Sperm-Guiding Unconventional Prostaglandins in C. elegans: Synthesis and Signaling
Abstract
1. Introduction
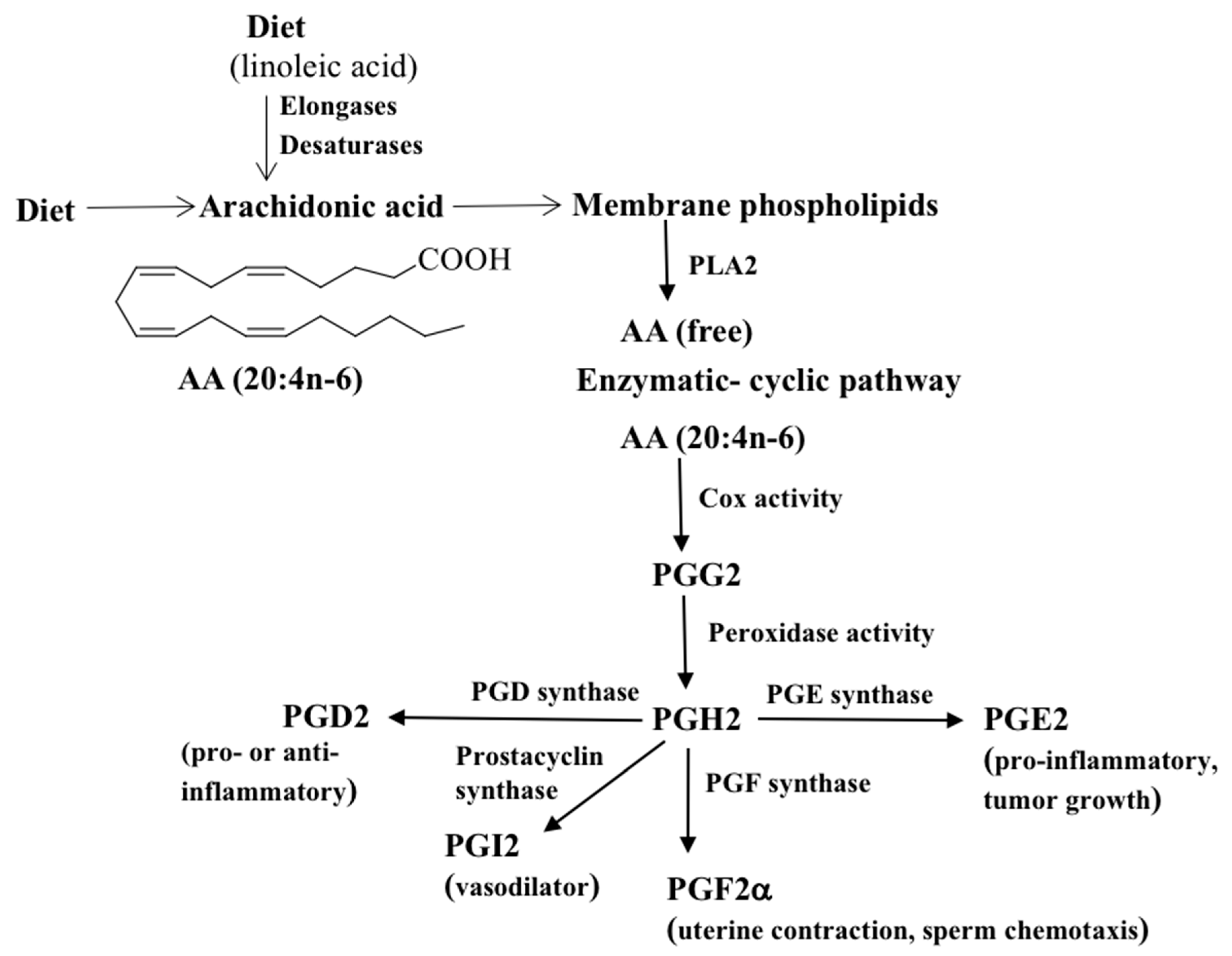
2. Prostaglandin Synthesis
2.1. Cox-Mediated PG Synthesis
2.2. Non-Enzymatic Pathways
2.3. Unconventional Cox-Independent Pathway
3. PUFAs, PGs and Reproduction
3.1. PUFAs
3.2. PG and Reproduction
4. Sperm Guidance Cues
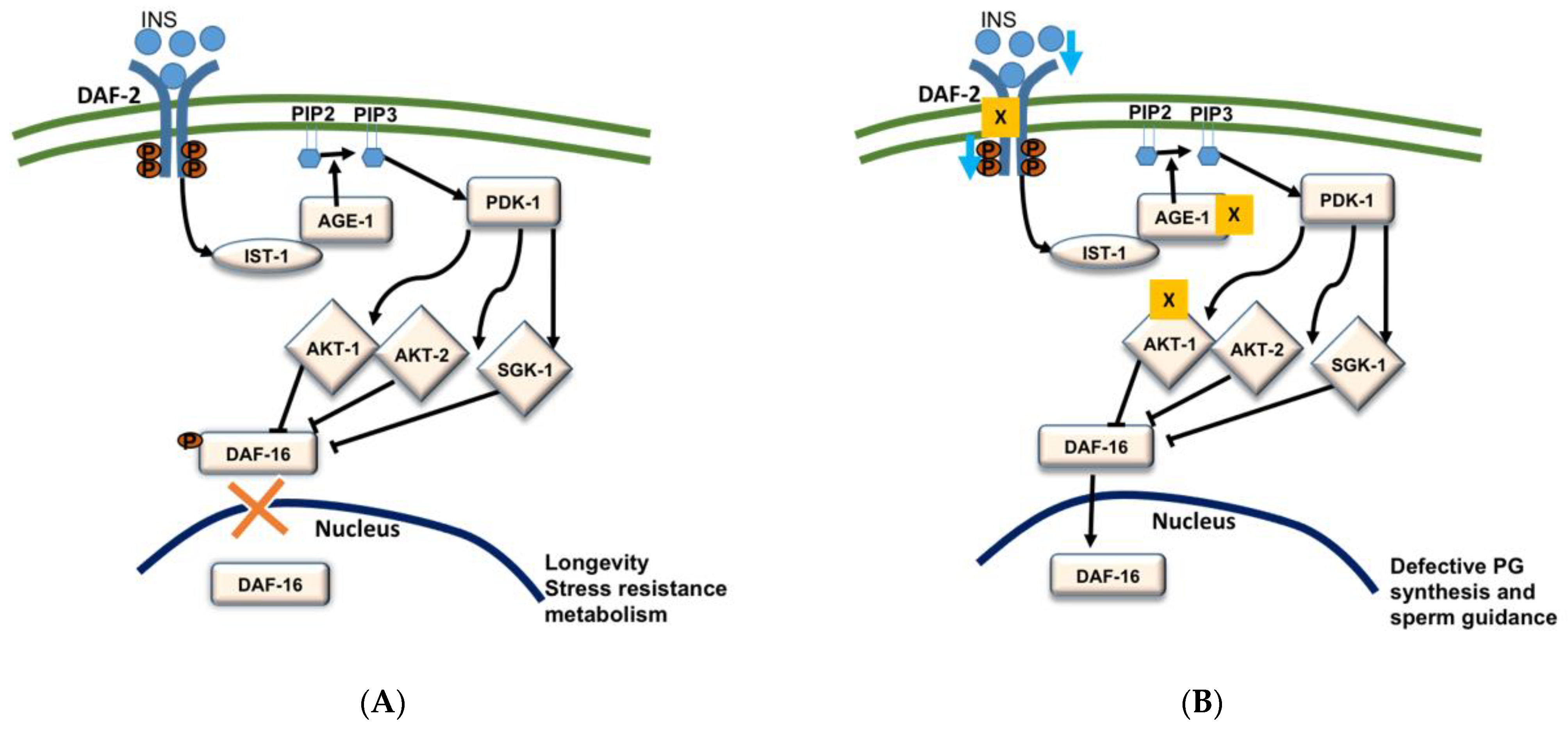
5. PG Signaling Pathways
5.1. Insulin/FOXO Signaling
5.2. DAF-7/TGF β Signaling
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, R.W. Prostaglandins in Semen: Their Occurrence and Possible Physiological Significance. Int. J. Androl. 1978, 1, 188–200. [Google Scholar] [CrossRef]
- Bygdeman, M.; Fredricsson, B.; Svanborg, K.; Samuelsson, B. The relation between fertility and prostaglandin content of seminal fluid in man. Fertil. Steril. 1970, 21, 622–629. [Google Scholar] [CrossRef]
- Bergstrom, S.; Samuelsson, B. Isolation of prostaglandin E1 from human seminal plasma. Prostaglandins and related factors. 11. J. Biol. Chem. 1962, 237, 3005–3006. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Herman, A.; Vane, J.R. Proceedings: Prostaglandin generation maintains the smooth muscle tone of the rabbit isolated jejunum. Br. J. Pharmacol. 1972, 44, 328P–329P. [Google Scholar]
- Zurier, R.B. Prostaglandins, inflammation, and asthma. Arch. Intern. Med. 1974, 133, 101–110. [Google Scholar] [CrossRef]
- Ragab, A.; Bitsch, C.; Thomas, J.M.F.; Bitsch, J.; Chap, H. Lipoxygenase conversion of arachidonic acid in males and inseminated females of the firebrat, Thermobia domestica (Thysanura). Insect. Biochem. 1987, 17, 863–870. [Google Scholar] [CrossRef]
- Valmsen, K.; Jarving, I.; Boeglin, W.E.; Varvas, K.; Koljak, R.; Pehk, T.; Brash, A.R.; Samel, N. The origin of 15R-prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. USA 2001, 98, 7700–7705. [Google Scholar] [CrossRef]
- Ells, R.; Kock, J.L.; Albertyn, J.; Pohl, C.H. Arachidonic acid metabolites in pathogenic yeasts. Lipids Health Dis. 2012, 11, 100. [Google Scholar] [CrossRef]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J., 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Awad, J.A.; Boss, H.J.; Blair, I.A.; Roberts, L.J., 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA 1992, 89, 10721–10725. [Google Scholar] [CrossRef]
- Edmonds, J.W.; Prasain, J.K.; Dorand, D.; Yang, Y.; Hoang, H.D.; Vibbert, J.; Kubagawa, H.M.; Miller, M.A. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell 2010, 19, 858–871. [Google Scholar] [CrossRef]
- Hoang, H.D.; Prasain, J.K.; Dorand, D.; Miller, M.A. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 2013, 9, e1003271. [Google Scholar] [CrossRef]
