others | |||||
| Dihydroquercetin | C15H12O7 | 304.0557 | 14,22 | 303.0510 | 303.0528; 285.0332; 260.9191; 151.9627; 125.0254; 110.9089 |
| Flavonols | |||||
| Quercetin-3,7,4′-triglucoside | C33H40O22 | 788.2041 | 13.10 | 787.1937 | 787.1943; 770.3506; 671.1474; 625.1403; 432.0439; 204.0667; 179.0487 |
| Quercetin-7,4′-diglucoside | C27H30O17 | 626.1483 | 13.47 | 625.1404 | 625.1301; 606.1779; 463.0867; 301.0267 |
| Quercetin-3,4′-diglucoside | C27H30O17 | 626.1483 | 13.68 | 625.1404 | 625.1348; 463.0867; 301.0370; 271.0606; 150.9868 |
| Isorhamnetin-3,4′-diglucoside | C28H32O17 | 640.1656 | 14.34 | 639.1556 | 639.1556; 477.0853; 313.0640; 303.0485 |
| Quercetin-3-rutinoside | C27H30O16 | 610.1545 | 15.34 | 609.1457 | 609.1471; 553.3327; 463.0928; 301.1196 |
| Quercetin-3-glucoside | C21H20O12 | 464.0963 | 15.66 | 463.0884 | 463.0888; 343.0571; 301.0319; 271.0205; 229.0334 |
| Quercetin-4-glucoside | C21H20O12 | 464.0963 | 17.28 | 463.0881 | 463.0897; 445.8403; 301.0342; 283.0054 |
| Isorhamnetin-3-glucoside | C22H22O12 | 478.1122 | 17.33 | 477.1037 | 477.1031; 315.0414; 271.0043; 299.0117; 243.0197 |
| Isorhamnetin-4-glucoside | C22H22O12 | 478.1122 | 18.21 | 477.1037 | 477.1051; 463.0893; 315.0393; 299.0150; 157.3284 |
| Quercetin | C15H10O7 | 302.0381 | 19.98 | 301.0343 | 301.0340; 273.0260; 245.0376; 178.9937; 151.0054;121.0313; 107.0123 |
| Isorhamnetin | C16H12O7 | 316.0583 | 21.97 | 315.0501 | 315.0508; 299.9147; 285.9066; 258.9187; 161.0463 |
| Daily Quercetin Supply (µg) | ||
|---|---|---|
| 134.64 | ||
| Colon Region | Days | Quercetin (µg/100 g fw of Slurry) |
| AC 1 | 4 d | 360.22 ± 18.82 b |
| 9 d | 118.51 ± 13.44 a | |
| 14 d | 529.73 ± 68.79 c | |
| TC 1 | 4 d | 5.24 ± 1.74 b |
| 9 d | 1.70 ± 0.28 a | |
| 14 d | 10.10 ± 0.51 c | |
| DC 1 | 4 d | 0.89 ± 0.21 a |
| 9 d | 1.54 ± 0.2 a | |
| 14 d | 2.37 ± 1.03 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Jalao, I.; Balderas, C.; Calvo, M.V.; Fontecha, J.; Sánchez-Moreno, C.; De Ancos, B. Impact of High-Pressure Processed Onion on Colonic Metabolism Using a Dynamic Gastrointestinal Digestion Simulator. Metabolites 2021, 11, 262. https://doi.org/10.3390/metabo11050262
Fernández-Jalao I, Balderas C, Calvo MV, Fontecha J, Sánchez-Moreno C, De Ancos B. Impact of High-Pressure Processed Onion on Colonic Metabolism Using a Dynamic Gastrointestinal Digestion Simulator. Metabolites. 2021; 11(5):262. https://doi.org/10.3390/metabo11050262
Chicago/Turabian StyleFernández-Jalao, Irene, Claudia Balderas, María V. Calvo, Javier Fontecha, Concepción Sánchez-Moreno, and Begoña De Ancos. 2021. "Impact of High-Pressure Processed Onion on Colonic Metabolism Using a Dynamic Gastrointestinal Digestion Simulator" Metabolites 11, no. 5: 262. https://doi.org/10.3390/metabo11050262
APA StyleFernández-Jalao, I., Balderas, C., Calvo, M. V., Fontecha, J., Sánchez-Moreno, C., & De Ancos, B. (2021). Impact of High-Pressure Processed Onion on Colonic Metabolism Using a Dynamic Gastrointestinal Digestion Simulator. Metabolites, 11(5), 262. https://doi.org/10.3390/metabo11050262







