Metabolomic Profile at Birth, Bronchiolitis and Recurrent Wheezing: A 3-Year Prospective Study
Abstract
1. Introduction
2. Results
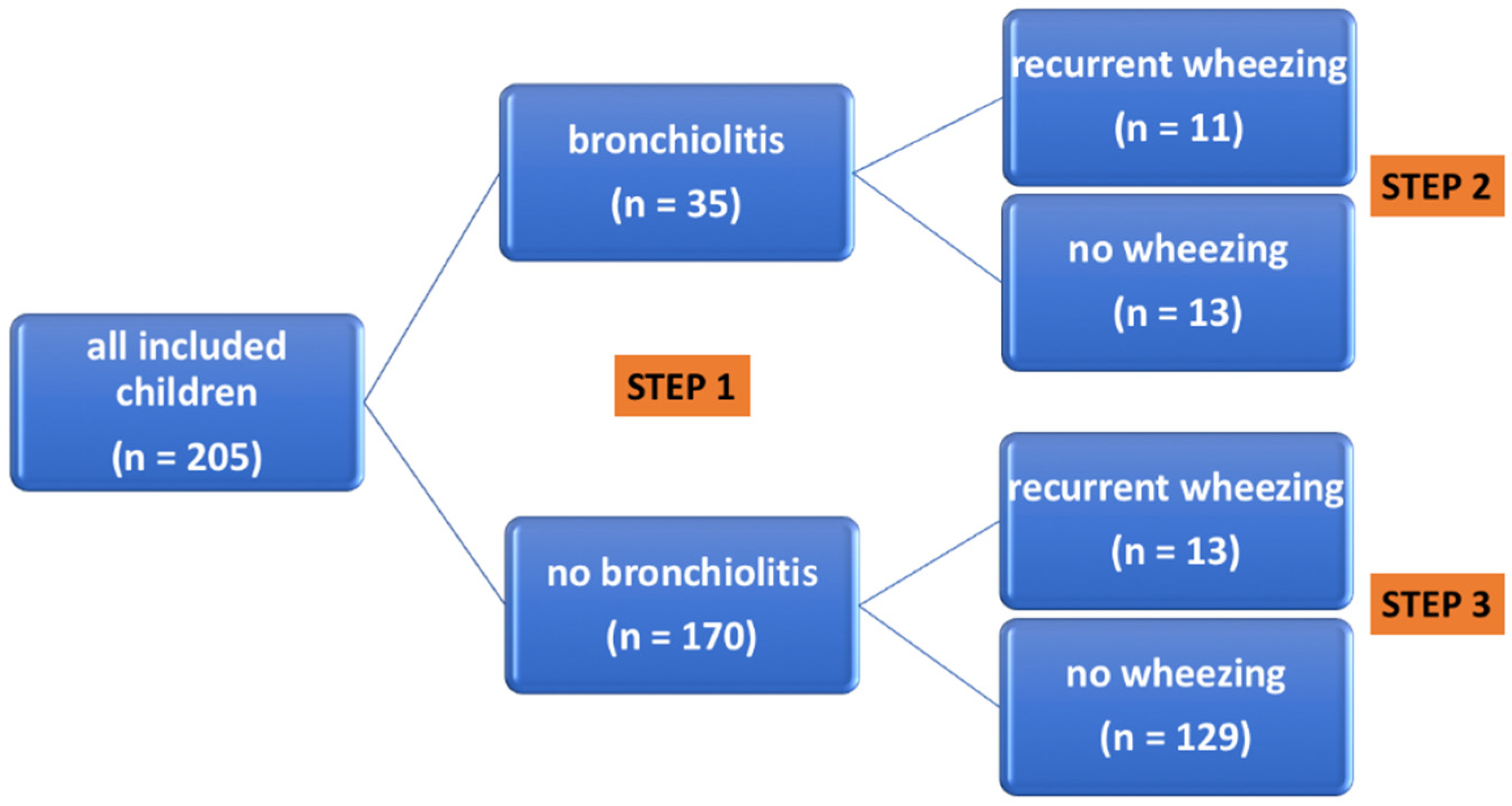
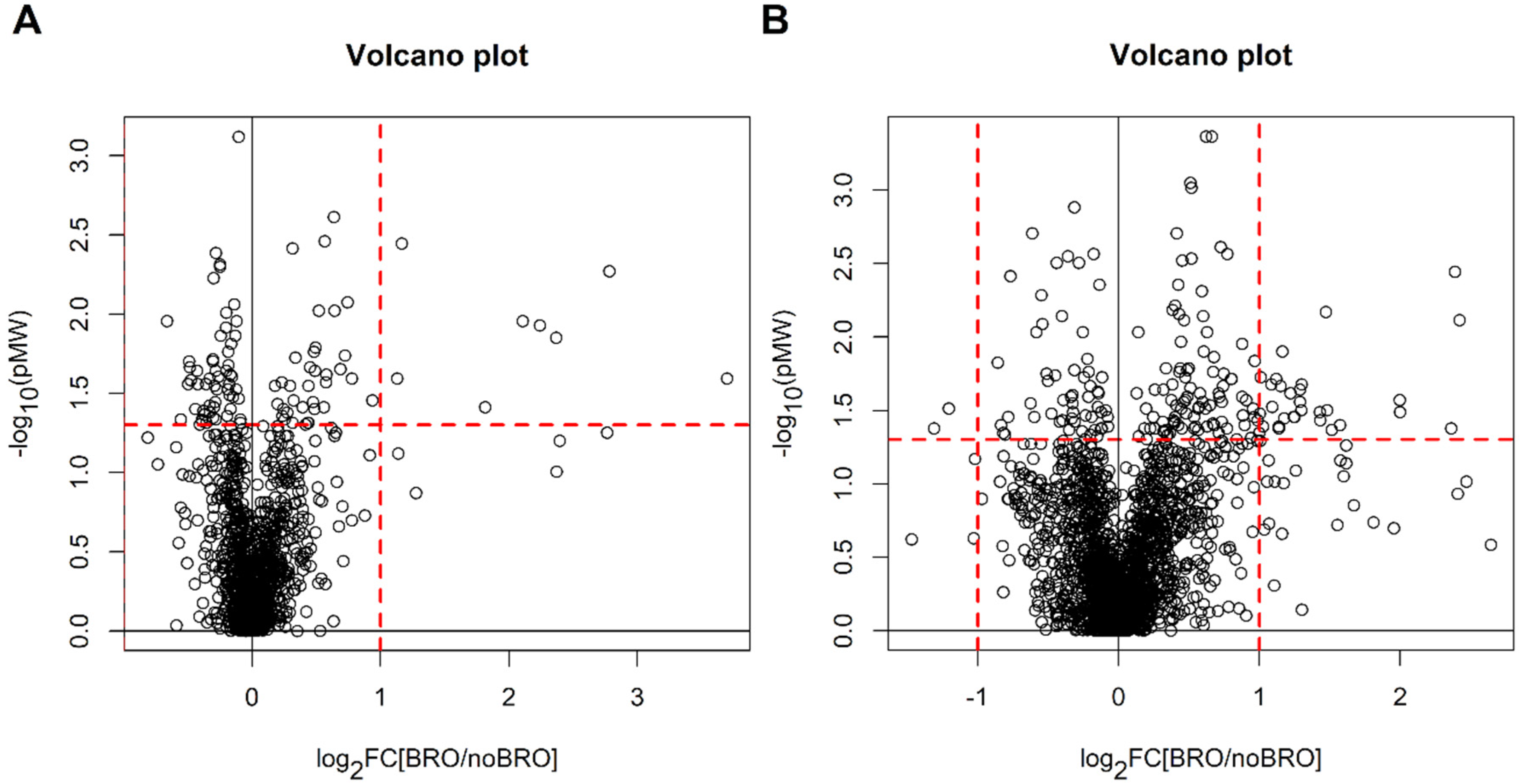
2.1. Newborns with Bronchiolitis vs. Newborns without Bronchiolitis
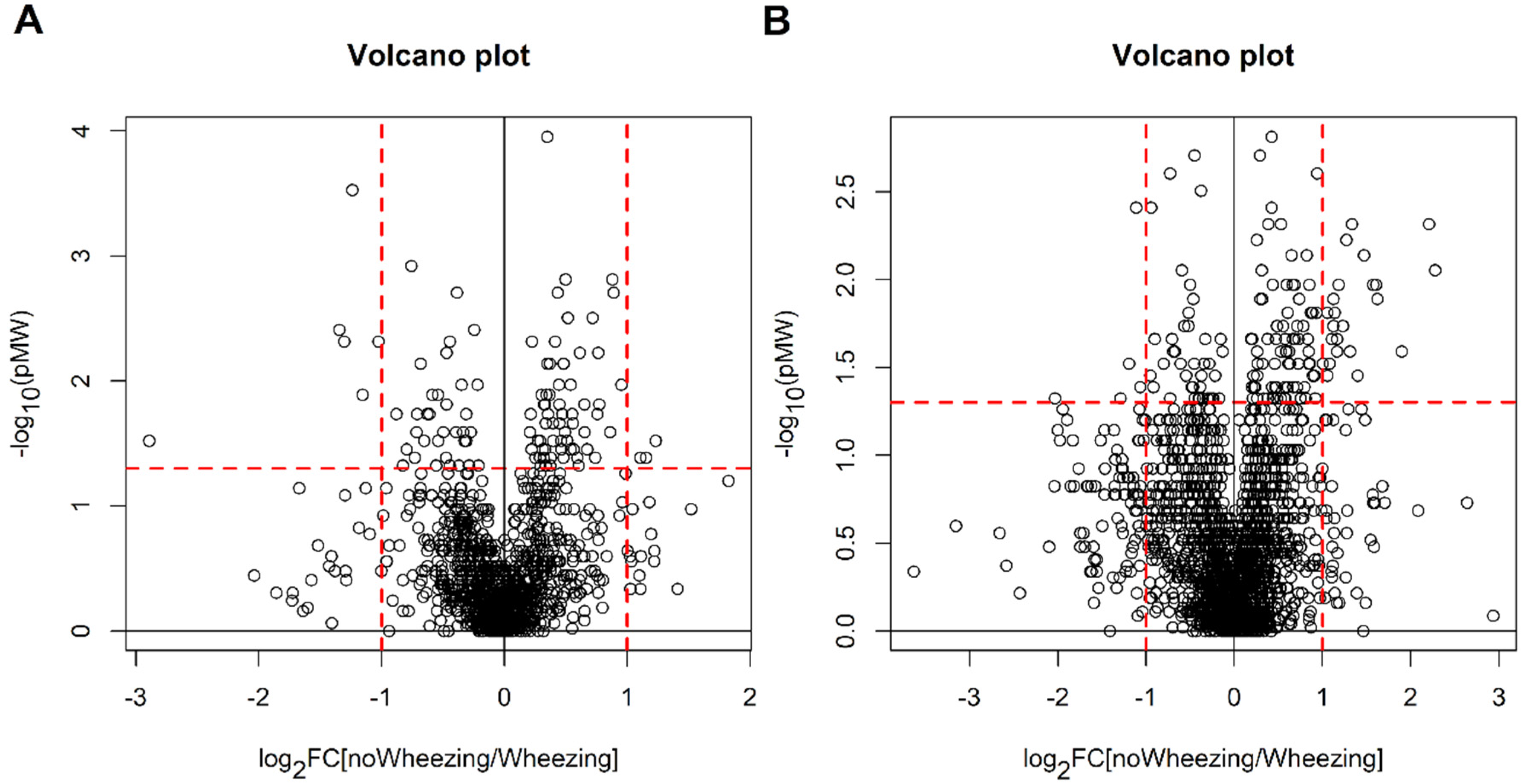
2.2. Children with Bronchiolitis with or without Recurrent Wheezing
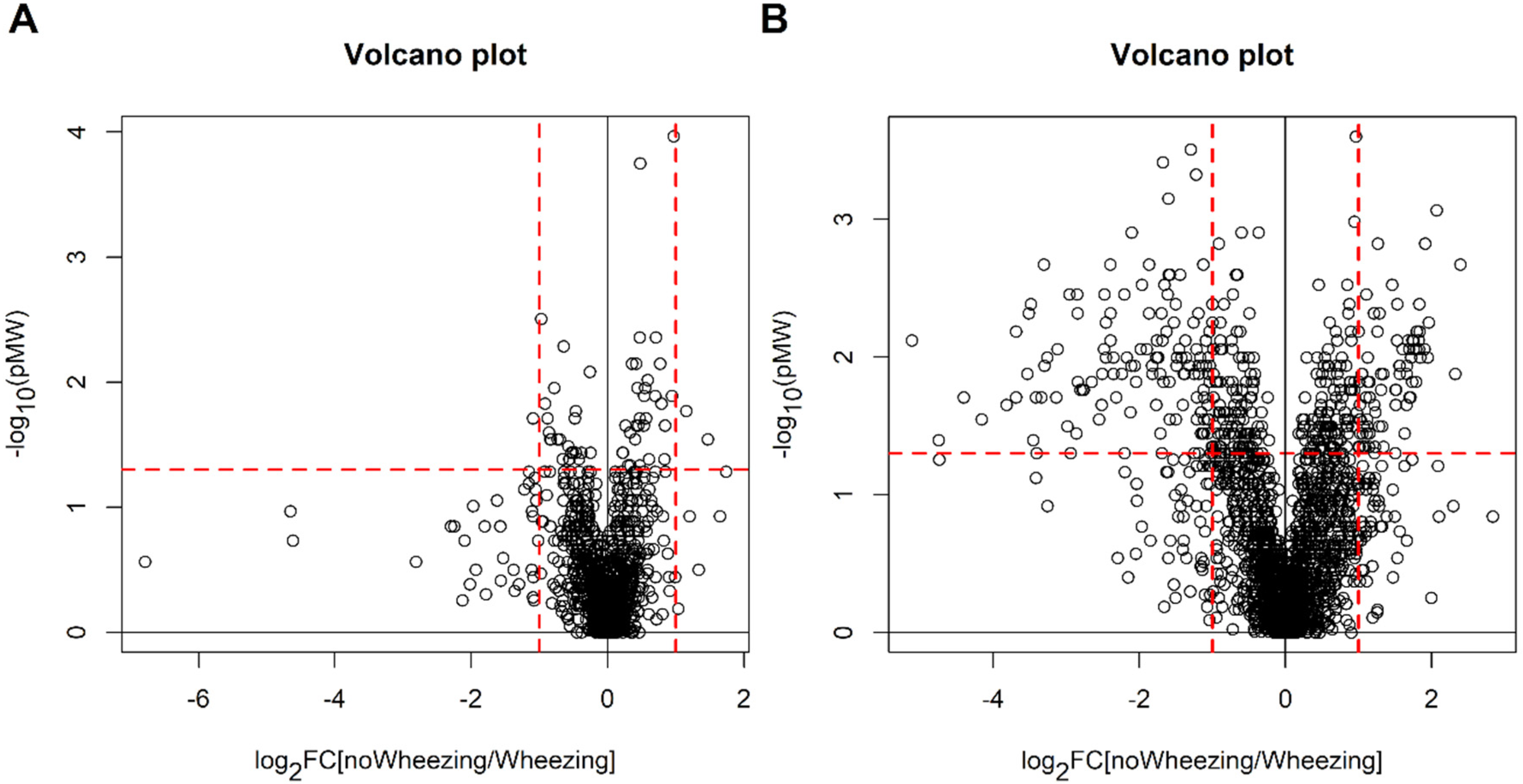
2.3. Children without Bronchiolitis with or without Recurrent Wheezing
3. Discussion
3.1. Metabolomic Profile at Birth, Acute Bronchiolitis and Development of Post-Bronchiolitis Wheezing
3.2. Metabolomic Profile at Birth and Development of Recurrent Wheezing Not Preceded by Bronchiolitis
3.3. Limits and Strengths of the Study
4. Materials and Methods
4.1. Study Population and Study Design
4.2. Metabolomic Analysis
4.2.1. Chemicals and Reagents
4.2.2. Urine Analysis
4.2.3. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, R.T.; Martinez, F.D. Respiratory syncytial virus and asthma: Still no final answer. Thorax 2010, 65, 1033–1034. [Google Scholar] [CrossRef][Green Version]
- Bont, L.; Ramilo, O. The relationship between RSV bronchiolitis and recurrent wheeze: The chicken and the egg. Early Hum. Dev. 2011, 87 (Suppl.1), S51–S54. [Google Scholar] [CrossRef]
