Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus hystrix DC) Leaves Essential Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Altitude, Rainfall and Temperature of Kaffir Lime Growing Locations
2.2. Soil pH and Nutrient Status of Kaffir Lime Growing Locations
2.3. Plant Nutrient Status of Kaffir Lime
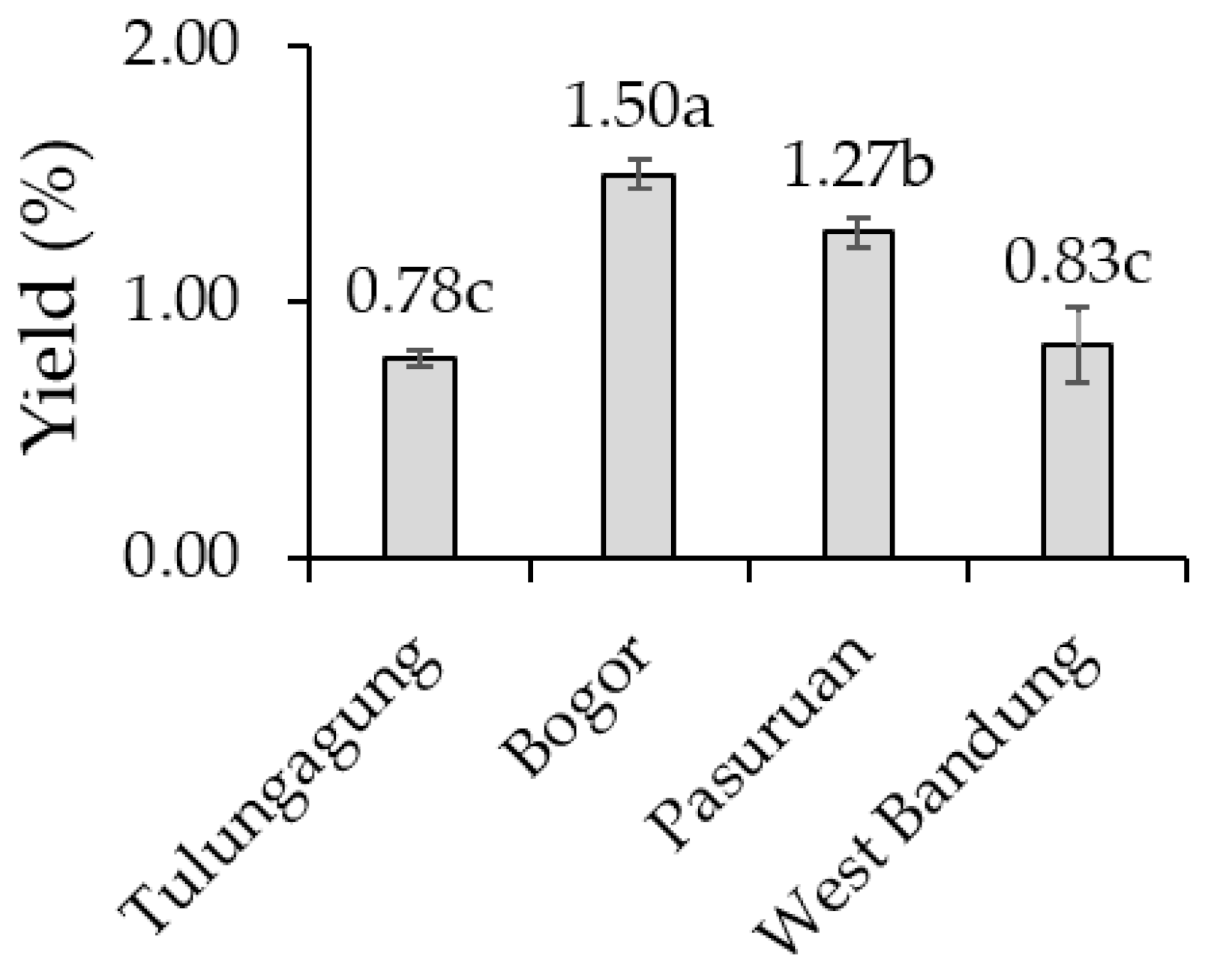
2.4. Essential Oil Yield of Kaffir Lime Leaves
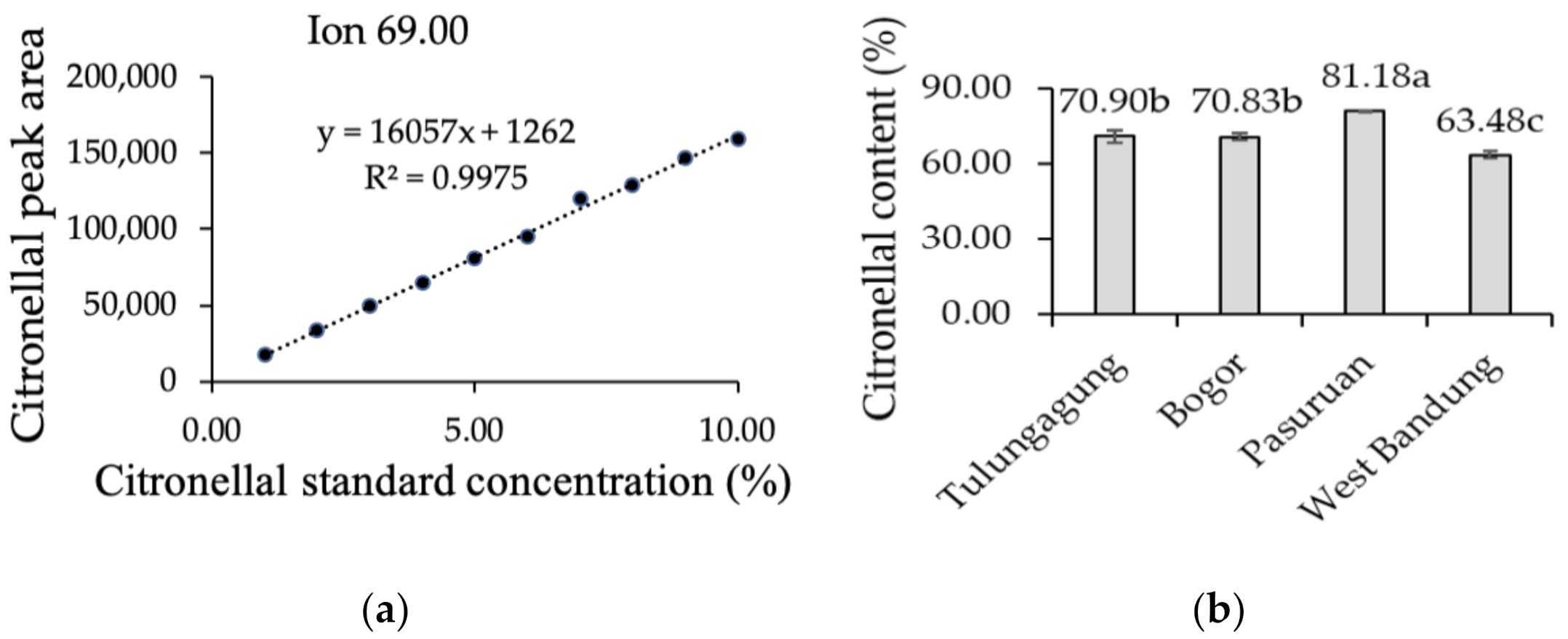
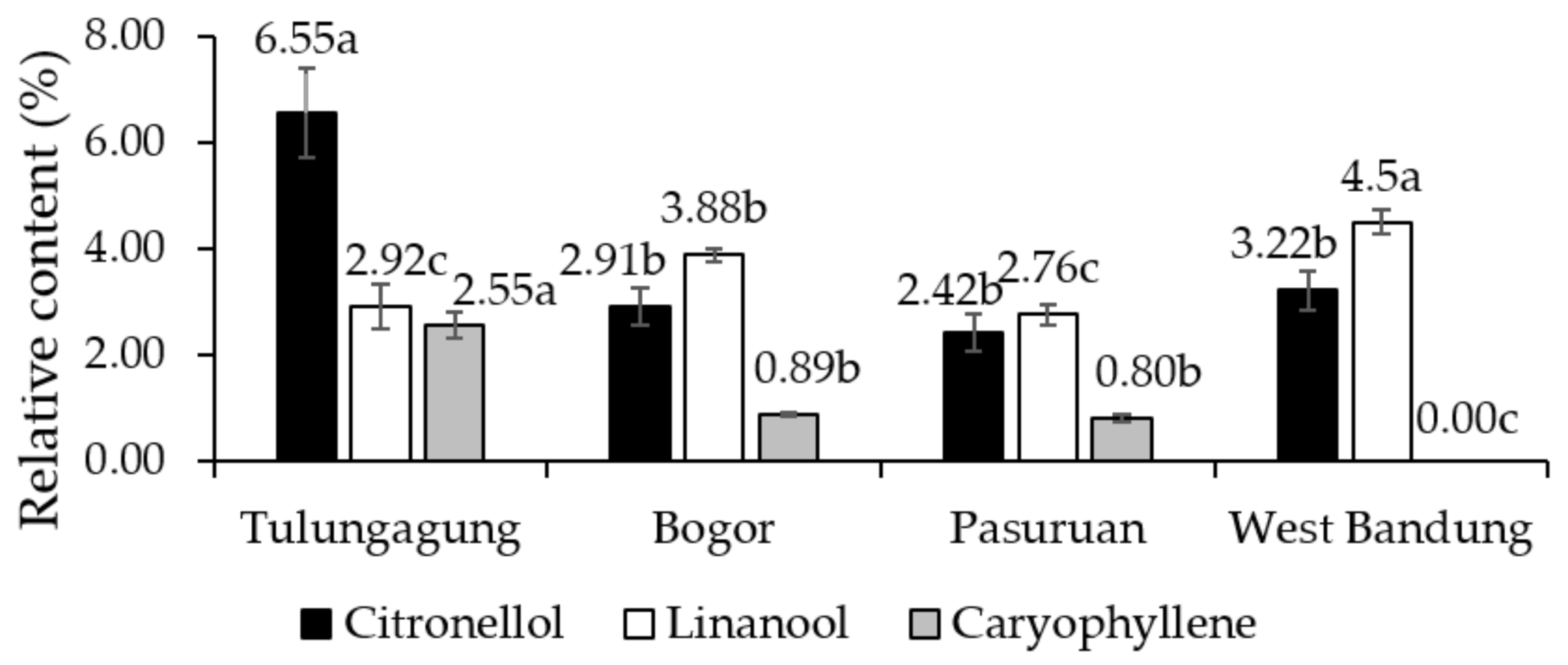
2.5. Main Composition of KLL Essential Oil
3. Materials and Methods
3.1. Study Site
3.2. Plant Material Preparation
3.3. Procedure of Essential Oil Analysis
3.4. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.H.; Yang, K.M.; Huang, T.Z.; Wu, M.L. Traditional small-size Citrus from Taiwan: Essential oils, bioactive compounds, and antioxidant capacity. Medicines 2017, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Apriantini, A.; Meijerink, J.; Witkamp, R.; Gabriele, B.; Fazio, A. In vitro anti-inflammatory and radical scavenging properties of chinotto (Citrus myrtifoloia Raf.) essential oils. Nutrients 2018, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon—A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, C.; Maso, S.D.; Parisi, O.; Dresen, F.; Zhiri, A.; Jijakli, M.H. Screening of antifungal and antibacterial activity of 90 commercial essential oils against 10 pathogens of agronomical importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial effects and mechanism of mandarin (Citrus retculata L.) essential oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef]
- Aliberti, L.; Caputo, L.; Feo, V.D.; Martino, L.D.; Nazzaro, F.; Souza, L.F. Chemical composition and in vitro antimicrobial, cytotoxic and central nervous system activities of the essential oils of Citrus medica L. cv ‘Liscia’ and C. medica cv. ‘Rugosa’ cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef]
- Eleni, M.; Antonios, M.; George, K.; Alexios-Leandros, S.; Prokopios, M. High-quality bergamot oil from Greece: Chemical analysis using chiral gas chromatography and larvicidal activity against the West Nile virus vector. Molecules 2009, 14, 839–849. [Google Scholar] [CrossRef]
- Chandharakool, S.; Koomhin, P.; Sinlapasorn, J.; Suanjan, S.; Phungsai, J.; Suttipromma, N.; Songsamoe, S.; Matan, N.; Sattayakhom, A. Effects of tangerine essential oil on brain waves, moods and sleep onset latency. Molecules 2020, 25, 4865. [Google Scholar] [CrossRef]
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D.; Agusta, D. Preliminary study on production, post-harvest, and marketing of kaffir lime (Citrus hystrix DC) in Tulungagung, Indonesia. J. Trop. Crop. Sci. 2019, 6, 138–143. [Google Scholar] [CrossRef]
- Lertsatitthanakorn, P.; Taweechaisupapong, S.; Aromdee, C.; Khunkitti, W. In vitro bioactivities of essential oils used for acne control. Int. J. Aromather. 2006, 16, 43–49. [Google Scholar] [CrossRef]
- Tunjung, W.A.S.; Cinatl, J., Jr.; Michaelis, M.; Smales, C.M. Anti-cancer effect of kaffir lime (Citrus hystrix DC) leaf extract in cervical cancer and neuroblastoma cell lines. Procedia Chem. 2015, 14, 465–468. [Google Scholar] [CrossRef]
