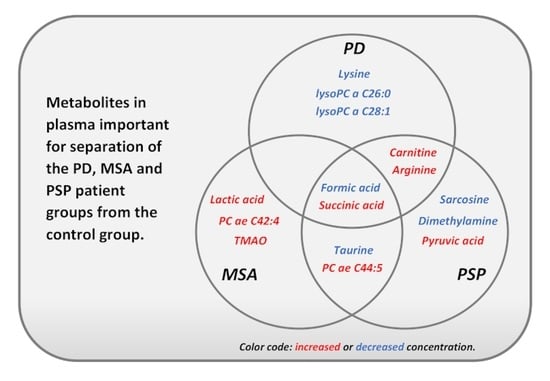
Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism
Abstract
:1. Introduction
2. Results
2.1. NMR-Based Analysis
2.2. MS-Based Analysis
2.3. Analysis of the Merged NMR- and MS-Data
3. Discussion
3.1. PD Patients versus Control Subjects
3.2. MSA Patients versus Control Subjects
3.3. PSP Patients versus Control Subjects
3.4. PD versus MSA Patients
3.5. PD versus PSP Patients
3.6. MSA versus PSP Patients
3.7. Discriminatory Metabolites and Disease Mechanisms
3.8. Concluding Remarks
4. Materials and Methods
4.1. Participants and Sample Collection
4.2. NMR Analysis
4.3. MS Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Kassubek, J.; Csoti, I.; Ehret, R.; Herbst, H.; Wellach, I.; Winkler, J.; Jost, W.H. Differentiation of atypical Parkinson syndromes. J. Neural Transm. 2017, 124, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Neuropathology of multiple system atrophy: New thoughts about pathogenesis. Mov. Disord. 2014, 29, 1720–1741. [Google Scholar] [CrossRef]
- Williams, D.R.; Lees, A.J. Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009, 8, 270–279. [Google Scholar] [CrossRef]
- Miki, Y.; Tsushima, E.; Foti, S.C.; Strand, K.M.; Asi, Y.T.; Yamamoto, A.K.; Bettencourt, C.; Oliveira, M.C.B.; De Pablo-Fernandez, E.; Jaunmuktane, Z.; et al. Identification of multiple system atrophy mimicking Parkinson’s disease or progressive supranuclear palsy. Brain 2021, 144, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Meijer, F.J.A.; Bloem, B.R.; Mahlknecht, P.; Seppi, K.; Goraj, B. Update on diffusion MRI in Parkinson’s disease and atypical parkinsonism. J. Neurol. Sci. 2013, 332, 21–29. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Nigro, S.; Quattrone, A.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nistico, R.; Salsone, M.; Novellino, F.; et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 54, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chelban, V.; Bocchetta, M.; Hassanein, S.; Haridy, N.A.; Houlden, H.; Rohrer, J.D. An update on advances in magnetic resonance imaging of multiple system atrophy. J. Neurol. 2019, 266, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, M.K.; Aerts, M.B.; Beenes, M.; Norgren, N.; Esselink, R.A.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF Neurofilament Light Chain but not FLT3 Ligand Discriminates Parkinsonian Disorders. Front. Neurol. 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.K.; Eeftens, J.M.; Aerts, M.B.; Esselink, R.A.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat. Disord. 2014, 20, 112–115. [Google Scholar] [CrossRef]
- Sako, W.; Murakami, N.; Izumi, Y.; Kaji, R. Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: Evidence from a meta-analysis. J. Neurol. Sci. 2015, 352, 84–87. [Google Scholar] [CrossRef]
- Sako, W.; Murakami, N.; Izumi, Y.; Kaji, R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: Evidence from a meta-analysis. Mov. Disord. 2014, 29, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Laurens, B.; Constantinescu, R.; Freeman, R.; Gerhard, A.; Jellinger, K.; Jeromin, A.; Krismer, F.; Mollenhauer, B.; Schlossmacher, M.G.; Shaw, L.M.; et al. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiol. Dis. 2015, 80, 29–41. [Google Scholar] [CrossRef]