- Manuck, T.A.; Levy, P.T.; Gyamfi-Bannerman, C.; Jobe, A.H.; Blaisdell, C.J. Prenatal and perinatal determinants of lung health and disease in early life: A National Heart, Lung, and Blood Institute workshop report. JAMA Pediatr. 2016, 170, e154577. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Scheltema, N.; Bont, L.; Baraldi, E. Early-life origins of chronic respiratory diseases: Understanding and promoting healthy ageing. Eur. Respir. J. 2014, 44, 1682–1696. [Google Scholar] [CrossRef]
- Prescott, S.L. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J. Allergy Clin. Immunol. 2013, 131, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Beigelman, A.; Calatroni, A.; Jackson, D.J.; Gergen, P.J.; O’Connor, G.T.; Kattan, M.; Wood, R.A.; Sandel, M.T.; Lynch, S.V.; et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am. J. Respir. Crit. Care Med. 2019, 199, 71–82. [Google Scholar] [CrossRef]
- Chawes, B.L.K.; Buchvald, F.; Bischoff, A.L.; Loland, L.; Hermansen, M.; Halkjaer, L.B.; Bønnelykke, K.; Bisgaard, H. Elevated exhaled nitric oxide in high-risk neonates precedes transient early but not persistent wheeze. Am. J. Respir. Crit. Care Med. 2010, 182, 138–142. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Perilongo, G.; Baraldi, E. Metabolomics: A new frontier for research in pediatrics. J. Pediatr. 2009, 154, 638–644. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Haremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, E123. [Google Scholar] [CrossRef]