- Ansori, A.N.M.; Supriyadi, A.P.; Kartjito, M.V.; Rizqi, F.; Adrianto, H. Biolarvacidal effectivities of polar and non-polar extract fraction from kaffir lime (Citrus hystrix) leaf against 3rd instar larvae of Aedes aegypti. J. Biol. Eng. Res. Rev. 2015, 2, 14–17. [Google Scholar]
- Kooltheat, N.; Kamuthachad, L.; Anthapanya, M.; Samakchan, N.; Sranujit, R.P.; Potup, P.; Ferrante, A.; Usuwanthim, K. Kaffir lime leaves extract inhibits biofilm formation by Streptococcus mutans. Nutrition 2016, 32, 486–490. [Google Scholar] [CrossRef]
- Hongratanaworakit, T.; Buchbauer, G. Chemical composition and stimulating effect of Citrus hystrix oil on humans. Flavour Fragr. J. 2007, 22, 443–449. [Google Scholar] [CrossRef]
- Wongpornchai, S. Kaffir lime leaf. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Fuglie, K.O. Sources of growth in Indonesian agriculture. J. Prod. Anal. 2010, 33, 225–240. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Plants of the same place do not have the same metabolic pace: Soil properties affect differently essential oil yields of plants growing wild in semiarid Mediterranean lands. Arab. J. Geosci. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Boaro, C.S.F.; Vieira, M.A.R.; Campos, F.G.; Ferreira, G.; De-la-Cruz-Chacon, I.; Marques, M.O.M. Factors influencing the production and chemical composition of essential oils in aromatic plants from Brazil. In Essential Oil Research; Malik, S., Ed.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Comm. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Arce, A.; Soto, A. Citrus essential oils: Extraction and deterpenation. Tree For. Sci. Biotech. 2008, 2, 1–9. [Google Scholar]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Hasani, R.; Mehregan, I.; Larijani, K.; Nejadsattari, T.; Scalone, R. Survey of the impacts of soil and climatic variations on the production of essential oils in Heracleum persicum. Biodivers. J. Biol. Divers. 2017, 18, 365–377. [Google Scholar] [CrossRef]
- Nejad, S.M.H.A.O.; Badi, H.N.; Mehrafarin, A.; Abdossi, V.; Khalighi-Sigaroodi, F. The impact of macro environmental factors on essential oils of Oliveria decumbens vent. from different regions of Iran. Jundishapur J. Nat. Pharm. Prod. 2019, 14, 1–7. [Google Scholar]
- Shams, M.; Ramezani, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dursun, A.; Yildirim, E. Effect of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). Indian J. Sci. Tech. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Rao, B.R.; Kaul, P.N.; Mallavarapu, G.R.; Ramesh, S. Effect of seasonal climatic changes on biomass yield and terpenoid composition of rose-scented geranium (Pelargonium species). Biochem. Syst. Ecol. 1996, 24, 627–635. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Effect of climatic factors on essential oil accumulation in two Lamiaceae species from Algerian semiarid lands. In Exploring the Nexus of Geoecology, Geography, Geoarchaeology, and Geotourism: Advances and Applications for Sustainable Development in Environmental Sciences and Agroforestry Research, Advances in Science, Technology and Innovation; Chennhouni, H., Errami, E., Rocha, F., Sabato, L., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Said-Al Ahl, H.A.H.; Abdou, M.A.A. Impact of water stress and phosphorus fertilizer on fresh herb and essential oil content of dragonhead. Inst. Agrophys. 2009, 23, 403–407. [Google Scholar]
