Metabolic Markers Demonstrate the Heterogeneity of Myosteatosis in Community-Dwelling Older Black Men from the Health ABC Study
Abstract
1. Introduction
2. Results
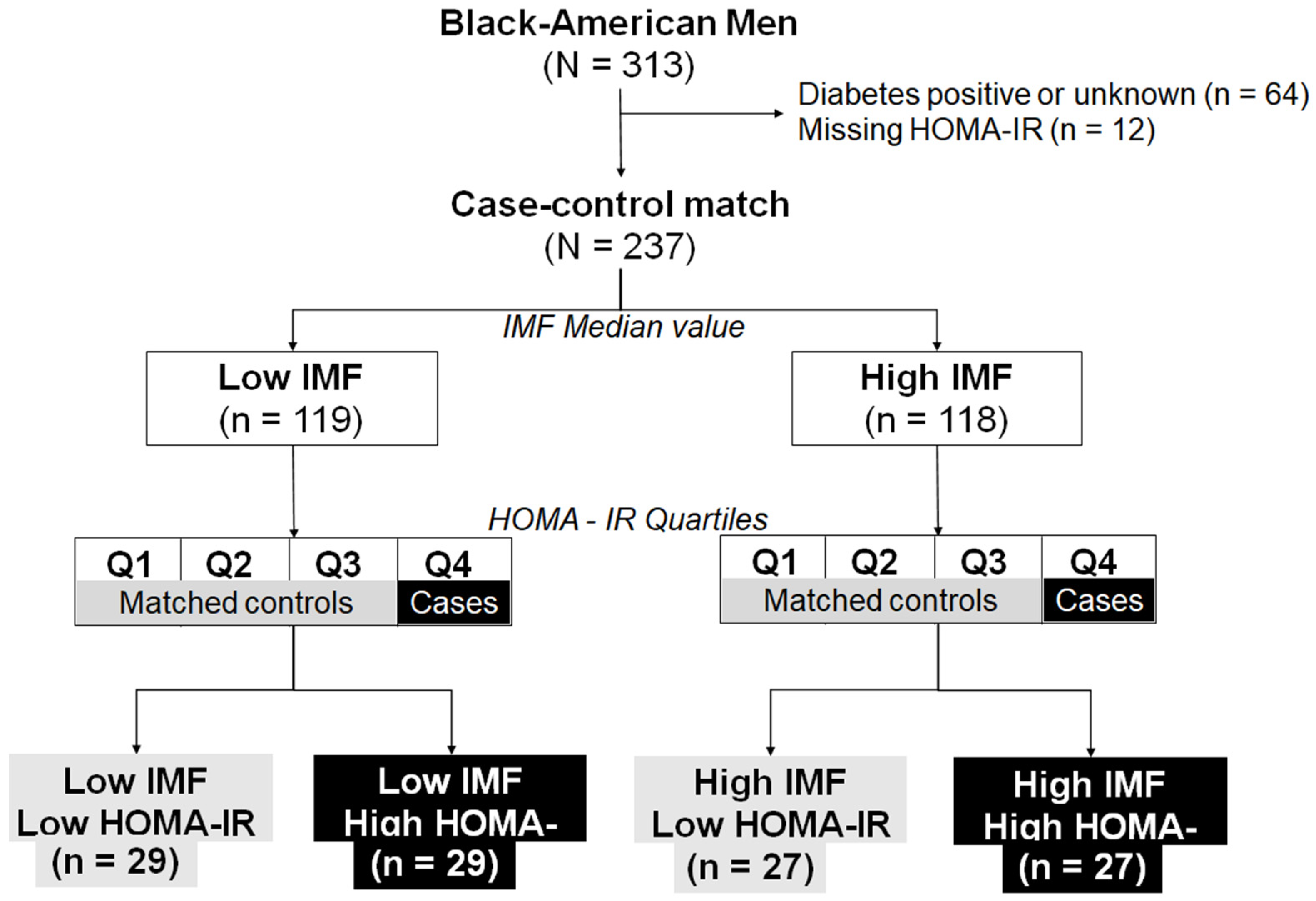
2.1. Participant Characteristics
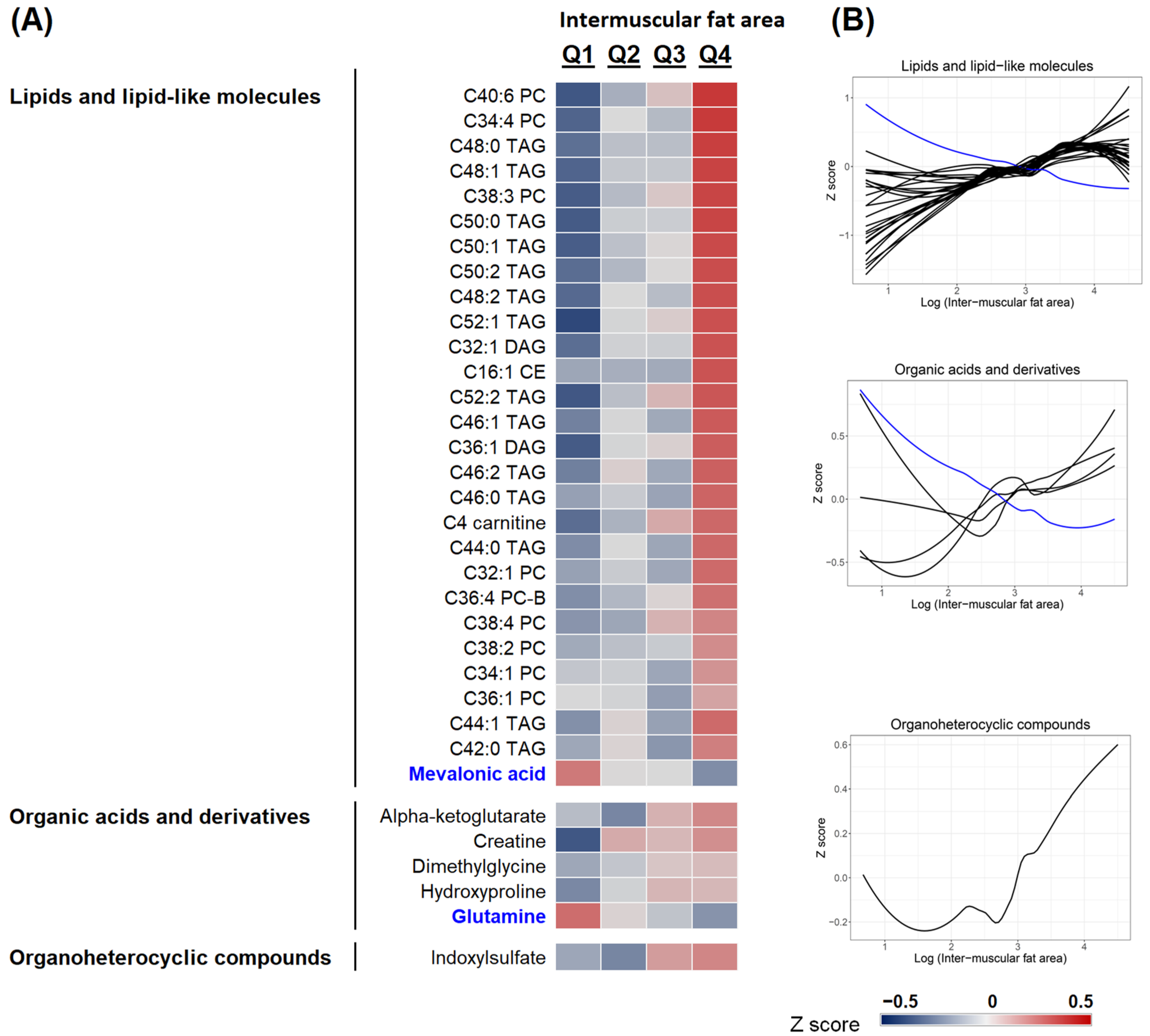
2.2. Metabolomics Profiling of Muscle Fat Deposition
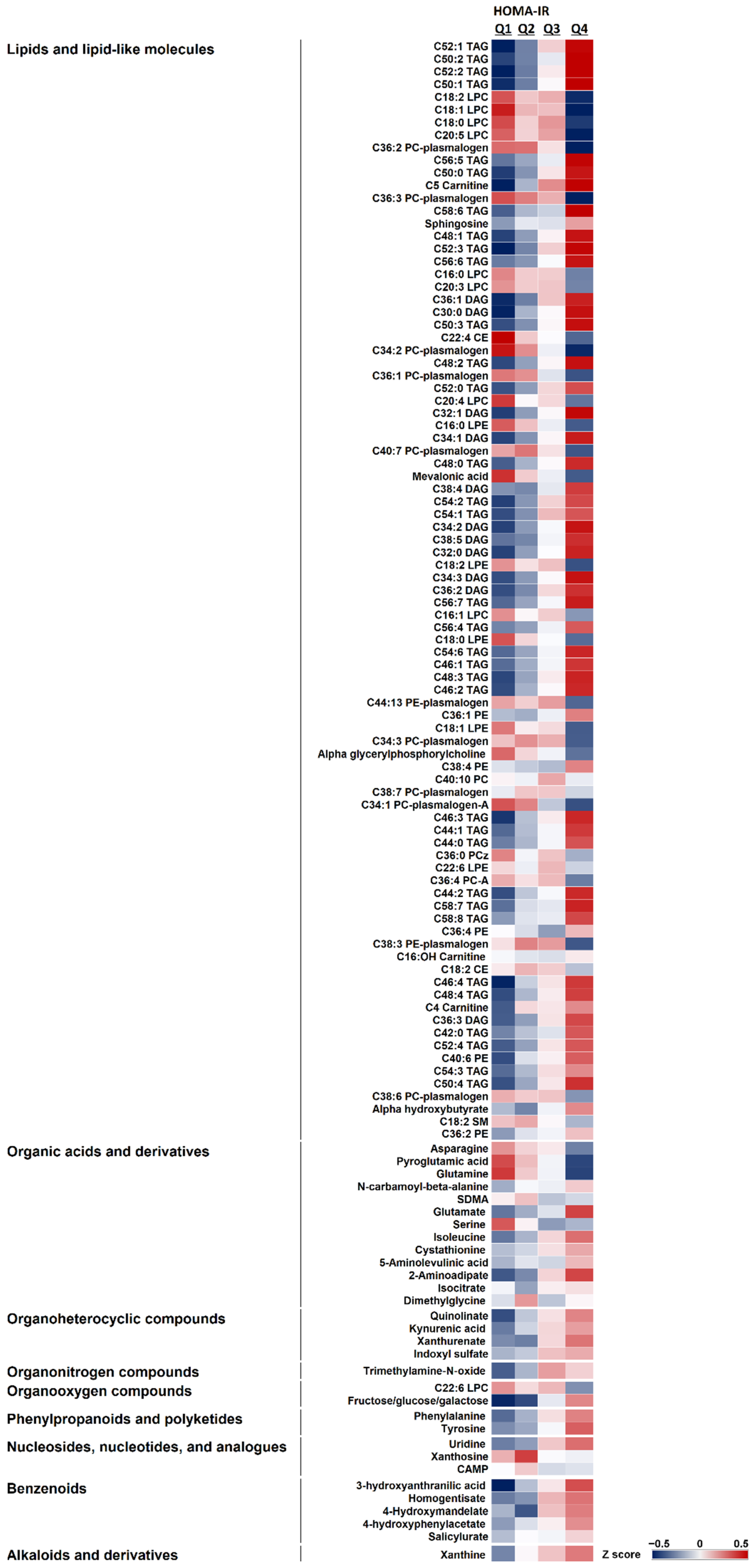
2.3. Metabolite Signature Associated with HOMA-IR
2.4. The Heterogeneity of IMF
2.4.1. Body Composition
2.4.2. Physical Activity and Performance
2.4.3. Blood Biomarkers
2.4.4. Plasma Metabolite Profiles
3. Discussion
3.1. Age-Related Increase in Skeletal Muscle Fat Infiltration Is Associated with Dysregulated Lipid Metabolism
3.2. Body Composition, Physical Performance, and Metabolic Heterogeneity of Myosteatosis
3.3. Clinical Implications of Heterogeneity of Myosteatosis
3.4. Strength and Limitations
4. Materials and Methods
4.1. Study Population
4.2. Measurement of Plasma Metabolites
4.3. Body Composition Assessment
4.4. Midthigh Cross-Sectional Area Assessment
4.5. Dietary Assessment
4.6. Physical Performance
4.7. Blood Biochemistry
4.8. Other Covariates
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miljkovic, I.; Kuipers, A.L.; Cvejkus, R.; Bunker, C.H.; Patrick, A.L.; Gordon, C.L.; Zmuda, J.M. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity (Silver Spring) 2016, 24, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Devries, M.C.; Safdar, A.; Hamadeh, M.J.; Tarnopolsky, M.A. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 119–128. [Google Scholar] [CrossRef] [PubMed]