- McKnight, K.; Hoang, H.D.; Prasain, J.K.; Brown, N.; Vibbert, J.; Hollister, K.A.; Moore, R.; Ragains, J.R.; Reese, J.; Miller, M.A. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science 2014, 344, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.; Poyser, N.L.; Wunderlich, M. Levels of prostaglandins in human endometrium during the normal menstrual cycle. J. Physiol. 1974, 236, 465–472. [Google Scholar] [CrossRef]
- Murata, T.; Maehara, T. Discovery of anti-inflammatory role of prostaglandin D2. J. Vet. Med. Sci. 2016, 78, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Kadowitz, P.J.; Chapnick, B.M.; Feigen, L.P.; Hyman, A.L.; Nelson, P.K.; Spannhake, E.W. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 408–413. [Google Scholar] [CrossRef]
- Blomqvist, A.; Engblom, D. Neural Mechanisms of Inflammation-Induced Fever. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2018, 24, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W.; Colville-Nash, P.R.; Willis, D.; Chivers, J.; Paul-Clark, M.J.; Willoughby, D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Hemler, M.; Lands, W.E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J. Biol. Chem. 1976, 251, 5575–5579. [Google Scholar] [CrossRef]
- Smith, W.L.; Lands, W.E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry 1972, 11, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.A. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.A. Protective effects of prostaglandins against gastric mucosal damage: Current knowledge and proposed mechanisms. Am. J. Physiol. 1983, 245, G601–G623. [Google Scholar] [CrossRef]
- Soll, A.H.; Weinstein, W.M.; Kurata, J.; McCarthy, D. Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann. Intern. Med. 1991, 114, 307–319. [Google Scholar] [CrossRef]
- Chen, C. COX-2′s new role in inflammation. Nat. Chem. Biol. 2010, 6, 401–402. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37s–42s. [Google Scholar] [CrossRef]
- Samuelsson, B.; Morgenstern, R.; Jakobsson, P.J. Membrane prostaglandin E synthase-1: A novel therapeutic target. Pharmacol. Rev. 2007, 59, 207–224. [Google Scholar] [CrossRef]
- Watanabe, K. Prostaglandin F synthase. Prostaglandins Other Lipid Mediat. 2002, 68–69, 401–407. [Google Scholar] [CrossRef]
- Seo, M.J.; Oh, D.K. Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 2017, 66, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F.; Kuhn, H.; Schewe, T. Enzymes and Factors Involved in the Biosynthesis of Eicosanoids; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Jabbour, H.N.; Sales, K.J. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol. Metab. 2004, 15, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.D.; Winters, G.M.; Rogers, W.J.; Ziccardi, M.H.; Li, V.; Keser, B.; Denison, M.S. Activation of the Ah receptor signaling pathway by prostaglandins. J. Biochem. Mol. Toxicol. 2001, 15, 187–196. [Google Scholar] [CrossRef]