- Garcia-Caparros, P.; Romero, M.J.; Llanderal, A.; Cermeno, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Verma, R.S.; Laiq-Ur-Rahman; Verma, R.K.; Chauhan, A.; Singh, A. Essential oil composition of Pelargonium graveolens L’Her ex Ait. Cultivars harvested in different season. J. Essent. Oil Res. 2013, 25, 372–379. [Google Scholar] [CrossRef]
- Boussaada, O.; Chemli, R. Seasonal variation of essential oil composition of Citrus aurantium L. var amara. J. Essent. Oil-Bear. Plants 2007, 10, 109–120. [Google Scholar] [CrossRef]
- Lawal, O.A.; Ogunwande, I.A.; Owolabi, M.S.; Giwa-Ajeniya, A.O.; Kasali, A.A.; Abudu, F.A.; Sanni, A.A.; Opoku, A.R. Comparative analysis of essential oils of Citrus aurantifolia Swingle and Citrus reticulata Blanco, from two different localities of Lagos State, Nigeria. Am. J. Essent. Oil Nat. Prod. 2014, 2, 8–12. [Google Scholar]
- Khalid, K.A.; Ahmed, A.M.A.; El-Gohary, A.E. Effect of growing seasons on the leaf essential oil composition of Citrus species that are cultivated in Egypt. J. Essent. Oil Res. 2020, 32, 1–12. [Google Scholar] [CrossRef]
- Korner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Gale, J. Plant, and altitude. Ann. Bot. 2004, 94, 199. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Embleton, T.W.; Jones, W.W.; Labanauskas, C.K.; Reuther, W.J. Leaf analysis is a diagnostic tool and guide to fertilization. In The Citrus Industry; Reuther, W.J., Ed.; University of California: Berkeley, CA, USA, 1973; Volume 3. [Google Scholar]
- Liao, L.; Dong, T.; Qiu, X.; Rong, Y.; Wang, Z.; Zhu, J. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. PLoS ONE 2019, 14, e0223356. [Google Scholar] [CrossRef]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef]
- Sanchez, C.A. Phosphorus. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 51–90. [Google Scholar]
- Merhaut, D.J. Magnesium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 145–181. [Google Scholar]
- Camp, A.F. Magnesium in citrus fertilization in Florida. Soil Sci. 1947, 63, 43–52. [Google Scholar] [CrossRef]
- Adnan, M.; Hayyat, M.S.; Imran, M.; Rehman, F.; Saeed, M.S.; Mehta, J.; Tampubolon, K. Impact of foliar application of magnesium fertilizer on agronomic crops: A review. Ind. J. Pure Appl. Biosci. 2020, 8, 281–288. [Google Scholar] [CrossRef]
- McColloch, R.C.; Bingham, F.T.; Aldrich, D.G. Relation of soil potassium and magnesium nutrition of citrus. Soil Sci. Soc. Proc. 1957, 21, 85–88. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A. Plant Nutrients for Citrus Trees; The Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Diaz-Gutierrez, C.; Trillos, A.T.; Villa, V.; Silva, Z.; Acevdeo, L.; Arroyave, C.; Poschenrieder, C.; Pelaez, C. Altitude and fertilization type: Concentration of nutrients and production of biomass in Stevia rebaudiana Bertoni. J. Plant Nutr. 2020, 44, 1–16. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A. Calcium (Ca) and Sulfur (S) for Citrus Trees; The Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Pilbeam, D.J.; Morley, P.S. Calcium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 121–144. [Google Scholar]
- Haneklaus, S.; Bloem, E.; Schnug, E.; de Kok, L.J.; Stulen, I. Sulfur. 2007. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 183–238. [Google Scholar]