- Schjødt, M.S.; Gürdeniz, G.; Chawes, B. The Metabolomics of childhood atopic diseases: A comprehensive pathway-specific review. Metabolites 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Baraldi, E.; Giordano, G.; Pirillo, P.; Stocchero, M.; Houben, M.; Bont, L. Metabolomic profile of amniotic fluid and wheezing in the first year of life-a healthy birth cohort study. J. Pediatr. 2018, 196, 264–269.e4. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Park, Y.; Brown, L.A.S.; Jones, D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J. Allergy Clin. Immunol. 2014, 133, 258–261.e8. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Carpi, D.; Stocchero, M.; Sterk, P.J.; Baraldi, E. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy 2013, 68, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Bozzetto, S.; Giordano, G.; El Mazloum, D.; Stocchero, M.; Pirillo, P.; Zanconato, S.; Baraldi, E. Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr. Allergy Immunol. 2018, 29, 375–382. [Google Scholar] [CrossRef]
- Wu, P.; Dupont, W.D.; Griffin, M.R.; Carroll, K.N.; Mitchel, E.F.; Gebretsadik, T.; Hartert, T.V. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Brunwasser, S.M.; Snyder, B.M.; Driscoll, A.J.; Fell, D.B.; Savitz, D.A.; Feikin, D.R.; Skidmore, B.; Bhat, N.; Bont, L.J.; Dupont, W.D.; et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: A systematic review and meta-analysis. Lancet Respir. Med. 2020, 8, 795–806. [Google Scholar] [CrossRef]
- Zomer-Kooijker, K.; Uiterwaal, C.S.P.M.; van der Gugten, A.C.; Wilbrink, B.; Bont, L.J.; van der Ent, C.K. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur. Respir. J. 2014, 44, 666–674. [Google Scholar] [CrossRef]