- Morrow, J.D.; Awad, J.A.; Kato, T.; Takahashi, K.; Badr, K.F.; Roberts, L.J., 2nd; Burk, R.F. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J. Clin. Investig. 1992, 90, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef]
- Milne, G.L.; Yin, H.; Morrow, J.D. Human biochemistry of the isoprostane pathway. J. Biol. Chem. 2008, 283, 15533–15537. [Google Scholar] [CrossRef] [PubMed]
- Waugh, R.J.; Morrow, J.D.; Roberts, L.J., 2nd; Murphy, R.C. Identification and relative quantitation of F2-isoprostane regioisomers formed in vivo in the rat. Free Radic. Biol. Med. 1997, 23, 943–954. [Google Scholar] [CrossRef]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Their role as an index of oxidant stress status in human pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Stern, A.; Roberts, L.J.; Morrow, J.D. The isoprostanes: Novel prostaglandin-like products of the free radical-catalyzed peroxidation of arachidonic acid. J. Biomed. Sci. 1999, 6, 226–235. [Google Scholar] [CrossRef]
- de Faria, A.P.; Fontana, V.; Modolo, R.; Barbaro, N.R.; Sabbatini, A.R.; Pansani, I.F.; Ferreira-Melo, S.E.; Moreno, H. Plasma 8-isoprostane levels are associated with endothelial dysfunction in resistant hypertension. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 433, 179–183. [Google Scholar] [CrossRef]
- Van ’t Erve, T.J.; Lih, F.B.; Kadiiska, M.B.; Deterding, L.J.; Eling, T.E.; Mason, R.P. Reinterpreting the best biomarker of oxida-tive stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med. 2015, 83, 245–251. [Google Scholar] [CrossRef]
- Watkins, M.T.; Patton, G.M.; Soler, H.M.; Albadawi, H.; Humphries, D.E.; Evans, J.E.; Kadowaki, H. Synthesis of 8-epi-prostaglandin F2alpha by human endothelial cells: Role of prostaglandin H2 synthase. Biochem. J. 1999, 344 Pt 3, 747–754. [Google Scholar] [CrossRef]
- Gao, L.; Zackert, W.E.; Hasford, J.J.; Danekis, M.E.; Milne, G.L.; Remmert, C.; Reese, J.; Yin, H.; Tai, H.H.; Dey, S.K.; et al. Formation of prostaglandins E2 and D2 via the isoprostane pathway: A mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J. Biol. Chem. 2003, 278, 28479–28489. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, E.; Hu, M.; Miller, M.A.; Prasain, J.K. Signature profile of cyclooxygenase-independent F2 series prostaglandins in C. elegans and their role in sperm motility. Sci. Rep. 2019, 9, 11750. [Google Scholar] [CrossRef]
- Pier, B.; Edmonds, J.W.; Wilson, L.; Arabshahi, A.; Moore, R.; Bates, G.W.; Prasain, J.K.; Miller, M.A. Comprehensive profiling of prostaglandins in human ovarian follicular fluid using mass spectrometry. Prostaglandins Other Lipid Mediat. 2018, 134, 7–15. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002, 27, 467–473. [Google Scholar] [CrossRef]
- Watts, J.L.; Browse, J. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2000, 272, 263–269. [Google Scholar] [CrossRef]