- Alam, S.M.; Naqvi, S.S.M.; Ansari, R. Impact of soil pH on nutrient uptake by crop plants. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Fageria, N.K.; Zimmermann, F.J.P. Influence of pH on growth and nutrient uptake by crop species in an oxisol. Comm. Soil Sci. Plant Anal. 1998, 29, 2675–2682. [Google Scholar] [CrossRef]
- Canaly, S.; Bartolomeo, E.D.; Trinchera, A.; Nissini, L.; Tittarelli, F.; Intrigliolo, F.; Roccuzzo, G.; Calabretta, M.L. Effect of different management strategies on soil quality of citrus orchards in Southern Italy. Soil Use Manag. 2009, 25, 34–42. [Google Scholar] [CrossRef]
- Valiki, S.R.H.; Ghanbari, S.; Akbarzadeh, M.; Alamdari, M.G.; Golmohamadzadeh, S. Effect of organic and chemical fertilizers on dry yield, essential oil and compounds on rosemary (Rosemarinus officinalis L.). Biol. Forum Int. J. 2015, 7, 773–782. [Google Scholar]
- Jenkinson, D. Soil organic matter and its dynamic. In Russel’s Soil Conditions and Plant Growth, 11th ed.; Wild Longman Group: London, UK, 1988. [Google Scholar]
- Yassen, A.A.; Khalid, K.A. Influence of organic fertilizers on the yield, essential oil, and mineral content of onion. Int. Agrophys. 2009, 23, 183–188. [Google Scholar]
- Darzi, M.T. Effect of organic manure and biofertilizer application on flowering and some yield traits of coriander (Coriandrum sativum). Int. J. Agric. Crop Sci. 2012, 4, 103–107. [Google Scholar] [CrossRef]
- Santos, M.F.; Mendonca, M.C.; Filho, J.L.A.S.C.; Dantas, I.B.; Silva-Mann, R.; Blank, A.F. Cattle manure and biofertilizer on the cultivation of lemon balm (Melissa officinalis L.). Rev. Braz. Plant Med. 2009, 11, 355–359. [Google Scholar] [CrossRef][Green Version]
- Ram, M.; Kumar, S. Yield improvement in the regenerated and transplanted Mint (Mentha arvensis) by recycling the organic wastes and manures. Biores. Techol. 1997, 97, 886–893. [Google Scholar] [CrossRef]
- Anwar, M.; Patra, D.D.; Chand, S.; Alpesh, K.; Naqvi, A.A.; Khanuja, S.P.S. Effect of organic manures and inorganic fertilizer on growth, herb and oil yield, nutrient accumulation, and oil quality of french basil. Comm. Soil Sci. Plant Anal. 2005, 36, 1737–1746. [Google Scholar] [CrossRef]
- Gerami, F.; Moghaddam, P.R.; Ghorbani, R.; Hassani, A. Effects of irrigation intervals ad organic manure on morphological traits, essential oil content, and yield of oregano (Origanum vulgare L.). Ann. Braz. Acad. Sci. 2016, 88, 2375–2385. [Google Scholar] [CrossRef]
- Singh, R.; Singh, R.; Soni, S.K.; Singh, S.P.; Chauhan, U.K.; Kalra, A. Vermicompost from biodegraded distillation waste improves soil properties and essential oil yield of Pogostemo cablin (patchouli) Benth. Appl. Soil Ecol. 2013, 70, 48–56. [Google Scholar] [CrossRef]
- Bondada, B.R.; Syvertsen, J.P. Leaf chlorophyll, net gas exchange and chloroplast ultrastructure of citrus leaves under different nitrogen status. Tree Physiol. 2003, 23, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.N.A.M.; Hassan, M.A.; Nahar, L.; Basar, N.; Jamil, S.; Sarker, S.D. Essential oils from the Malaysian citrus (Rutaceae) medicinal plants. Medicines 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Nurzyinska-Wierdak, R.; Rozek, E.; Borowski, B. Response of different basil cultivars to nitrogen and potassium fertilization: Total and mineral nitrogen content in herb. Acta Sci. Pol. Hortorum Cultus 2011, 10, 217–232. [Google Scholar]