- Magdalinou, N.K.; Paterson, R.W.; Schott, J.M.; Fox, N.C.; Mummery, C.; Blennow, K.; Bhatia, K.; Morris, H.R.; Giunti, P.; Warner, T.T.; et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1240–1247. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012, 135, 1900–1913. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Wu, J.F.; Wuolikainen, A.; Trupp, M.; Jonsson, P.; Marklund, S.L.; Andersen, P.M.; Forsgren, L.; Öhman, A. NMR analysis of the CSF and plasma metabolome of rigorously matched amyotrophic lateral sclerosis, Parkinson’s disease and control subjects. Metabolomics 2016, 12, 101. [Google Scholar] [CrossRef]
- Öhman, A.; Forsgren, L. NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson’s disease and controls. Neurosci. Lett. 2015, 594, 36–39. [Google Scholar] [CrossRef]
- Molina, J.A.; Jimenez-Jimenez, F.J.; Gomez, P.; Vargas, C.; Navarro, J.A.; Orti-Pareja, M.; Gasalla, T.; Benito-Leon, J.; Bermejo, F.; Arenas, J. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson’s disease. J. Neurol. Sci. 1997, 150, 123–127. [Google Scholar] [CrossRef]
- Figura, M.; Kusmierska, K.; Bucior, E.; Szlufik, S.; Koziorowski, D.; Jamrozik, Z.; Janik, P. Serum amino acid profile in patients with Parkinson’s disease. PLoS ONE 2018, 13, e0191670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1081, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.X.; Hu, L.; Wei, Y.D.; Chen, G.H. Metabolomics profiling reveals altered lipid metabolism and identifies a panel of lipid metabolites as biomarkers for Parkinson’s disease related anxiety disorder. Neurosci. Lett. 2021, 745, 135626. [Google Scholar] [CrossRef]
- Luan, H.; Liu, L.F.; Tang, Z.; Zhang, M.; Chua, K.K.; Song, J.X.; Mok, V.C.; Li, M.; Cai, Z. Comprehensive urinary metabolomic profiling and identification of potential noninvasive marker for idiopathic Parkinson’s disease. Sci. Rep. 2015, 5, 13888. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Jimenez, F.J.; Molina, J.A.; Vargas, C.; Gomez, P.; Navarro, J.A.; Benito-Leon, J.; Orti-Pareja, M.; Gasalla, T.; Cisneros, E.; Arenas, J. Neurotransmitter amino acids in cerebrospinal fluid of patients with Parkinson’s disease. J. Neurol. Sci. 1996, 141, 39–44. [Google Scholar] [CrossRef]
- Mally, J.; Szalai, G.; Stone, T.W. Changes in the concentration of amino acids in serum and cerebrospinal fluid of patients with Parkinson’s disease. J. Neurol. Sci. 1997, 151, 159–162. [Google Scholar] [CrossRef]
- Nagesh Babu, G.; Gupta, M.; Paliwal, V.K.; Singh, S.; Chatterji, T.; Roy, R. Serum metabolomics study in a group of Parkinson’s disease patients from northern India. Clin. Chim. Acta 2018, 480, 214–219. [Google Scholar] [CrossRef]