- Broughton, S.; Bhat, R.; Roberts, A.; Zuckerman, M.; Rafferty, G.; Greenough, A. Diminished lung function, RSV infection, and respiratory morbidity in prematurely born infants. Arch. Dis. Child 2006, 91, 26–30. [Google Scholar] [CrossRef]
- Zar, H.J.; Nduru, P.; Stadler, J.A.M.; Gray, D.; Barnett, W.; Lesosky, M.; Myer, L.; Nicol, M.P. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: Epidemiology and effect on lung health. Lancet Glob. Health 2020, 8, e1316–e1325. [Google Scholar] [CrossRef]
- Barlotta, A.; Pirillo, P.; Stocchero, M.; Donato, F.; Giordano, G.; Bont, L.; Zanconato, S.; Carraro, S.; Baraldi, E. Metabolomic profiling of infants with recurrent wheezing after bronchiolitis. J. Infect. Dis. 2019, 219, 1216–1223. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Gebretsadik, T.; Watanabe, M.; Brunwasser, S.; Anderson, L.J.; Moore, M.L.; Larkin, E.K.; Peebles, R.S.; Hartert, T.V. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics 2018, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Lin, G.; Cheng, M.-L.; Chiang, M.-H.; Tsai, M.-H.; Su, K.-W.; Hua, M.-C.; Lia, S.-L.; Lai, S.-H.; Yao, T.-C.; et al. Longitudinal urinary metabolomic profiling reveals metabolites for asthma development in early childhood. Pediatr. Allergy Immunol. 