- Rao, E.V.S.P.; Puttana, K.; Rao, R.S.G.; Ramesh, S. Nitrogen and potassium nutrition of French basil (Ocimum basilicum Linn.). J. Spices Arom. Crops 2007, 16, 99–105. [Google Scholar]
- Emongor, V.E.; Chweya, J.A.; Keya, S.O.; Munavu, R.M. Effect of nitrogen and phosphorus on the essential oil yield and quality of chamomile (Matricaria chamomilla L.) flowers. East Afr. Agric. For. J. 1990, 55, 261–264. [Google Scholar] [CrossRef]
- Puttanna, K.; Rao, E.V.S.P.; Singh, R.; Ramesh, S. Influence of nitrogen and potassium fertilization on yield and quality of rosemary in relation to harvest number. Comm. Soil Sci. Plant Anal. 2010, 41, 190–198. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield and essential oil of oregano (Origanum vulgare ssp. hirtum). Ind. Crops Prod. 2009, 29, 559–608. [Google Scholar] [CrossRef]
- Ramezani, S.; Rezaei, M.R.; Sotoudehnia, P. Improved growth, yield, and essential oil content of basil grown under different levels of phosphorus sprays in the field. J. Appl. Biol. Sci. 2009, 3, 96–101. [Google Scholar]
- Pandey, V.; Patra, D.D. Crop productivity, aroma profile, and antioxidant activity in Pelargonium graveolens L’Her. under integrated supply of various organic and chemical fertilizer. Ind. Crops Prod. 2015, 67, 257–263. [Google Scholar] [CrossRef]
- Prasad, A.; Kumar, S.; Pandey, A. Microbial and chemical sources of phosphorus supply modulate the field and chemical composition of volatile oil of sweet basil (Ocimum basilicum L.). Biol. Fertil. Soils 2012, 47, 853–861. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambia, J.L.; Lopez-Gresa, M.P.; Blazquez, M.A.; Granell, A. Volatile compounds in Citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Waikedre, J.; Dugay, A.; Barrachina, I.; Herrenknecht, C.; Cabalion, P.; Fournet, A. Chemical composition and antimicrobial activity of the essential oils from New Caledonian Citrus macroptera and Citrus hystrix. Chem. Biodiv. 2010, 7, 871–877. [Google Scholar] [CrossRef]
- Nor, O.M. Volatile aroma compounds in Citrus hystrix oil. J. Trop. Agric. Food Sci. 1999, 27, 225–229. [Google Scholar]
- Jantan, I.; Ahmad, A.S.; Ahmad, A.R.; Ali, N.A.M.; Ayop, N. Chemical composition of some citrus oils from Malaysia. J. Essent. Oil Res. 1996, 8, 627–632. [Google Scholar] [CrossRef]
- Riyadi, E. Profiling the Volatile Compounds of Variety of Essential oils Species from Indonesia. Master’s Thesis, Institut Pertanian Bogor, Bogor, Indonesia, 6 June 2012. [Google Scholar]
- Lenardao, E.J.; Botteselle, G.V.; De Azambuja, F.; Perin, G.; Jacob, R.G. Citronellal as key compound in organic synthesis. Tetrahedron 2007, 63, 6671–6712. [Google Scholar] [CrossRef]
- Lota, M.L.; Serra, D.R.D.; Tomi, F.; Jacquemond, C.; Casanova, J. Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 2002, 50, 796–805. [Google Scholar] [CrossRef]
- Jabalpurwala, F.A.; Smoot, J.M.; Rouseff, R.L. A comparison of citrus blossom volatiles. Phytochemistry 2009, 70, 1428–1434. [Google Scholar] [CrossRef]
- Rammanee, K.; Hongpattarakere, T. Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastucture alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess Technol. 2011, 4, 1050–1059. [Google Scholar] [CrossRef]