- St-Jean-Pelletier, F.; Pion, C.H.; Leduc-Gaudet, J.-P.; Sgarioto, N.; Zovilé, I.; Barbat-Artigas, S.; Reynaud, O.; Alkaterji, F.; Lemieux, F.C.; Grenon, A.; et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J. Cachexia Sarcopenia Muscle 2017, 8, 213–228. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Kelley, D.E.; Thaete, F.L.; He, J.; Ross, R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. 2000, 89, 104–110. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Théron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B.; et al. Skeletal Muscle Lipid Content and Oxidative Activity in Relation to Muscle Fiber Type in Aging and Metabolic Syndrome. J. Gerontol. Ser. A 2014, 70, 566–576. [Google Scholar] [CrossRef]
- Beavers, K.M.; Beavers, D.P.; Houston, D.K.; Harris, T.B.; Hue, T.F.; Koster, A.; Newman, A.B.; Simonsick, E.M.; Studenski, S.A.; Nicklas, B.J.; et al. Associations between body composition and gait-speed decline: Results from the Health, Aging, and Body Composition study. Am. J. Clin. Nutr. 2013, 97, 552–560. [Google Scholar] [CrossRef]
- Stretch, C.; Aubin, J.-M.; Mickiewicz, B.; Leugner, D.; Al-Manasra, T.; Tobola, E.; Salazar, S.; Sutherland, F.R.; Ball, C.G.; Dixon, E.; et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS ONE 2018, 13, e0196235. [Google Scholar] [CrossRef]
- Moore, C.D.; Craven, B.C.; Thabane, L.; Laing, A.C.; Frank-Wilson, A.W.; Kontulainen, S.A.; Papaioannou, A.; Adachi, J.D.; Giangregorio, L.M. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 2015, 15, 32–41. [Google Scholar] [PubMed]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.H.; Lobo, D.N. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Bittel, D.C.; Bittel, A.J.; Tuttle, L.J.; Hastings, M.K.; Commean, P.K.; Mueller, M.J.; Cade, W.T.; Sinacore, D.R. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J. Diabetes Complicat. 2015, 29, 250–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farsijani, S.; Santanasto, A.J.; Miljkovic, I.; Boudreau, R.