- Kubagawa, H.M.; Watts, J.L.; Corrigan, C.; Edmonds, J.W.; Sztul, E.; Browse, J.; Miller, M.A. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 2006, 8, 1143–1148. [Google Scholar] [CrossRef]
- Grant, B.; Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 1999, 10, 4311–4326. [Google Scholar] [CrossRef]
- Han, S.M.; Cottee, P.A.; Miller, M.A. Sperm and oocyte communication mechanisms controlling C. elegans fertility. Dev. Dyn. 2010, 239, 1265–1281. [Google Scholar]
- Stone, S.; Khamashta, M.A.; Nelson-Piercy, C. Nonsteroidal anti-inflammatory drugs and reversible female infertility: Is there a link? Drug Saf. 2002, 25, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.A.; Imamura, T.; Luedke, C.; Vogt, S.K.; Olson, L.M.; Nelson, D.M.; Sadovsky, Y.; Muglia, L.J. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc. Natl. Acad. Sci. USA 1998, 95, 11875–11879. [Google Scholar] [CrossRef]
- Norman, R.J. Reproductive consequences of COX-2 inhibition. Lancet 2001, 358, 1287–1288. [Google Scholar] [CrossRef]
- McCracken, J.A.; Custer, E.E.; Lamsa, J.C. Luteolysis: A neuroendocrine-mediated event. Physiol. Rev. 1999, 79, 263–323. [Google Scholar] [CrossRef]
- Davis, B.J.; Lennard, D.E.; Lee, C.A.; Tiano, H.F.; Morham, S.G.; Wetsel, W.C.; Langenbach, R. Anovulation in Cyclooxygenase-2-Deficient Mice Is Restored by Prostaglandin E2 and Interleukin-1β*. Endocrinology 1999, 140, 2685–2695. [Google Scholar] [CrossRef]
- Harris, S.M.; Aschenbach, L.C.; Skinner, S.M.; Dozier, B.L.; Duffy, D.M. Prostaglandin E2 receptors are differentially expressed in subpopulations of granulosa cells from primate periovulatory follicles. Biol. Reprod. 2011, 85, 916–923. [Google Scholar] [CrossRef]
- Hizaki, H.; Segi, E.; Sugimoto, Y.; Hirose, M.; Saji, T.; Ushikubi, F.; Matsuoka, T.; Noda, Y.; Tanaka, T.; Yoshida, N.; et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc. Natl. Acad. Sci. USA 1999, 96, 10501–10506. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Harris, S.M.; Duffy, D.M. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology 2014, 155, 1466–1475. [Google Scholar] [CrossRef]
- Perez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef]
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef]
- Avila, F.W.; Wolfner, M.F. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc. Natl. Acad. Sci. USA 2009, 106, 15796–15800. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- Armon, L.; Caplan, S.R.; Eisenbach, M.; Friedrich, B.M. Testing human sperm chemotaxis: How to detect biased motion in population assays. PLoS ONE 2012, 7, e32909. [Google Scholar] [CrossRef][Green Version]
- Armon, L.; Ben-Ami, I.; Ron-El, R.; Eisenbach, M. Human oocyte-derived sperm chemoattractant is a hydrophobic molecule associated with a carrier protein. Fertil. Steril. 2014, 102, 885–890. [Google Scholar] [CrossRef][Green Version]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. Elife 2014, 3, e02403. [Google Scholar] [CrossRef]
