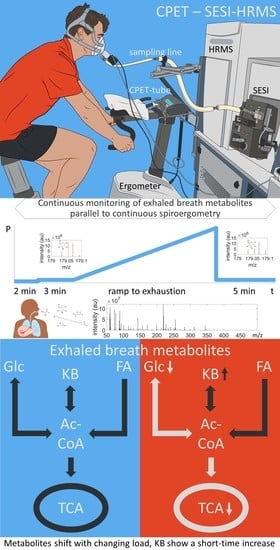
Real-Time Monitoring of Metabolism during Exercise by Exhaled Breath
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
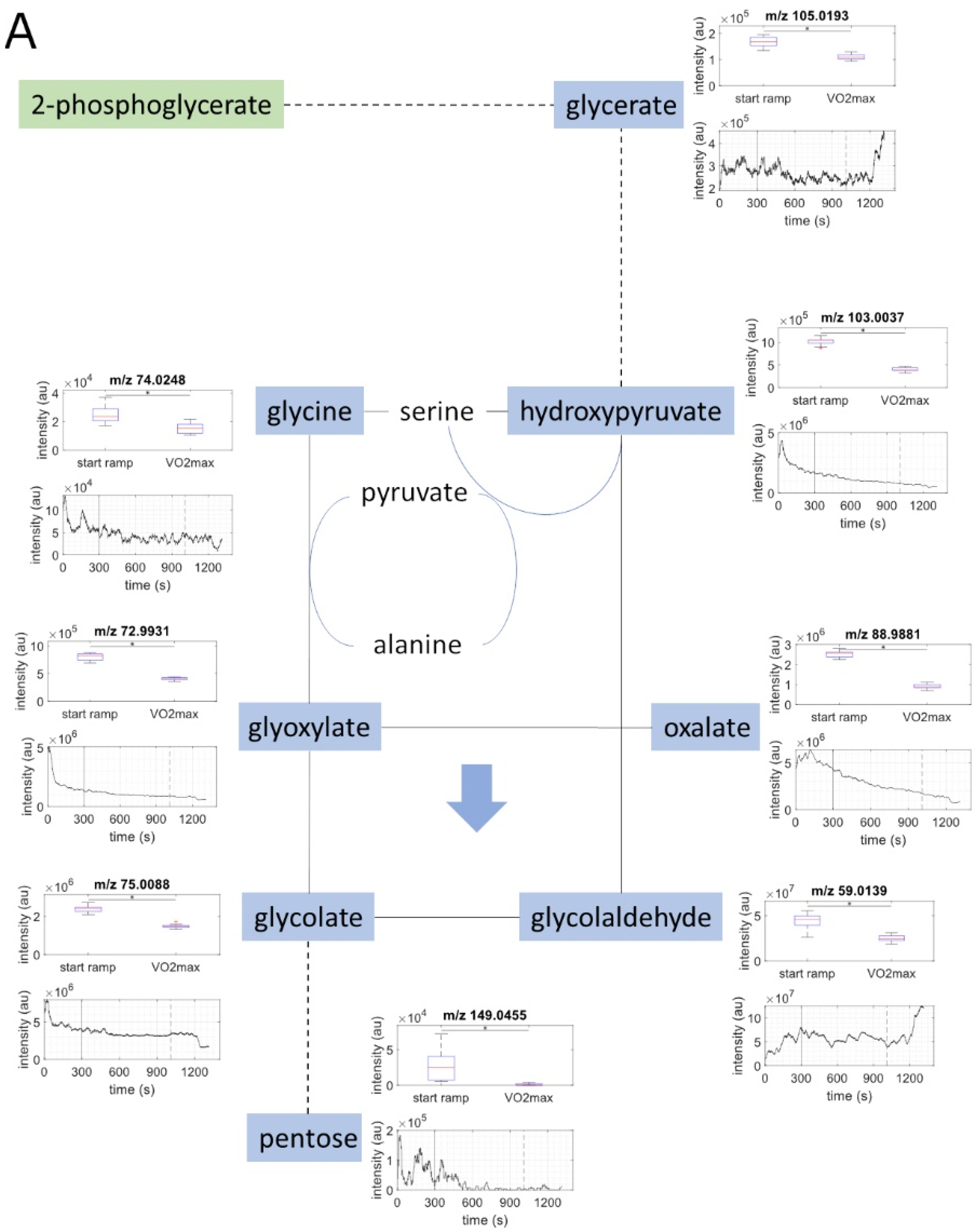