- Mori, A.; Ishikawa, K.I.; Saiki, S.; Hatano, T.; Oji, Y.; Okuzumi, A.; Fujimaki, M.; Koinuma, T.; Ueno, S.I.; Imamichi, Y.; et al. Plasma metabolite biomarkers for multiple system atrophy and progressive supranuclear palsy. PLoS ONE 2019, 14, e0223113. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, P.A.; Li, J.; Lu, M.; Beach, T.G.; Adler, C.H.; Guo, L.; Arizona Parkinson’s Disease, C. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov. Disord. 2013, 28, 1653–1660. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Jonsson, P.; Ohrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J. Parkinsons Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 29–44. [Google Scholar] [CrossRef]
- Armengou, A.; Hurtado, O.; Leira, R.; Obon, M.; Pascual, C.; Moro, M.A.; Lizasoain, I.; Castillo, J.; Davalos, A. L-arginine levels in blood as a marker of nitric oxide-mediated brain damage in acute stroke: A clinical and experimental study. J. Cereb. Blood Flow Metab. 2003, 23, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Virarkar, M.; Alappat, L.; Bradford, P.G.; Awad, A.B. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013, 53, 1157–1167. [Google Scholar] [CrossRef]
- Graham, S.F.; Chevallier, O.P.; Elliott, C.T.; Holscher, C.; Johnston, J.; McGuinness, B.; Kehoe, P.G.; Passmore, A.P.; Green, B.D. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 2015, 10, e0119452. [Google Scholar] [CrossRef]
- Papes, F.; Surpili, M.J.; Langone, F.; Trigo, J.R.; Arruda, P. The essential amino acid lysine acts as precursor of glutamate in the mammalian central nervous system. FEBS Lett. 2001, 488, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Strijbis, K.; Vaz, F.M.; Distel, B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life 2010, 62, 357–362. [Google Scholar] [CrossRef]
- Jaspers, Y.R.J.; Ferdinandusse, S.; Dijkstra, I.M.E.; Barendsen, R.W.; van Lenthe, H.; Kulik, W.; Engelen, M.; Goorden, S.M.I.; Vaz, F.M.; Kemp, S. Comparison of the Diagnostic Performance of C26:0-Lysophosphatidylcholine and Very Long-Chain Fatty Acids Analysis for Peroxisomal Disorders. Front. Cell Dev. Biol. 2020, 8, 690. [Google Scholar] [CrossRef]
- Stoessel, D.; Schulte, C.; Teixeira Dos Santos, M.C.; Scheller, D.; Rebollo-Mesa, I.; Deuschle, C.; Walther, D.; Schauer, N.; Berg, D.; Nogueira da Costa, A.; et al. Promising Metabolite Profiles in the Plasma and CSF of Early Clinical Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Miletic Vukajlovic, J.; Drakulic, D.; Pejic, S.; Ilic, T.V.; Stefanovic, A.; Petkovic, M.; Schiller, J. Increased plasma phosphatidylcholine/lysophosphatidylcholine ratios in patients with Parkinson’s disease. Rapid Commun. Mass Spectrom. 2020, 34, e8595. [Google Scholar] [CrossRef] [PubMed]
- Menzie, J.; Pan, C.; Prentice, H.; Wu, J.Y. Taurine and central nervous system disorders. Amino Acids 2014, 46, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Engelborghs, S.; Marescau, B.; De Deyn, P.P. Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson’s disease. Neurochem. Res. 2003, 28, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, Y.; Tong, Q.; Jiang, S.; Xu, Q.; Ding, J.; Zhang, L.; Zhang, R.; Zhang, K. Reduced plasma taurine level in Parkinson’s disease: Association with motor severity and levodopa treatment. Int. J. Neurosci. 