- Ward, G.E.; Brokaw, C.J.; Garbers, D.L.; Vacquier, V.D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 1985, 101, 2324–2329. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Kashikar, N.D.; Weyand, I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 2008, 70, 93–117. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Murata, M.; Inaba, K.; Morisawa, M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA 2002, 99, 14831–14836. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C.; Bowden, B.F.; Meehan, G.V.; Konig, G.M.; Carroll, A.R.; Tapiolas, D.M.; Aliño, P.M.; Heaton, A.; De Nys, R.; Leone, P.A.; et al. Chemical aspects of mass spawning in corals. I. Sperm-attractant molecules in the eggs of the scleractinian coral Montipora digitata. Mar. Biol. 1994, 118, 177–182. [Google Scholar] [CrossRef]
- Riffell, J.A.; Krug, P.J.; Zimmer, R.K. Fertilization in the sea: The chemical identity of an abalone sperm attractant. J. Exp. Biol. 2002, 205 Pt 10, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004, 117 Pt 24, 5835–5845. [Google Scholar] [CrossRef]
- Guerrero, A.; Nishigaki, T.; Carneiro, J.; Yoshiro, T.; Wood, C.D.; Darszon, A. Tuning sperm chemotaxis by calcium burst timing. Dev. Biol. 2010, 344, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.; Van, Q.; Weyand, I.; Hagen, V.; Beyermann, M.; Matsumoto, M.; Hoshi, M.; Hildebrand, E.; Kaupp, U.B. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 2005, 24, 2741–2752. [Google Scholar] [CrossRef]
- Iqbal, M.; Shivaji, S.; Vijayasarathy, S.; Balaram, P. Synthetic peptides as chemoattractants for bull spermatozoa structure activity correlations. Biochem. Biophys. Res. Commun. 1980, 96, 235–242. [Google Scholar] [CrossRef]
- Villanueva-Diaz, C.; Arias-Martinez, J.; Bermejo-Martinez, L.; Vadillo-Ortega, F. Progesterone induces human sperm chemotaxis. Fertil. Steril. 1995, 64, 1183–1188. [Google Scholar] [CrossRef]
- Teves, M.E.; Barbano, F.; Guidobaldi, H.A.; Sanchez, R.; Miska, W.; Giojalas, L.C. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil. Steril. 2006, 86, 745–749. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Teves, M.E.; Uñates, D.R.; Anastasía, A.; Giojalas, L.C. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS ONE 2008, 3, e3040. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Minoura, H.; Tanaka, K.; Shibahara, T.; Hayashi, N.; Toyoda, N. The effect of RANTES on human sperm chemotaxis. Hum. Reprod. 2002, 17, 1441–1446. [Google Scholar] [CrossRef]
- Tamba, S.; Yodoi, R.; Segi-Nishida, E.; Ichikawa, A.; Narumiya, S.; Sugimoto, Y. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc. Natl. Acad. Sci. USA 2008, 105, 14539–14544. [Google Scholar] [CrossRef] [PubMed]