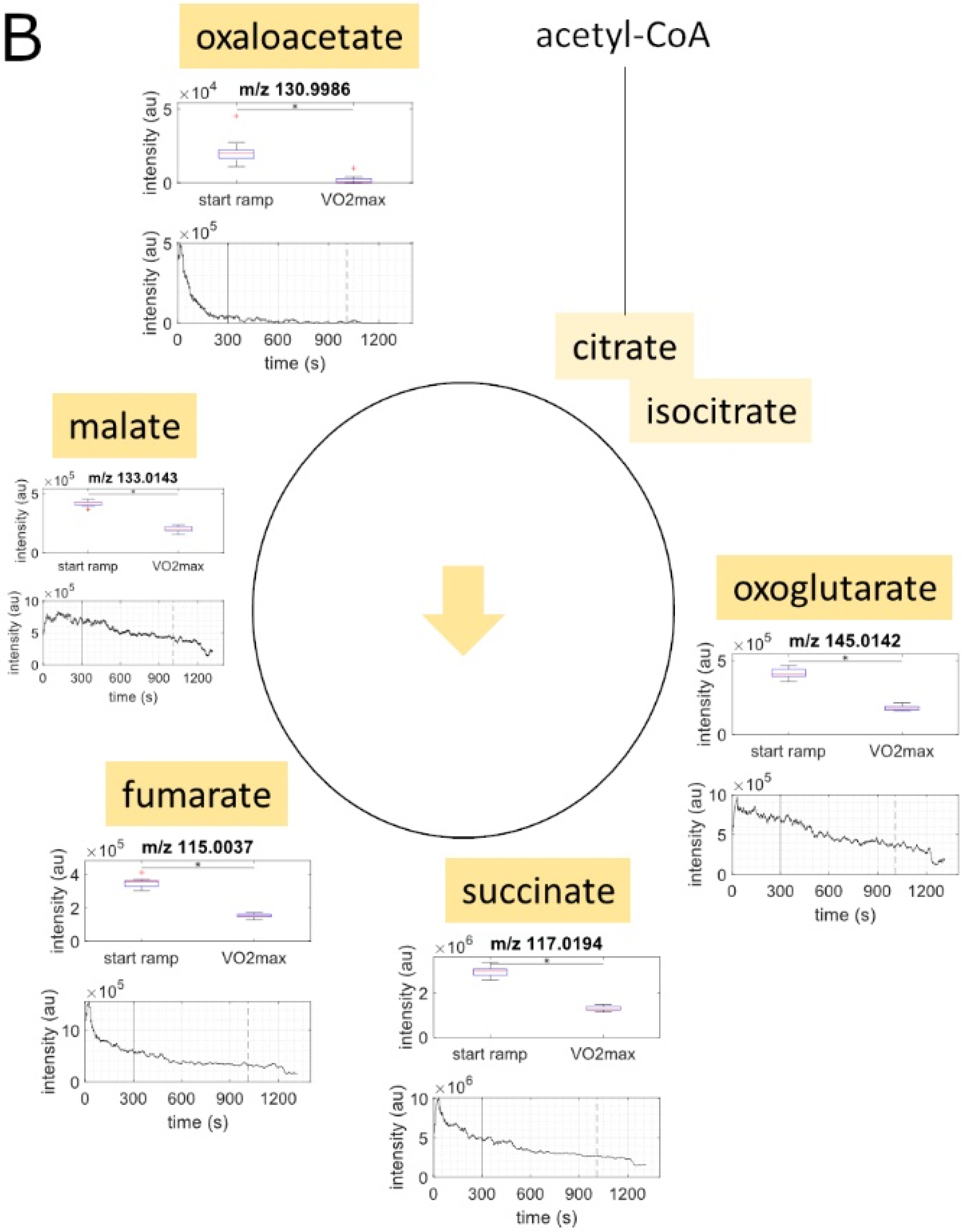
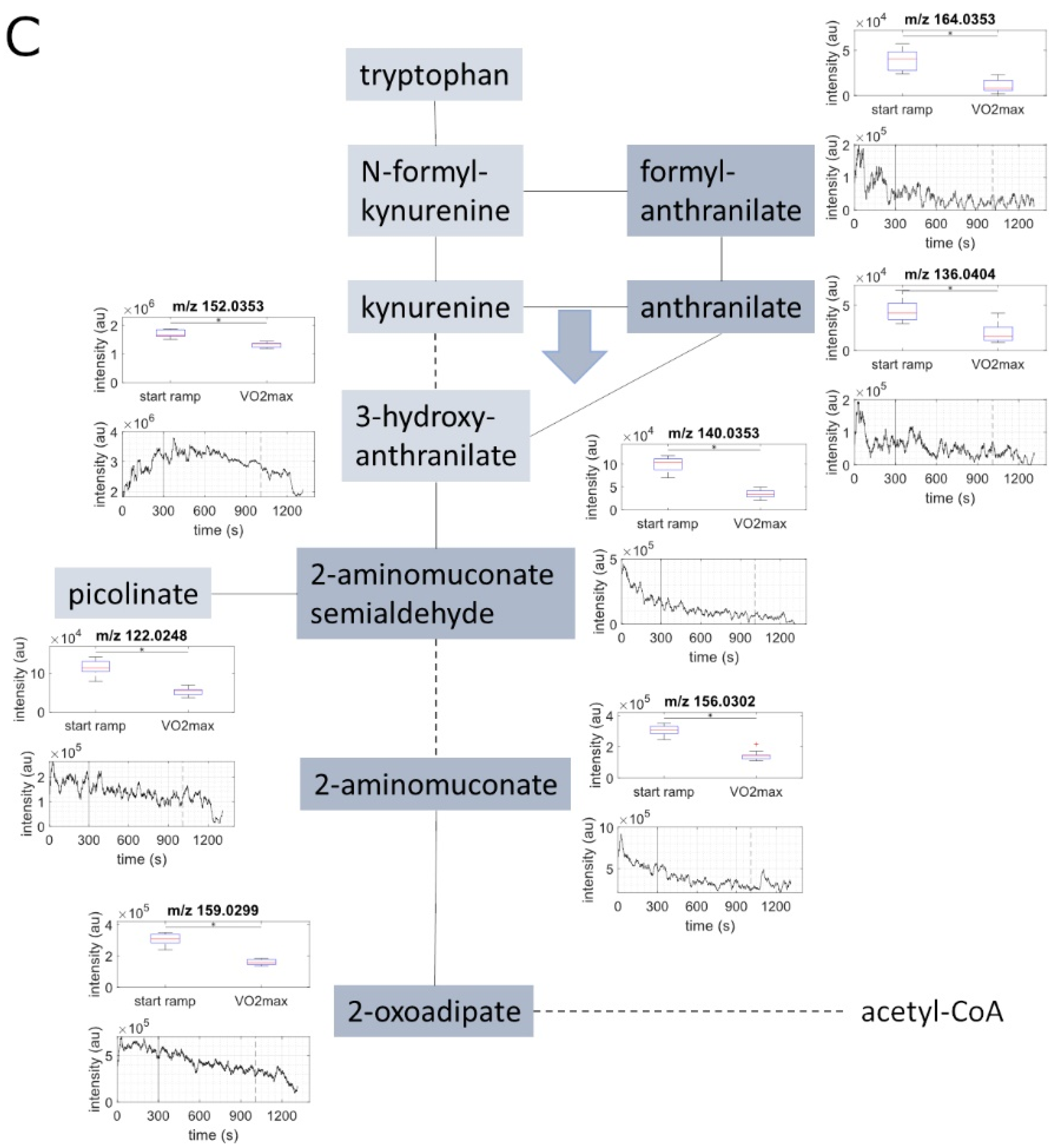
2.2. Metabolite Concentration of Several Pathways Decreased with Increasing Load
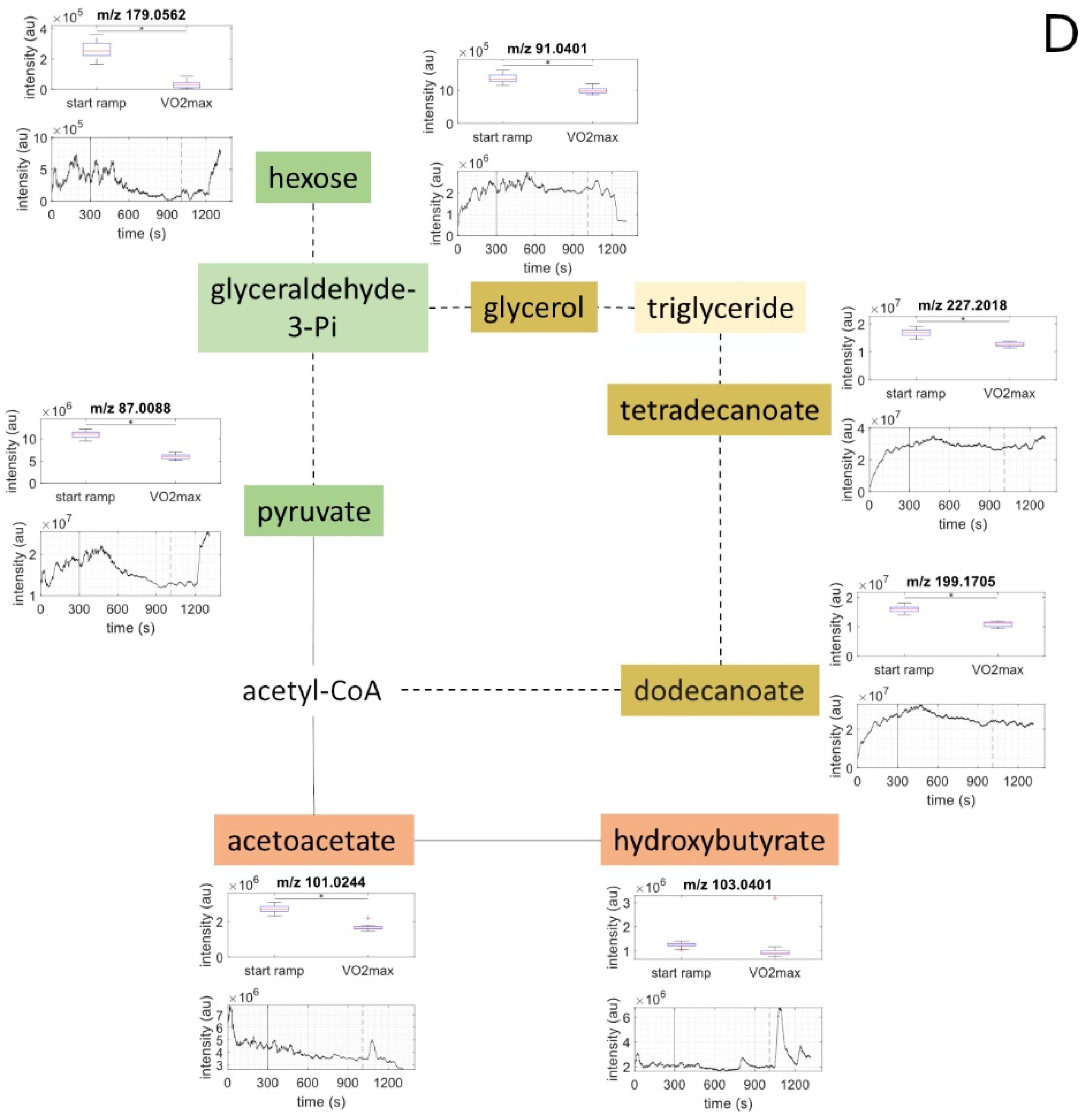
2.3. Metabolites of Glycolysis and Oxo Fatty Acids Showed Inverse Behavior to Ketone Bodies
2.4. Ketone Bodies Correlated with Performance
2.5. Metabolic Aerobic Threshold was Associated with Decrease of Carbohydrates and Fatty Acids
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. CPET and Spirometry
4.3. SESI-HRMS
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 7 May 2021).