2016, 126, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Erkut, C.; Pan-Montojo, F.; Boland, S.; Stewart, M.P.; Müller, D.J.; Wurst, W.; Hyman, A.A.; Kurzchalia, T.V. Products of the Parkinson’s disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol. Open 2014, 3, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nho, K.; Kueider-Paisley, A.; Arnold, M.; MahmoudianDehkordi, S.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmuller, G.; et al. Serum metabolites associated with brain amyloid beta deposition, cognition and dementia progression. Brain Commun. 2021, 3, fcab139. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Pizzato, S.; Piovesan, F.; Trevisan, C.; Veronese, N.; Manzato, E. Effects of acetyl-L-carnitine in diabetic neuropathy and other geriatric disorders. Aging Clin. Exp. Res. 2018, 30, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G.; et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer’s Dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzada, P.R.; Paula Lima, A.C.; Mendonca-Silva, D.L.; Noel, F.; De Mello, F.G.; Ferreira, S.T. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: Activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004, 18, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.M.; da Costa Ferreira, G.; Schuck, P.F.; Tonin, A.M.; Zanatta, A.; de Souza Wyse, A.T.; Dutra-Filho, C.S.; Wannmacher, C.M.; Wajner, M. Evidence that 3-hydroxyisobutyric acid inhibits key enzymes of energy metabolism in cerebral cortex of young rats. Int. J. Dev. Neurosci. 2008, 26, 293–299. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Gilman, S.; Low, P.A.; Quinn, N.; Albanese, A.; Ben-Shlomo, Y.; Fowler, C.J.; Kaufmann, H.; Klockgether, T.; Lang, A.E.; Lantos, P.L.; et al. Consensus statement on the diagnosis of multiple system atrophy. J. Neurol. Sci. 1999, 163, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Litvan, I.; Agid, Y.; Jankovic, J. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Bowser, R.; Turner, M.; Berry, J.; Brettschneider, J.; Connor, J.; Costa, J.; Cudkowicz, M.; Glass, J.; Jahn, O.; et al. Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph. Lateral. Scler. 2012, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Bylesjo, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS (R) models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0--A comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef] [Green Version]
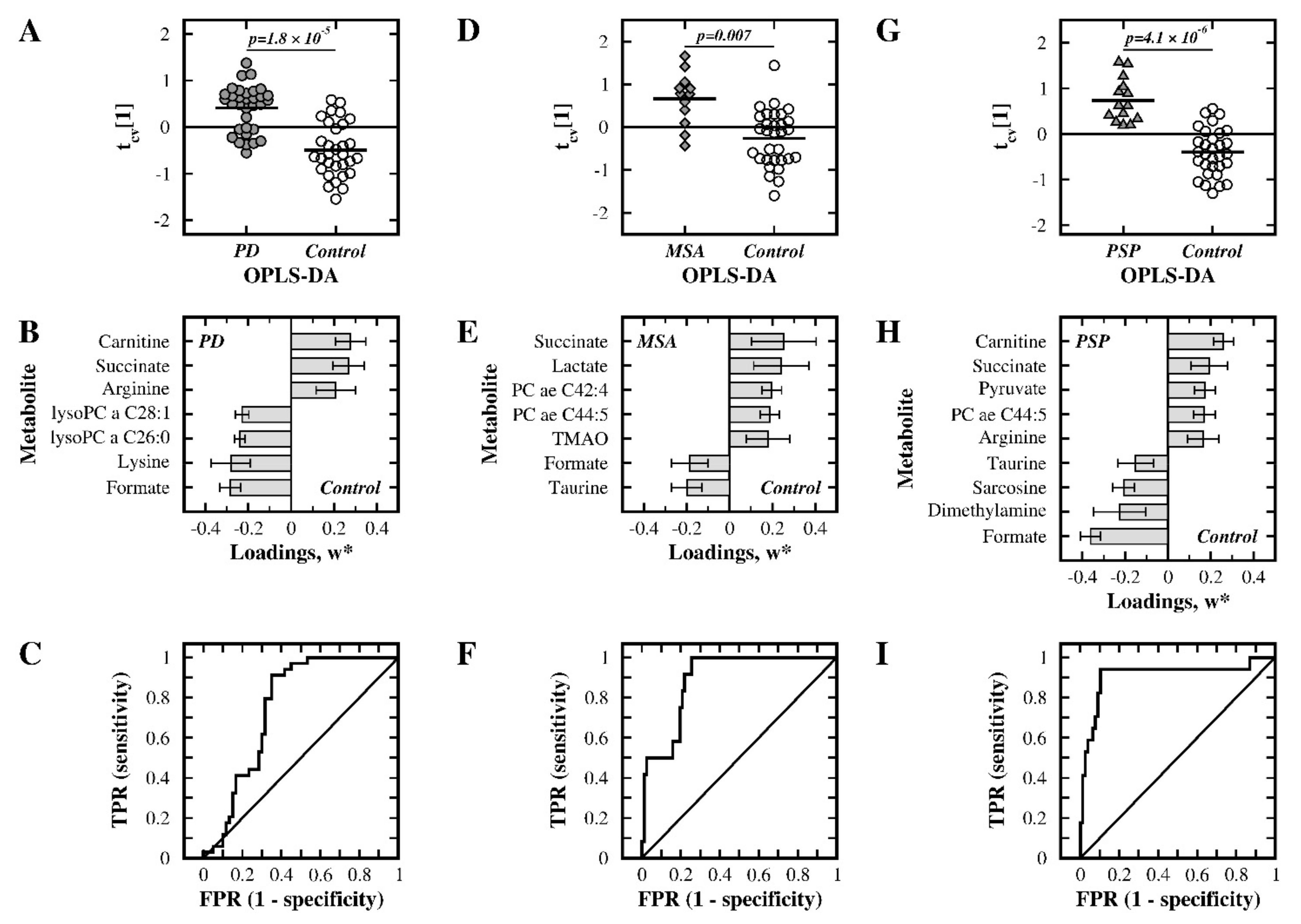

| Comparison | Variables | Apred | Aorth | R2X | R2Y | Q2Y | CV-ANOVA | AUC |
|---|---|---|---|---|---|---|---|---|
| PD:Control | All | 1 | 2 | 0.290 | 0.801 | 0.417 | 1.8 × 10−5 | 0.76:0.98 |
| MSA:Control | All | 1 | 1 | 0.246 | 0.753 | 0.302 | 0.007 | 0.89:0.90 |
| PSP:Control | All | 1 | 1 | 0.248 | 0.811 | 0.524 | 4.1 × 10–6 | 0.91:0.89 |
| PD:MSA | Selected | 1 | 0 | 0.243 | 0.430 | 0.355 | 1.0 × 10–4 | 0.70:0.87 |
| PD:PSP | Selected | 1 | 0 | 0.236 | 0.546 | 0.467 | 9.9 × 10–7 | 0.73:0.91 |
| MSA:PSP | Selected | 1 | 1 | 0.301 | 0.717 | 0.626 | 1.2 × 10–5 | 0.56:0.89 |