- Ratseewo, J.; Tangkhawanit, E.; Meeso, N.; Kaewseejan, N.; Siriamornpun, S. Changes in antioxidant properties and volatile compounds of kaffir lime leaf as affected by cooking processes. Int. Food. Res. J. 2016, 23, 188–196. [Google Scholar]
- Tinjan, P.; Jirapakkul, W. Comparative study on extraction methods of free and glycosidically bound volatile compounds from kaffir lime leaves by solvent extraction and solid phase extraction. Kasetsart J. Nat. Sci. 2007, 41, 300–306. [Google Scholar]
- Taylor, W.G.; Schreck, C.E. Chiral-phase capillary gas chromatography and mosquito repellent activity of some oxazolidine derivatives of (+)- and (-)-citronellol. J. Pharm. Sci. 1985, 74, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Deepa, B.; Anuradha, C.V. Linalool, a plant derived monoterpene alcohol, rescues kidney from diabetes-induced nephropathic changes via blood glucose reduction. Diabetol. Croat. 2011, 40, 121–137. [Google Scholar]
- Fisher, K.; Rowe, C.; Phillips, C.A. The survival of three strains of Arcobacter butzleri in the presence of lemon, oranges and bergamot essential oils and their components in vitro and on food. Lett. Appl. Microbiol. 2007, 44, 495–499. [Google Scholar] [CrossRef]
- Sonboli, A.; Eftekhar, F.; Yousefzadi, M.; Kanani, M.R. Antibacterial activity and chemical composition of the essential oil of Grammosciadium platycarpum Boiss. from Iran. Z. Naturforsch C J. Biosci. 2005, 60, 30–34. [Google Scholar] [CrossRef]
- Yang, Z.; Bengtsson, M.; Witzgall, P. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J. Chem. Ecol. 2004, 30, 619–629. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. Hortorum Cultus 2013, 12, 3–16. [Google Scholar]
- Shaikh, M.N.; Suryawanshi, Y.C.; Mokat, D.N. Volatile profiling and essential oil yield of cymbopogon citratus (DC) stapf treated with rizhosphere fungi and some important fertilizers. J. Essent. Oil-Bear. Plants 2019, 22, 477–483. [Google Scholar] [CrossRef]
- Mohd-Yusoff, Z.; Muhammad, Z.; Kasuan, N.; Rahiman, M.H.F.; Taib, M.N. Effect of temperature on kaffir lime oil by using hydro-diffusion steam distillation system. Malays. J. Anal. Sci. 2013, 17, 326–339. [Google Scholar]
- Cheong, M.-W.; Loke, X.-Q.; Liu, S.-Q.; Pramudya, K.; Curran, P.; Yu, B. Characterization of volatile compounds and aroma profiles of Malaysian pomelo (Citrus grandis (L.) Osbeck) blossom and peel. J. Essent. Oil Res. 2011, 23, 34–44. [Google Scholar] [CrossRef]
- Opdyke, D.L. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1973, 11, 1011–1081. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect on β-caryophyllene on anticancer activity on α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Kakarapharthi, P.S.; Srinivas, K.V.N.S.; Kumar, J.K.; Kumar, A.N.; Rajput, D.K.; Sarma, V.U.M. Variation in the essential oil content and composition of citronella (Cymbopogon winterianus Jowitt.) in the relation to time harvest and weather conditions. Ind. Crops Prod. 2014, 61, 240–248. [Google Scholar] [CrossRef]
- Ngunut Climate: Average Temperature, Weather by Month, Ngunut Weather Averages—Climate-Data. Available online: http://en.climate-data.org/asia/indonesia/east-java/ngunut-611805/ (accessed on 5 March 2019).