- Yang, X.; Li, Y.; Ren, X.; Xiong, X.; Wu, L.; Li, J.; Wang, J.; Gao, Y.; Shang, H.; Xing, Y. Effects of exercise-based cardiac rehabilitation in patients after percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Sci. Rep. 2017, 7, 44789. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Chen, S.; Durstine, J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.-S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular Choreography of Acute Exercise. Cell 2020, 181, 1112–1130. [Google Scholar] [CrossRef]
- Nayor, M.; Shah, R.V.; Miller, P.E.; Blodgett, J.B.; Tanguay, M.; Pico, A.R.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Deik, A.; et al. Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation 2020, 142, 1905–1924. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of Exhaled Breath for Disease Detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Chippendale, W.E.T.; Dryahina, K.; Spanel, P. SIFT-MS Analysis of Nose-Exhaled Breath; Mouth Contamination and the Influence of Exercise. Curr. Anal. Chem. 2013, 9, 565–575. [Google Scholar] [CrossRef]
- King, J.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Teschl, S.; Teschl, G.; Miekisch, W.; Schubert, J.; Hinterhuber, H.; Amann, A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009, 3, 027006. [Google Scholar] [CrossRef]
- Heaney, L.M.; Lindley, M.R. Translation of exhaled breath volatile analyses to sport and exercise applications. Metab. Off. J. Metab. Soc. 2017, 13, 139. [Google Scholar] [CrossRef]
- Grapov, D.; Fiehn, O.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Keim, N.L.; Newman, J.W.; Hunter, G.R.; et al. Exercise plasma metabolomics and xenometabolomics in obese, sedentary, insulin-resistant women: Impact of a fitness and weight loss intervention. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E999–E1014. [Google Scholar] [CrossRef]
- Grapov, D.; Fiehn, O.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Keim, N.L.; Hunter, G.R.; Fernandez, J.R.; et al. Impact of a weight loss and fitness intervention on exercise-associated plasma oxylipin patterns in obese, insulin-resistant, sedentary women. Physiol. Rep. 2020, 8, e14547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Gaisl, T.; Miladinovic, D.; Marcinkevics, R.; Osswald, M.; Bauer, S.; Buhmann, J.; Zenobi, R.; Sinues, P.; Brown, S.A.; et al. Rapid and reversible control of human metabolism by individual sleep states. Cell Rep. 2021, 37, 109903. [Google Scholar] [CrossRef]
- Tejero Rioseras, A.; Singh, K.D.; Nowak, N.; Gaugg, M.T.; Bruderer, T.; Zenobi, R.; Sinues, P.M. Real-Time Monitoring of Tricarboxylic Acid Metabolites in Exhaled Breath. Anal. Chem. 2018, 90, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
- Anokhina, T.N.; Anaev, E.K.; Revelsky, A.I.; Revelsky, I.A.; Chuchalin, A.G. Saturated fatty acids in exhaled breath condensate in COPD patients. Eur. Respir. J. 2012, 40, P2207. [Google Scholar]
- Gaugg, M.T.; Bruderer, T.; Nowak, N.; Eiffert, L.; Martinez-Lozano Sinues, P.; Kohler, M.; Zenobi, R. Mass-Spectrometric Detection of Omega-Oxidation Products of Aliphatic Fatty Acids in Exhaled Breath. Anal. Chem. 2017, 89, 10329–10334. [Google Scholar] [CrossRef]