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B. The Relationship between Inter-muscular Fat and Physical Performance Is Moderated by Muscle Area in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Zavaroni, I.; Bonora, E.; Pagliara, M.; Dall’Aglio, E.; Luchetti, L.; Buonanno, G.; Bonati, P.A.; Bergonzani, M.; Gnudi, L.; Passeri, M.; et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N. Engl. J. Med. 1989, 320, 702–706. [Google Scholar] [CrossRef]
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 168, 4761–4768. [Google Scholar] [CrossRef]
- Miljkovic-Gacic, I.; Gordon, C.L.; Goodpaster, B.H.; Bunker, C.H.; Patrick, A.L.; Kuller, L.H.; Wheeler, V.W.; Evans, R.W.; Zmuda, J.M. Adipose tissue infiltration in skeletal muscle: Age patterns and association with diabetes among men of African ancestry. Am. J. Clin. Nutr. 2008, 87, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Cirulli, E.T.; Guo, L.; Swisher, C.L.; Shah, N.; Huang, L.; Napier, L.A.; Kirkness, E.F.; Spector, T.D.; Caskey, C.T.; Thorens, B.; et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019, 29, 488–500.e2. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Theriault, R.; Watkins, S.C.; Kelley, D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000, 49, 467–472. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Lattuada, G.; Piemonti, L.; Garancini, M.P.; Ragogna, F.; Villa, M.; Mannino, S.; Crosignani, P.; Bosi, E.; Luzi, L.; et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: The Cremona Study. Diabetes Care 2011, 34, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Murphy, R.A.; Moore, S.C.; Playdon, M.; Meirelles, O.; Newman, A.B.; Milijkovic, I.; Kritchevsky, S.B.; Schwartz, A.; Goodpaster, B.H.; Sampson, J.; et al. Metabolites Associated with Lean Mass and Adiposity in Older Black Men. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1352–1359. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Marron, M.M.; Harris, T.B.; Boudreau, R.M.; Clish, C.B.; Moore, S.C.; Murphy, R.A.; Murthy, V.L.; Sanders, J.L.; Shah, R.V.; Tseng, G.; et al. Metabolites Associated with Vigor to Frailty Among Community-Dwelling Older Black Men. Metabolites 2019, 9, 83. [Google Scholar] [CrossRef]
- Visser, M.; Fuerst, T.; Lang, T.; Salamone, L.; Harris, T.B. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J. Appl. Physiol. (1985) 1999, 87, 1513–1520. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; A Tylavsky, F.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Strotmeyer, E.S.; De Rekeneire, N.; Schwartz, A.V.; Faulkner, K.A.; Resnick, H.E.; Goodpaster, B.H.; Shorr, R.I.; Vinik, A.I.; Harris, T.B.; Newman, A.B. The Relationship of Reduced Peripheral Nerve Function and Diabetes with Physical Performance in Older White and Black Adults. The Health, Aging, and Body Composition (Health ABC) Study. Diabetes Care 2008, 31, 1767–1772. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, But Not Muscle Mass, Is Associated with Mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. Ser. A 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Newman, A.B.; Haggerty, C.L.; Goodpaster, B.; Harris, T.; Kritchevsky, S.; Nevitt, M.; Miles, T.P.; Visser, M. Strength and muscle quality in a well-functioning cohort of older adults: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2003, 51, 323–330. [Google Scholar] [CrossRef]
- Katsiaras, A.; Newman, A.B.; Kriska, A.; Brach, J.; Krishnaswami, S.; Feingold, E.; Kritchevsky, S.B.; Li, R.; Harris, T.B.; Schwartz, A.; et al. Skeletal muscle fatigue, strength, and quality in the elderly: The Health ABC Study. J. Appl. Physiol. (1985) 2005, 99, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Simonsick, E.M.; Kritchevsky, S.; Yaffe, K.; Newman, A.B. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J. Am. Geriatr. Soc. 2004, 52, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Saito, K.; Maekawa, K.; Pappan, K.L.; Urata, M.; Ishikawa, M.; Kumagai, Y.; Saito, Y. Differences in metabolite profiles between blood matrices, ages, and sexes among Caucasian individuals and their inter-individual variations. Metabolomics 2014, 10, 402–413. [Google Scholar] [CrossRef]
- Saito, K.; Maekawa, K.; Kinchen, J.M.; Tanaka, R.; Kumagai, Y.; Saito, Y. Gender- and Age-Associated Differences in Serum Metabolite Profiles among Japanese Populations. Biol. Pharm. Bull. 2016, 39, 1179–1186. [Google Scholar] [CrossRef]

| IMF Quartiles | Q1 ≤11.81 cm2 | Q2 11.82–18.01 cm2 | Q3 18.02–27.11 cm2 | Q4 ≥27.12 cm2 | p |
|---|---|---|---|---|---|
| (n = 78) | (n = 78) | (n = 79) | (n = 78) | ||
| Age, y | 73.9 ± 2.8 | 73.1 ± 2.6 | 73.5 ± 2.9 | 73.4 ± 2.8 | 0.341 |
| Medications, n | 4.5 ± 3.4 | 4.6 ± 4.8 | 4.8 ± 3.1 | 5.2 ± 4.0 | 0.212 |
| Body composition | |||||
| Weight, kg | 68.0 ± 9.4 | 77.2 ± 9.8 | 83.6 ± 9.6 | 95.8 ± 13.0 | <0.001 § |
| BMI, kg/m2 | 23.4 ± 3.1 | 25.7 ± 3.1 | 28.0 ± 2.9 | 31.4 ± 3.8 | <0.001 § |
| Total fat mass, kg | 16.1 ± 4.7 | 20.8 ± 5.0 | 24.7 ± 4.3 | 31.2 ± 7.0 | <0.001 |
| Total lean mass, kg | 49.2 ± 6.2 | 53.6 ± 6.0 | 56.1 ± 6.4 | 61.5 ± 7.2 | <0.001 § |
| ALM, kg | 22.2 ± 3.3 | 24.4 ± 3.2 | 25.3 ± 3.2 | 28.3 ± 3.9 | <0.001 § |
| Muscle area, cm2 | 251.3 ± 44.7 | 274.5 ± 41.1 | 280.9 ± 41.7 | 311.0 ± 50.1 | <0.001 § |
| IMF, cm2 | 8.5 ± 2.3 | 14.9 ± 1.6 | 22.0 ± 2.7 | 39.4 ± 13.6 | <0.001 |
| SFA, cm2 | 71.1 ± 30.3 | 84.5 ± 31.1 | 104.6 ± 33.3 | 126.8 ± 39.9 | <0.001 § |
| Diet | |||||
| Energy, kcal/d | 2272 ± 1102 | 2171 ± 920 | 2229 ± 1030 | 2038 ± 784 | 0.740 |
| Fat intake, %kcal/d | 34.8 ± 8.0 | 35.2 ± 6.7 | 34.4 ± 7.2 | 34.1 ± 8.0 | 0.810 § |