2018, 29, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Chawes, B.L.; Giordano, G.; Pirillo, P.; Rago, D.; Rasmussen, M.A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Baraldi, E. Neonatal urine metabolic profiling and development of childhood asthma. Metabolites 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Thomson, A.H. Acute bronchiolitis. BMJ 2007, 335, 1037–1041. [Google Scholar] [CrossRef] [PubMed]

| Bronchiolitis (n = 35) | No Bronchiolitis (n = 170) | p | Post-Bronchiolitis Recurrent Wheezing (n = 11) | No Post-Bronchiolitis Wheezing (n = 13) | p | |
|---|---|---|---|---|---|---|
| Males | 29 (83%) | 103 (61%) | 0.012 | 10 (91%) | 9 (69%) | 0.327 |
| Atopic parents | 17 (49%) | 81 (48%) | 1.000 | 5 (45%) | 6 (46%) | 1.000 |
| Smoke exposure | 9 (26%) | 70 (41%) | 0.126 | 3 (27%) | 3 (23%) | 1.000 |
| Vitamin D supplementation | 27 (77%) | 135 (79%) | 0.470 | 6 (55%) | 6 (46%) | 1.000 |
| Exclusive breast feeding for 6 months | 12 (34%) | 51 (30%) | 0.454 | 4 (36%) | 4 (31%) | 1.000 |
| Siblings | 26 (74%) | 108 (64%) | 0.248 | 10 (91%) | 11 (85%) | 1.000 |
| Pets in the house | 14 (40%) | 70 (41%) | 0.849 | 3 (27%) | 8 (62%) | 0.123 |
| Mold in the house | 20 (57%) | 71 (42%) | 0.135 | 8 (73%) | 5 (38%) | 0.123 |
| Dust mite exposure | 30 (86%) | 152 (89%) | 0.557 | 10 (91%) | 11 (85%) | 1.000 |
| Hospitalization for bronchiolitis | 6 (17%) | -- | -- | 3 (27%) | 3 (23%) | 1.000 |
| Recurrent Wheezing (n = 13) | No Wheezing (n = 129) | p | |
|---|---|---|---|
| Males | 5 (38%) | 80 (62%) | 0.138 |
| Atopic parents | 8 (62%) | 63 (49%) | 0.562 |
| Smoke exposure | 8 (62%) | 50 (39%) | 0.142 |
| Vitamin D supplementation | 11 (85%) | 111 (86%) | 1.000 |