- Schader, J.F.; Haid, M.; Cecil, A.; Schoenfeld, J.; Halle, M.; Pfeufer, A.; Prehn, C.; Adamski, J.; Nieman, D.C.; Scherr, J. Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites 2020, 10, 87. [Google Scholar] [CrossRef]
- Gibala, M.J.; MacLean, D.A.; Graham, T.E.; Saltin, B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am. J. Physiol. -Endocrinol. Metab. 1998, 275, E235–E242. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol.-Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Lenard, N.R.; Gettys, T.W.; Dunn, A.J. Activation of β2- and β3-adrenergic receptors increases brain tryptophan. J. Pharmacol. Exp. Ther. 2003, 305, 653–659. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef]
- Poole, R.C.; Halestrap, A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol.-Cell Physiol. 1993, 264, C761–C782. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Hamilton, J.A. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Churchward-Venne, T.A.; Bailey, D.; van Loon, L.J.C. Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Med. 2017, 47, 383–391. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. (Auckl. N. Z.) 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Hepatic glucose production during exercise. Adv. Exp. Med. Biol. 1998, 441, 117–127. [Google Scholar]
- Miura, Y. The biological significance of ω-oxidation of fatty acids. Proc. Jpn. Acad. Ser. B 2013, 89, 370–382. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Danaher, J.; Gerber, T.; Wellard, R.M.; Stathis, C.G.; Cooke, M.B. The use of metabolomics to monitor simultaneous changes in metabolic variables following supramaximal low volume high intensity exercise. Metab. Off. J. Metab. Soc. 2015, 12, 7. [Google Scholar] [CrossRef][Green Version]
- Zafeiridis, A.; Chatziioannou, A.C.; Sarivasiliou, H.; Kyparos, A.; Nikolaidis, M.G.; Vrabas, I.S.; Pechlivanis, A.; Zoumpoulakis, P.; Baskakis, C.; Dipla, K.; et al. Global Metabolic Stress of Isoeffort Continuous and High Intensity Interval Aerobic Exercise: A Comparative 1H NMR Metabonomic Study. J. Proteome Res. 2016, 15, 4452–4463. [Google Scholar] [CrossRef]
- Garcia-Gomez, D.; Gaisl, T.; Bregy, L.; Cremonesi, A.; Sinues, P.M.; Kohler, M.; Zenobi, R. Real-Time Quantification of Amino Acids in the Exhalome by Secondary Electrospray Ionization-Mass Spectrometry: A Proof-of-Principle Study. Clin. Chem. 2016, 62, 1230–1237. [Google Scholar] [CrossRef]
- Chicharro, J.L.; Lucía, A.; Pérez, M.; Vaquero, A.F.; Ureña, R. Saliva Composition and Exercise. Sports Med. 1998, 26, 17–27. [Google Scholar] [CrossRef]
- King, J.; Koc, H.; Unterkofler, K.; Mochalski, P.; Kupferthaler, A.; Teschl, G.; Teschl, S.; Hinterhuber, H.; Amann, A. Physiological modeling of isoprene dynamics in exhaled breath. J. Theor. Biol. 2010, 267, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Crook, S.; Kaltsakas, G.; Louvaris, Z.; Berton, D.; Urquhart, D.S.; Kampouras, A.; Rabinovich, R.A.; Verges, S.; Kontopidis, D.; et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur. Respir. Rev. 2019, 28, 180101. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- David Groppe. MATLAB Central File Exchange. 2021. Available online: https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh (accessed on 8 December 2021).