| Protein intake, %kcal/d | 13.2 ± 2.7 | 13.8 ± 3.0 | 14.6 ± 3.4 | 13.9 ± 3.2 | 0.075 § |
| CHO intake, %kcal/d | 53.3 ± 10.2 | 51.9 ± 8.2 | 52.0 ± 8.7 | 52.5 ± 9.1 | 0.771 § |
| Physical activity & performance | |||||
| PA, kcal/kg/wk | 78.5 ± 62.3 | 95.9 ± 93.5 | 76.5 ± 69.0 | 84.2 ± 79.4 | 0.703 |
| Fast 6-m walk, m/s | 1.16 ± 0.21 | 1.15 ± 0.22 | 1.15 ± 0.20 | 1.14 ± 0.17 | 0.979 § |
| Chair stand, sec | 0.36 ± 0.12 | 0.35 ± 0.11 | 0.35 ± 0.11 | 0.34 ± 0.11 | 0.547 |
| Balance, 0–90 | 71.9 ± 23.4 | 69.5 ± 23.3 | 72.7 ± 19.9 | 73.9 ± 20.0 | 0.708 |
| Grip, N | 385.1 ± 105.7 | 409.9 ± 102.3 | 410.1 ± 99.8 | 422.5 ± 116.4 | 0.231 § |
| Leg strength, N.m | 126.9 ± 35.2 | 140.0 ± 38.1 | 139.0 ± 34.3 | 146.1 ± 38.5 | 0.022 § |
| Blood biochemistry | |||||
| Glucose, mg/dL | 96.5 ± 18.4 | 106.5 ± 32.4 | 112.8 ± 44.2 | 113.1 ± 36.0 | 0.002 |
| Insulin, μIU/mL | 6.18 ± 6.46 | 7.63 ± 4.58 | 7.92 ± 3.94 | 10.33 ± 5.04 | <0.001 |
| HOMA-IR | 1.48 ± 1.75 | 1.99 ± 1.38 | 2.18 ± 1.46 | 2.70 ± 1.51 | <0.001 |
| Triglycerides, mg/dL | 102.4 ± 60.0 | 118.2 ± 51.8 | 118.3 ± 64.4 | 121.9 ± 51.3 | 0.003 |
| Total cholesterol, mg/dL | 185.3 ± 31.6 | 202.9 ± 36.0 | 190.5 ± 34.2 | 193.3 ± 36.8 | 0.022 |
| HDL, mg/dL | 55.1 ± 14.7 | 51.3 ± 15.0 | 51.7 ± 13.9 | 49.9 ± 15.2 | 0.080 |
| LDL, mg/dL | 110.3 ± 32.0 | 128.4 ± 34.4 | 115.3 ± 31.7 | 119.0 ± 33.9 | 0.017 |
| Low IMF Low HOMA | Low IMF High HOMA | PWithin | High IMF Low HOMA | High IMF High HOMA | PWithin | PBetween | |
|---|---|---|---|---|---|---|---|
| (n = 29) | (n = 29) | (n = 27) | (n = 27) | ||||
| Age, y | 73.3 ± 2.7 | 72.8 ± 2.6 | 0.436 ¥ | 73.9 ± 2.8 | 73.1 ± 2.4 | 0.333 Ɫ | 0.475 |
| Body composition | |||||||
| Weight, kg | 70.6 ± 9 | 76.2 ± 8.2 | 0.017 ¥ | 85.6 ± 15.2 | 96.5 ± 13.5 | 0.008 ¥ | <0.001 § |
| Height, m | 1.74 ± 0.06 | 1.71 ± 0.06 | 0.099 ¥ | 1.72 ± 0.07 | 1.75 ± 0.08 | 0.101 ¥ | 0.092 § |