| Exclusive breast feeding for 6 months | 3 (23%) | 47 (36%) | 0.543 |
| Siblings | 7 (54%) | 83 (64%) | 0.549 |
| Pets in the house | 8 (62%) | 53 (41%) | 0.239 |
| Mold in the house | 4 (31%) | 57 (44%) | 0.396 |
| Dust mite exposure | 10 (77%) | 117 (91%) | 0.142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carraro, S.; Ferraro, V.A.; Maretti, M.; Giordano, G.; Pirillo, P.; Stocchero, M.; Zanconato, S.; Baraldi, E. Metabolomic Profile at Birth, Bronchiolitis and Recurrent Wheezing: A 3-Year Prospective Study. Metabolites 2021, 11, 825. https://doi.org/10.3390/metabo11120825
Carraro S, Ferraro VA, Maretti M, Giordano G, Pirillo P, Stocchero M, Zanconato S, Baraldi E. Metabolomic Profile at Birth, Bronchiolitis and Recurrent Wheezing: A 3-Year Prospective Study. Metabolites. 2021; 11(12):825. https://doi.org/10.3390/metabo11120825
Chicago/Turabian StyleCarraro, Silvia, Valentina Agnese Ferraro, Michela Maretti, Giuseppe Giordano, Paola Pirillo, Matteo Stocchero, Stefania Zanconato, and Eugenio Baraldi. 2021. "Metabolomic Profile at Birth, Bronchiolitis and Recurrent Wheezing: A 3-Year Prospective Study" Metabolites 11, no. 12: 825. https://doi.org/10.3390/metabo11120825
APA StyleCarraro, S., Ferraro, V. A., Maretti, M., Giordano, G., Pirillo, P., Stocchero, M., Zanconato, S., & Baraldi, E. (2021). Metabolomic Profile at Birth, Bronchiolitis and Recurrent Wheezing: A 3-Year Prospective Study. Metabolites, 11(12), 825. https://doi.org/10.3390/metabo11120825