| Characteristic | Subjects (N = 13) |
|---|---|
| Sex = male (%) | 6 (46.2) |
| Age (median [IQR]), years | 30.0 [27.0, 31.0] |
| BMI (median [IQR]), kg/m2 | 22.8 [22.3, 24.0] |
| Weight (median [IQR]), kg | 70.0 [62.5, 77.7] |
| Smoking = no (%) | 13 (100.0) |
| Spirometry FVC 1 (median [IQR]), L | 5.3 [4.3, 6.4] |
| FVC 1 % predicted (median [IQR]) | 124.0 [110.0, 128.0] |
| FEV1 2 (median [IQR]) | 4.3 [3.7, 5.0] |
| FEV1 2 % predicted (median [IQR]) | 110.0 [106.0, 120.0] |
| FEV1/FVC 3 (median [IQR]) | 81.0 [76.0, 84.0] |
| Exercise per Week Activity number (median [IQR]) | 4.5 [2.0, 6.0] |
| Activity duration (median [IQR]), min | 150.0 [120.0, 210.0] |
| Activity intensity 4 = moderate (%) | 13 (100.0) |
| Adapted Borg scale (0–10) Dyspnea start (median [IQR]) | 0.0 [0.0, 0.5] |
| Legs start (median [IQR]) | 1.0 [0.5, 1.0] |
| Dyspnea end (median [IQR]) | 9.5 [9.0, 10.0] |
| Legs end (median [IQR]) | 9.0 [7.5, 9.8] |
| Cardiopulmonary exercise test HR 5 at VT1 6 (median [IQR]), beats per min | 141.0 [123.0, 144.0] |
| HR 5 at VT2 7 (median [IQR]), beats per min | 168.5 [163.8, 179.0] |
| Maximal HR 5 (median [IQR]), beats per min | 185.0 [183.0, 190.0] |
| Power at VT1 6 (median [IQR]), W | 138.0 [88.0, 190.0] |
| Power at VT2 7 (median [IQR]), W | 234.5 [213.5, 296.2] |
| Maximal Power (median [IQR]), W | 290.0 [261.0, 366.0] |
| VO2max 8 (median [IQR]), mL/min/kg | 50.0 [47.0, 53.0] |
| Sum Formula | Compound Name | Measured Mass (M-H) 1 | Exact Mass (M-H) 1 | Mass Error (ppm) 2 | Identified Based on | Course of Metabolites during Exercise 3 |
|---|---|---|---|---|---|---|
| C6H12O6 | hexose | 179.0562 | 179.0556 | 3.56 | exact mass & pathway mapping, and Nowak, N. et al. [19] | ↓ |
| C5H10O4 | deoxypentose | 133.0507 | 133.0501 | 4.63 | exact mass & pathway mapping | ↓ |
| C5H10O5 | pentose | 149.0455 | 149.0450 | 3.37 | exact mass & pathway mapping, and Nowak, N. et al. [19] | ↓ |
| C3H4O3 | pyruvate | 87.0088 | 87.0082 | 6.68 | exact mass & pathway mapping | ↓ |
| C2H4O2 | glycolaldehyde | 59.0139 | 59.0133 | 10.09 | exact mass & pathway mapping | ↓ |
| C3H6O4 | glycerate | 105.0193 | 105.0188 | 4.92 | exact mass & pathway mapping | ↓ |
| C3H4O4 | hydroxypyruvate | 103.0037 | 103.0031 | 5.5 | exact mass & pathway mapping | ↓ |
| C2H2O3 | glyoxylate | 72.9931 | 72.9926 | 7.28 | exact mass & pathway mapping | ↓ |
| C2H2O4 | oxalate | 88.9881 | 88.9875 | 6.93 | exact mass & pathway mapping | ↓ |
| C2H3O3 | glycolate | 75.0088 | 75.0082 | 7.75 | exact mass & pathway mapping | ↓ |
| C2H4NO2 | glycine | 74.0248 | 74.0242 | 8.06 | exact mass & pathway mapping | ↓ |
| C4H6O3 | acetoacetate /oxobutyric acid | 101.0244 | 101.0239 | 5.26 | exact mass & pathway mapping | ↓ |
| C4H8O3 | hydroxybutyrate | 103.0401 | 103.0395 | 5.64 | exact mass & pathway mapping | Constant |
| C4H4O5 | oxaloacetate | 130.9986 | 130.9980 | 4.21 | exact mass & pathway mapping, and Rioseras, A. et al. [20] | ↓ |