| Metabolite | PD | MSA | PSP | PD | PD | MSA | Pathway or Physiological Function |
|---|---|---|---|---|---|---|---|
| vs. Control | MSA | vs. PSP | PSP | ||||
| Formic acid | 🡇 | 🡇 | 🡇 | 🡅 | 🡅 | 🡅 | Pyruvate met. Methane met., Kynurenine pathway |
| Succinate | 🡅 | 🡅 | 🡅 | Citric acid cycle | |||
| Carnitine | 🡅 | 🡅 | 🡇 | 🡇 | Carnitine biosynthesis, Mitochondrial shuttle system | ||
| L-ArginineNMR | 🡅 | 🡅 | 🡇 | Arg and Pro metabolism, linked to oxidative stress | |||
| L-LysineNMR | 🡇 | 🡇 | Carnitine biosynthesis, Lys synthesis/degradation | ||||
| lysoPC a C26:0 | 🡇 | Lipid metabolism | |||||
| lysoPC a C28:1 | 🡇 | Lipid metabolism | |||||
| TaurineNMR | 🡇 | 🡇 | 🡅 | 🡅 | Neurotransmitter, Neuroprotective | ||
| Lactic acid | 🡅 | Anaerobic glucose and pyruvate metabolism | |||||
| PC ae C44:5 | 🡅 | 🡅 | Lipid metabolism | ||||
| PC ae C42:4 | 🡅 | Lipid metabolism | |||||
| TMAO | ↑ | Methane metabolism | |||||
| Sarcosine | 🡇 | 🡅 | 🡅 | Arg/Pro metabolism, Gly/Ser/Thr metabolism | |||
| Dimethylamine | 🡇 | 🡅 | ↑ | Methane metabolism. Link to oxidative stress regulation | |||
| Pyruvic acid | 🡅 | Energy source for citric acid cycle | |||||
| Acetylcarnitine | 🡅 | 🡇 | MCH FA shuttle system, Glu/Gln/GABA synthesis | ||||
| GlycineMS | 🡇 | ↑ | GSH metabolism | ||||
| Acetoacetate | ↑ | ↓ | Ketone body, propanoate and Leu metabolism | ||||
| 2-HB | 🡅 | Lipid oxidation, oxidative stress | |||||
| 3-HB | ↑ | Ketone body metabolism | |||||
| 3-HIB | ↑ | Linked to brain metabolism and neurotransmission | |||||
| CreatinineMS | 🡅 | Creatine phosphate metabolism | |||||
| GlutamateMS | 🡅 | Glu/Gln/GABA synthesis, Arg synthesis, GSH met. | |||||
| L-GlutamineNMR | ↓ | Glu/Gln/GABA synthesis, Arg synthesis | |||||
| L-HistidineNMR | 🡅 | ↑ | Link to neurotransmission and oxidative stress | ||||
| L-HistidineMS | 🡅 | Link to neurotransmission and oxidative stress | |||||
| L-ValineMS | 🡅 | BCAA synthesis, neurotransmitter synthesis | |||||
| lysoPC a C28:0 | 🡇 | Lipid metabolism | |||||
| Glucose | ↓ | Glucose metabolism |
| Control | PD | MSA | PSP | |
|---|---|---|---|---|
| Number of subjects | 31 | 34 | 12 | 17 |
| Gender, Male/Female | 17/14 | 19/15 | 8/4 | 9/8 |
| Age, mean ± SD | 68.2 ± 6.6 | 69 ± 6.8 | 74.4 ± 9.2 | 75.2 ± 7.3 |
| Levodopa treatment | 0 | 1 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathan, M.; Wu, J.; Lakso, H.-Å.; Forsgren, L.; Öhman, A. Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism. Metabolites 2021, 11, 860. https://doi.org/10.3390/metabo11120860
Pathan M, Wu J, Lakso H-Å, Forsgren L, Öhman A. Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism. Metabolites. 2021; 11(12):860. https://doi.org/10.3390/metabo11120860
Chicago/Turabian StylePathan, Meerakhan, Junfang Wu, Hans-Åke Lakso, Lars Forsgren, and Anders Öhman. 2021. "Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism" Metabolites 11, no. 12: 860. https://doi.org/10.3390/metabo11120860
APA StylePathan, M., Wu, J., Lakso, H.-Å., Forsgren, L., & Öhman, A. (2021). Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism. Metabolites, 11(12), 860. https://doi.org/10.3390/metabo11120860