- Prigen Climate: Average Temperature, Weather by Month, Prigen Weather Averages—Climate-Data. Available online: http://en.climate-data.org/asia/indonesia/east-java/prigen-977149/ (accessed on 5 March 2019).
- Cikole Climate: Average Temperature, Weather by Month, Cikole Weather Averages—Climate-Data. Available online: http://en.climate-data.org/asia/indonesia/west-java/cikole-603731/ (accessed on 5 March 2019).
- Ciomas Climate: Average Temperature, Weather by Month, Ciomas Weather Averages—Climate-Data. Available online: http://en.climate-data.org/asia/indonesia/west-java/ciomas-614839/ (accessed on 5 March 2019).
| Sampling Locations (Village, District) | Locations’ Coordinates (Latitude, Longitude, Altitude) |
|---|---|
| Ngunut, Tulungagung | 8°6′27″ S, 112°0′35″ E, 109 m asl |
| Pasirkuda, Bogor | 6°36′36″ S, 106°46′47″ E, 239 m asl |
| Jatiarjo, Pasuruan | 7°45′5″ S, 112°40′6″ E, 803 m asl |
| Cikole, West Bandung | 6°48′12″ S, 107°39′16″ E, 1189 m asl |
| Variables | Al | AR | MR | Tav | Tmin |
|---|---|---|---|---|---|
| AR | 0.17 | ||||
| MR | 0.17 | 1 | |||
| Tav | −0.98 ** | −0.04 | −0.04 | ||
| Tmin | −0.95 ** | 0.01 | 0.01 | 0.98 ** | |
| Tmax | −0.92 ** | −0.07 | −0.07 | 0.90 ** | 0.87 ** |
| Locations | Tav (°C) | Tmin (°C) | Tmax (°C) | MR (mm) | AR (mm) |
|---|---|---|---|---|---|
| Tulungagung | 25.3 | 20.1 | 30.1 | 145 | 1743 |
| Bogor | 25.4 | 20.1 | 30.1 | 342 | 4104 |
| Pasuruan | 22.1 | 17.4 | 26.9 | 261 | 3126 |
| West Bandung | 20.3 | 15 | 24.1 | 250 | 3001 |
| Locations | pH H2O | pH KCL | C-Organic (%) | N Total (%) | P Total (mg P2O5 100 g−1) | K Total (mg K2O 100 g−1) |
|---|---|---|---|---|---|---|
| Tulungagung | 5.72 ± 0.04 b | 4.87 ± 0.08 c | 1.49 ± 0.02 b | 0.2 ± 0.01 b | 139.03 ± 1.20 c | 34.96 ± 3.96 b |
| Bogor | 6.24 ± 0.11 a | 5.66 ± 0.22 a | 2.37 ± 0.02 a | 0.27 ± 0.01 a | 242.23 ± 18.45 b | 163.60 ± 13.56 a |
| Pasuruan | 6.27 ± 0.04 a | 5.24 ± 0.12 b | 1.46 ± 0.06 b | 0.19 ± 0.01 b | 136.36 ± 9.46 c | 38.21 ± 4.62 b |
| West Bandung | 4.76 ± 0.09 c | 3.89 ± 0.10 d | 0.97 ± 0.05 c | 0.16 ± 0.01 c | 352.25 ± 17.72 a | 166.57 ± 10.51 a |
| Variables | C-Organic | N Total | P Total | K Total | pH H2O |
|---|---|---|---|---|---|
| N total | 0.98 ** | ||||
| P total | −0.25 | −0.23 | |||
| K total | 0.18 | 0.19 | 0.90 ** | ||
| pH H2O | 0.75 | 0.72 | −0.73 | −0.40 | |
| pH KCl | 0.88 ** | 0.86 ** | −0.61 | −0.22 | 0.97 ** |
| Locations | C-Organic (%) | N Total (%) | P Total (%) | K Total (%) | Mg (%) | Ca (%) | S (%) |
|---|---|---|---|---|---|---|---|
| Tulungagung | 40.36 ± 0.61 a | 1.83 ± 0.06 b | 0.14 ± 0.00 c | 2.18 ± 0.09 a | 0.17 ± 0.01 d | 2.70 ± 0.28 b | 0.38 ± 0.02 a |
| Bogor | 41.97 ± 1.18 a | 2.5 ± 0.06 a | 0.25 ± 0.01 a | 1.64 ± 0.05 d | 0.39 ± 0.02 a | 2.12 ± 0.30 c | 0.42 ± 0.17 a |
| Pasuruan | 40.22 ± 2.10 a | 2.4 ± 0.09 a | 0.17 ± 0.01 b | 1.99 ± 0.03 b | 0.26 ± 0.01 b | 3.29 ± 0.14 a | 0.15 ± 0.02 b |
| West Bandung | 40.94 ± 1.23 a | 1.72 ± 0.01 b | 0.15 ± 0.02 c | 1.85 ± 0.06 c | 0.20 ± 0.01 c | 1.82 ± 0.36 c | 0.34 ± 0.12 ab |
| Leaves Nutrient | C | N | P | K | Mg | Ca | Na | S | Fe | Mn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 0.18 | ||||||||||
| P | 0.40 | 0.80 ** | |||||||||
| K | −0.44 | −0.48 | −0.78 | ||||||||
| Mg | 0.37 | 0.83 ** | 0.98 ** | −0.80 ** | |||||||
| Ca | −0.44 | 0.36 | −0.17 | 0.46 | −0.14 | ||||||