| C4H6O5 | malate | 133.0143 | 133.0137 | 4.52 | exact mass & pathway mapping, and Rioseras, A. et al. [20] | ↓ |
| C4H4O4 | fumarate | 115.0037 | 115.0031 | 4.93 | exact mass & pathway mapping, and Rioseras, A. et al. [20] | ↓ |
| C4H6O4 | succinate | 117.0194 | 117.0188 | 5.27 | exact mass & pathway mapping, and Rioseras, A. et al. [20] | ↓ |
| C5H6O5 | oxoglutarate | 145.0142 | 145.0137 | 3.46 | exact mass & pathway mapping, and Rioseras, A. et al. [20] | ↓ |
| C6H7NO4 | 2-aminomuconate | 156.0302 | 156.0297 | 3.32 | exact mass & pathway mapping | ↓ |
| C6H8O5 | oxoadipate | 159.0299 | 159.0293 | 3.47 | exact mass & pathway mapping | ↓ |
| C8H7NO3 | formylanthranilate | 164.0353 | 164.0348 | 3.24 | exact mass & pathway mapping | ↓ |
| C7H7NO2 | anthranilate | 136.0404 | 136.0399 | 4.02 | exact mass & pathway mapping | ↓ |
| C6H7NO3 | 2-aminomuconate semialdehyde | 140.0353 | 140.0348 | 3.8 | exact mass & pathway mapping | ↓ |
| C7H7NO3 | 3-hydroxyanthranilate | 152.0353 | 152.0348 | 3.5 | exact mass & pathway mapping | ↓ |
| C6H5NO2 | picolinate | 122.0248 | 122.0242 | 4.89 | exact mass & pathway mapping | ↓ |
| C3H8O3 | glycerole | 91.0401 | 91.03952 | 6.38 | exact mass & pathway mapping | ↓ |
| C12H24O2 | dodecanoic acid /lauric acid | 199.1705 | 199.1698 | 3.49 | exact mass & pathway mapping | ↓ |
| C14H28O2 | tetradecanoic acid /myristic acid | 227.2018 | 227.2011 | 3.06 | exact mass & pathway mapping | ↓ |
| C18H36O2 | octadecanoic acid | 283.2643 | 283.2637 | 2.1 | exact mass, and Anokhina, T.N. et al. [21] | ↓ |
| C16H32O2 | hexadecanoic acid | 255.2329 | 255.2324 | 1.94 | exact mass, and Anokhina, T.N. et al. [21] | ↓ |
| C10H20O3 | hyroxydecanoic acid | 187.1341 | 187.1334 | 3.64 | exact mass and Gaugg, M. et al. [22] | ↓ |
| C10H16O3 | oxodecenoic acid | 183.1028 | 183.1021 | 3.72 | exact mass | ↓ |
| C12H20O3 | oxododecenoic acid | 211.1340 | 211.1334 | 2.75 | exact mass | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osswald, M.; Kohlbrenner, D.; Nowak, N.; Spörri, J.; Sinues, P.; Nieman, D.; Sievi, N.A.; Scherr, J.; Kohler, M. Real-Time Monitoring of Metabolism during Exercise by Exhaled Breath. Metabolites 2021, 11, 856. https://doi.org/10.3390/metabo11120856
Osswald M, Kohlbrenner D, Nowak N, Spörri J, Sinues P, Nieman D, Sievi NA, Scherr J, Kohler M. Real-Time Monitoring of Metabolism during Exercise by Exhaled Breath. Metabolites. 2021; 11(12):856. https://doi.org/10.3390/metabo11120856
Chicago/Turabian StyleOsswald, Martin, Dario Kohlbrenner, Nora Nowak, Jörg Spörri, Pablo Sinues, David Nieman, Noriane Andrina Sievi, Johannes Scherr, and Malcolm Kohler. 2021. "Real-Time Monitoring of Metabolism during Exercise by Exhaled Breath" Metabolites 11, no. 12: 856. https://doi.org/10.3390/metabo11120856
APA StyleOsswald, M., Kohlbrenner, D., Nowak, N., Spörri, J., Sinues, P., Nieman, D., Sievi, N. A., Scherr, J., & Kohler, M. (2021). Real-Time Monitoring of Metabolism during Exercise by Exhaled Breath. Metabolites, 11(12), 856. https://doi.org/10.3390/metabo11120856