- Ernesto, J.I.; Weigel Munoz, M.; Battistone, M.A.; Vasen, G.; Martinez-Lopez, P.; Orta, G.; Figueiras-Fierro, D.; De la Vega-Beltran, J.L.; Moreno, I.A.; Guidobaldi, H.A.; et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 2015, 210, 1213–1224. [Google Scholar] [CrossRef]
- Bian, F.; Mao, G.; Guo, M.; Mao, G.; Wang, J.; Li, J.; Han, Y.; Chen, X.; Zhang, M.; Xia, G. Gradients of natriuretic peptide precursor A (NPPA) in oviduct and of natriuretic peptide receptor 1 (NPR1) in spermatozoon are involved in mouse sperm chemotaxis and fertilization. J. Cell. Physiol. 2012, 227, 2230–2239. [Google Scholar] [CrossRef]
- Eisenbach, M. Towards understanding the molecular mechanism of sperm chemotaxis. J. Gen. Physiol. 2004, 124, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Tiwary, E.; Prasain, J.K.; Miller, M.; Serra, R. Mechanisms of TGFß in prostaglandin synthesis and sperm guidance in Caenorhabditis elegans. Dev. Dyn. 2021, 250, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef]
- Sun, F.; Bahat, A.; Gakamsky, A.; Girsh, E.; Katz, N.; Giojalas, L.C.; Tur-Kaspa, I.; Eisenbach, M. Human sperm chemotaxis: Both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum. Reprod. 2005, 20, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Publicover, S.J.; Giojalas, L.C.; Teves, M.E.; de Oliveira, G.S.; Garcia, A.A.; Barratt, C.L.; Harper, C.V. Ca2+ signalling in the control of motility and guidance in mammalian sperm. Front. Biosci. 2008, 13, 5623–5637. [Google Scholar] [CrossRef]
- Seifert, R.; Flick, M.; Bonigk, W.; Alvarez, L.; Trotschel, C.; Poetsch, A.; Muller, A.; Goodwin, N.; Pelzer, P.; Kashikar, N.D.; et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 2015, 34, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krahling, M.; Muller, A.; Kaupp, U.B.; Strunker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Jeschke, J.K.; Biagioni, C.; Schierling, T.; Wagner, I.V.; Börgel, F.; Schepmann, D.; Schüring, A.; Kulle, A.E.; Holterhus, P.M.; von Wolff, M.; et al. The Action of Reproductive Fluids and Contained Steroids, Prostaglandins, and Zn2+ on CatSper Ca2+ Channels in Human Sperm. Front. Cell Dev. Biol. 2021, 9, 699554. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef]
- Samuel, A.D.; Murthy, V.N.; Hengartner, M.O. Calcium dynamics during fertilization in C. elegans. BMC Dev. Biol. 2001, 1, 8. [Google Scholar] [CrossRef]
- Edmonds, J.W.; McKinney, S.L.; Prasain, J.K.; Miller, M.A. The gap junctional protein INX-14 functions in oocyte precursors to promote C. elegans sperm guidance. Dev. Biol. 2011, 359, 47–58. [Google Scholar] [CrossRef]
- Álvarez-Castro, P.; Sangiao-Alvarellos, S.; Brandón-Sandá, I.; Cordido, F. Endocrine function in obesity. Endocrinol. Y Nutr. 2011, 58, 422–432. [Google Scholar] [CrossRef]