| BMI, kg/m2 | 23.4 ± 2.4 | 26.2 ± 2.7 | <0.001 ¥ | 29.0 ± 4.5 | 31.4 ± 3.3 | 0.016 Ɫ | <0.001 § |
| Total fat mass, kg | 16.8 ± 3.3 | 21.4 ± 4.0 | <0.001 Ɫ | 26.2 ± 7.3 | 31.5 ± 7.0 | 0.010 Ɫ | <0.001 |
| Total lean mass, kg | 51.2 ± 6.8 | 52.2 ± 5.5 | 0.536 ¥ | 56.9 ± 8.3 | 62.0 ± 7.7 | 0.025 ¥ | <0.001 § |
| ALM, kg | 23.5 ± 3.8 | 23.3 ± 2.8 | 0.743 ¥ | 26.1 ± 4.4 | 28.9 ± 4.4 | 0.022 ¥ | <0.001 § |
| Muscle area, cm2 | 253.3 ± 35.8 | 271.8 ± 38.9 | 0.066 ¥ | 286.8 ± 53.5 | 326.8 ± 49.9 | 0.006 ¥ | <0.001 § |
| IMF, cm2 | 12.8 ± 3.0 | 12.8 ± 3.0 | 0.966 ¥ | 33.7 ± 15.5 | 36.0 ± 15.0 | 0.337 Ɫ | <0.001 |
| SFA, cm2 | 73.8 ± 24.6 | 90.6 ± 30.4 | 0.025 ¥ | 113.4 ± 44.3 | 127.5 ± 30.3 | 0.178 ¥ | <0.001 § |
| Physical activity & performance | |||||||
| PA, kcal/kg/wk | 113.9 ± 90.4 | 77.5 ± 88.4 | 0.053 Ɫ | 104.6 ± 92.3 | 79.8 ± 97.3 | 0.135 | 0.096 |
| Fast 6-m walk, m/s | 1.09 ± 0.24 | 1.20 ± 0.18 | 0.049 ¥ | 1.12 ± 0.17 | 1.15 ± 0.19 | 0.474 ¥ | 0.161 § |
| 20-m walk, m/s | 1.24 ± 0.27 | 1.35 ± 0.18 | 0.090 ¥ | 1.30 ± 0.19 | 1.36 ± 0.25 | 0.222 Ɫ | 0.207 |
| Chair stand, sec | 0.34 ± 0.10 | 0.37 ± 0.09 | 0.269 ¥ | 0.35 ± 0.10 | 0.34 ± 0.11 | 0.959 Ɫ | 0.600 |
| Balance, 0–90 | 67.3 ± 20.5 | 71.2 ± 24.2 | 0.370 Ɫ | 75.3 ± 15.8 | 73.9 ± 16.6 | 0.758 Ɫ | 0.615 |
| Grip, N | 387.0 ± 90.9 | 430.0 ± 116.6 | 0.163 ¥ | 377.4 ± 82.7 | 467.6 ± 120.9 | 0.006 ¥ | 0.016 § |
| Torque, N.m | 131.7 ± 31.6 | 142.7 ± 42.4 | 0.309 ¥ | 130.1 ± 31.3 | 159.7 ± 41.0 | 0.010 ¥ | 0.034 |
| Specific torque, N.m/cm2 | 1.02 ± 0.20 | 1.02 ± 0.24 | 0.901 Ɫ | 0.91 ± 0.24 | 0.97 ± 0.21 | 0.326 ¥ | 0.239 |
| Blood Biochemistry | |||||||
| Glucose, mg/dL | 90.8 ± 15.4 | 108.1 ± 20.6 | 0.001 Ɫ | 88.3 ± 9.8 | 107.2 ± 16.4 | <0.001 Ɫ | <0.001 |
| Insulin, μIU/mL | 3.33 ± 1.41 | 12.78 ± 8.13 | <0.001 Ɫ | 5.72 ± 2.63 | 15.91 ± 2.90 | <0.001 Ɫ | <0.001 |