| Na | 0.44 | 0.61 | 0.88 ** | −0.70 | 0.88 ** | −0.31 | |||||
| S | 0.18 | −0.14 | 0.23 | −0.32 | 0.15 | −0.37 | 0.41 | ||||
| Fe | −0.11 | −0.80 ** | −0.52 | −0.02 | −0.53 | −0.56 | −0.45 | 0.20 | |||
| Mn | −0.05 | −0.85 ** | −0.52 | 0.00 | −0.53 | −0.64 | −0.38 | 0.28 | 0.98 ** | ||
| Cu | −0.07 | −0.64 | −0.19 | −0.21 | −0.22 | −0.70 | −0.06 | 0.45 | 0.81 ** | 0.84 ** | |
| Zn | 0.00 | −0.87 ** | −0.51 | 0.16 | −0.55 | −0.58 | −0.24 | 0.55 | 0.78 | 0.87 ** | 0.75 |
| Locations | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | Na (%) |
|---|---|---|---|---|---|
| Tulungagung | 375.96 ± 116.35 b | 57.41 ± 7.17 b | 2.21 ± 0.23 b | 23.83 ± 1.35 a | 0.003 ± 0.001 b |
| Bogor | 9.30 ± 8.47 c | 23.96 ± 1.65 c | 2.08 ± 1.89 b | 14.63 ± 2.07 b | 0.008 ± 0.001 a |
| Pasuruan | 4.38 ± 2.48 c | 7.74 ± 1.07 d | 0.09 ± 0.01 c | 7.49 ± 0.40 c | 0.003 ± 0.001 b |
| West Bandung | 1497.33 ± 282.94 a | 133.81 ± 10.11 a | 4.57 ± 0.89 a | 26.37 ± 2.11 a | 0.003 ± 0.001 b |
| Variables | Y | Clal | Clol | Llol | Car |
|---|---|---|---|---|---|
| Al | −0.22 | −0.20 | −0.58 | 0.47 | −0.83 ** |
| AR | 0.83 ** | 0.06 | −0.85 ** | 0.44 | −0.67 |
| MR | 0.83 ** | 0.06 | −0.49 | 0.26 | −0.39 |
| Tav | 0.33 | 0.17 | 0.49 | −0.39 | 0.75 |
| Tmin | 0.41 | 0.25 | 0.41 | −0.41 | 0.70 |
| Tmax | 0.29 | 0.28 | 0.45 | −0.46 | 0.73 |
| Variables | Y | Clal | Clol | Llol | Car |
|---|---|---|---|---|---|
| C-org | 0.77 ** | 0.25 | −0.13 | −0.11 | 0.21 |
| N | 0.73 | 0.20 | −0.08 | −0.09 | 0.25 |
| P | −0.13 | −0.41 | −0.35 | 0.98 ** | −0.73 |
| K | 0.23 | −0.69 | −0.45 | 0.95 ** | −0.68 |
| pH H2O | 0.73 | 0.81 ** | −0.17 | −0.67 | 0.34 |
| pH KCl | 0.78 ** | 0.65 | −0.15 | −0.51 | 0.33 |
| Variable | Y | Clal | Clol | Llol | Car |
|---|---|---|---|---|---|
| C-org | 0.30 | −0.16 | −0.18 | 0.33 | −0.17 |
| N | 0.93 ** | 0.66 | −0.58 | −0.30 | −0.12 |
| P | 0.86 ** | 0.14 | −0.49 | 0.18 | −0.21 |
| K | −0.67 | 0.26 | 0.69 | −0.66 | 0.66 |
| Mg | 0.92 ** | 0.20 | −0.57 | 0.18 | −0.28 |
| Ca | 0.18 | 0.83 ** | 0.04 | −0.88 ** | 0.39 |
| Na | 0.71 | −0.07 | −0.24 | 0.26 | −0.05 |
| S | 0.00 | −0.63 | 0.33 | 0.41 | 0.17 |
| Fe | −0.66 | −0.80 ** | 0.05 | 0.70 | −0.45 |
| Mn | −0.70 | −0.87 ** | 0.17 | 0.72 | −0.34 |
| Cu | −0.45 | −0.89 ** | 0.14 | 0.75 | −0.29 |
| Zn | −0.74 | −0.88 ** | 0.58 | 0.53 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efendi, D.; Budiarto, R.; Poerwanto, R.; Santosa, E.; Agusta, A. Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus hystrix DC) Leaves Essential Oil. Metabolites 2021, 11, 260. https://doi.org/10.3390/metabo11050260
Efendi D, Budiarto R, Poerwanto R, Santosa E, Agusta A. Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus hystrix DC) Leaves Essential Oil. Metabolites. 2021; 11(5):260. https://doi.org/10.3390/metabo11050260
Chicago/Turabian StyleEfendi, Darda, Rahmat Budiarto, Roedhy Poerwanto, Edi Santosa, and Andria Agusta. 2021. "Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus hystrix DC) Leaves Essential Oil" Metabolites 11, no. 5: 260. https://doi.org/10.3390/metabo11050260
APA StyleEfendi, D., Budiarto, R., Poerwanto, R., Santosa, E., & Agusta, A. (2021). Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus hystrix DC) Leaves Essential Oil. Metabolites, 11(5), 260. https://doi.org/10.3390/metabo11050260