- Murphy, C.T.; Hu, P.J. Insulin/insulin-like growth factor signaling in C. elegans. WormBook 2013, 1–43. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Oh, S.W.; Tissenbaum, H.A. Worming pathways to and from DAF-16/FOXO. Exp. Gerontol. 2006, 41, 928–934. [Google Scholar] [CrossRef]
- Landis, J.N.; Murphy, C.T. Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev. Dyn. 2010, 239, 1405–1412. [Google Scholar]
- Gems, D.; Sutton, A.J.; Sundermeyer, M.L.; Albert, P.S.; King, K.V.; Edgley, M.L.; Larsen, P.L.; Riddle, D.L. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 1998, 150, 129–155. [Google Scholar] [CrossRef]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Ruvkun, G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics 1998, 148, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- DePina, A.S.; Iser, W.B.; Park, S.S.; Maudsley, S.; Wilson, M.A.; Wolkow, C.A. Regulation of Caenorhabditis elegans vitellogenesis by DAF-2/IIS through separable transcriptional and posttranscriptional mechanisms. BMC Physiol. 2011, 11, 11. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Schroeder, F.C. Ascaroside signaling in C. elegans. WormBook 2013, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Lim, C.S.; Johnsen, R.; Albert, P.S.; Pilgrim, D.; Riddle, D.L. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 1996, 274, 1389–1391. [Google Scholar] [CrossRef]
- Inoue, T.; Thomas, J.H. Targets of TGF-beta signaling in Caenorhabditis elegans dauer formation. Dev. Biol. 2000, 217, 192–204. [Google Scholar] [CrossRef]
- Gumienny, T.L.; Savage-Dunn, C. TGF-beta signaling in C. elegans. WormBook 2013, 1–34. [Google Scholar] [CrossRef]
- Greer, E.R.; Perez, C.L.; Van Gilst, M.R.; Lee, B.H.; Ashrafi, K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008, 8, 118–131. [Google Scholar] [CrossRef]
- Hu, M.; Crossman, D.; Prasain, J.K.; Miller, M.A.; Serra, R.A. Transcriptomic Profiling of DAF-7/TGF beta Pathway Mutants in C. elegans. Genes 2020, 11, 288. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwary, E.; Hu, M.; Prasain, J.K. Sperm-Guiding Unconventional Prostaglandins in C. elegans: Synthesis and Signaling. Metabolites 2021, 11, 853. https://doi.org/10.3390/metabo11120853
Tiwary E, Hu M, Prasain JK. Sperm-Guiding Unconventional Prostaglandins in C. elegans: Synthesis and Signaling. Metabolites. 2021; 11(12):853. https://doi.org/10.3390/metabo11120853
Chicago/Turabian StyleTiwary, Ekta, Muhan Hu, and Jeevan K. Prasain. 2021. "Sperm-Guiding Unconventional Prostaglandins in C. elegans: Synthesis and Signaling" Metabolites 11, no. 12: 853. https://doi.org/10.3390/metabo11120853
APA StyleTiwary, E., Hu, M., & Prasain, J. K. (2021). Sperm-Guiding Unconventional Prostaglandins in C. elegans: Synthesis and Signaling. Metabolites, 11(12), 853. https://doi.org/10.3390/metabo11120853