| HOMA-IR | 0.74 ± 0.32 | 3.38 ± 2.23 | <0.001 Ɫ | 1.27 ± 0.65 | 4.23 ± 1.17 | <0.001 Ɫ | <0.001 § |
| Triglycerides, mg/dL | 95.8 ± 31.8 | 150.3 ± 82.3 | 0.002 ¥ | 98.0 ± 31.9 | 138.4 ± 49.2 | 0.001 ¥ | <0.001 § |
| TChol, mg/dL | 194.4 ± 36.5 | 201.5 ± 29.2 | 0.414 ¥ | 193.2 ± 37.3 | 180.9 ± 29.6 | 0.183 ¥ | 0.227 |
| HDL, mg/dL | 57.6 ± 12.9 | 47.1 ± 13.2 | 0.003 ¥ | 55.8 ± 15.2 | 45.9 ± 10.7 | 0.005 Ɫ | <0.001 |
| LDL, mg/dL | 117.7 ± 32.7 | 126.1 ± 28.7 | 0.305 ¥ | 117.9 ± 36.8 | 107.3 ± 24.1 | 0.217 ¥ | 0.158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farsijani, S.; Marron, M.M.; Miljkovic, I.; Baugh, M.E.; Kritchevsky, S.B.; Newman, A.B. Metabolic Markers Demonstrate the Heterogeneity of Myosteatosis in Community-Dwelling Older Black Men from the Health ABC Study. Metabolites 2021, 11, 224. https://doi.org/10.3390/metabo11040224
Farsijani S, Marron MM, Miljkovic I, Baugh ME, Kritchevsky SB, Newman AB. Metabolic Markers Demonstrate the Heterogeneity of Myosteatosis in Community-Dwelling Older Black Men from the Health ABC Study. Metabolites. 2021; 11(4):224. https://doi.org/10.3390/metabo11040224
Chicago/Turabian StyleFarsijani, Samaneh, Megan M. Marron, Iva Miljkovic, Mary Elizabeth Baugh, Stephen B. Kritchevsky, and Anne B. Newman. 2021. "Metabolic Markers Demonstrate the Heterogeneity of Myosteatosis in Community-Dwelling Older Black Men from the Health ABC Study" Metabolites 11, no. 4: 224. https://doi.org/10.3390/metabo11040224
APA StyleFarsijani, S., Marron, M. M., Miljkovic, I., Baugh, M. E., Kritchevsky, S. B., & Newman, A. B. (2021). Metabolic Markers Demonstrate the Heterogeneity of Myosteatosis in Community-Dwelling Older Black Men from the Health ABC Study. Metabolites, 11(4), 224. https://doi.org/10.3390/metabo11040224







