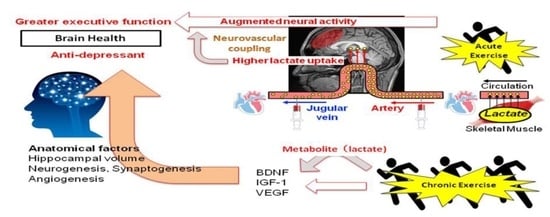
Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine
Abstract
:1. Introduction
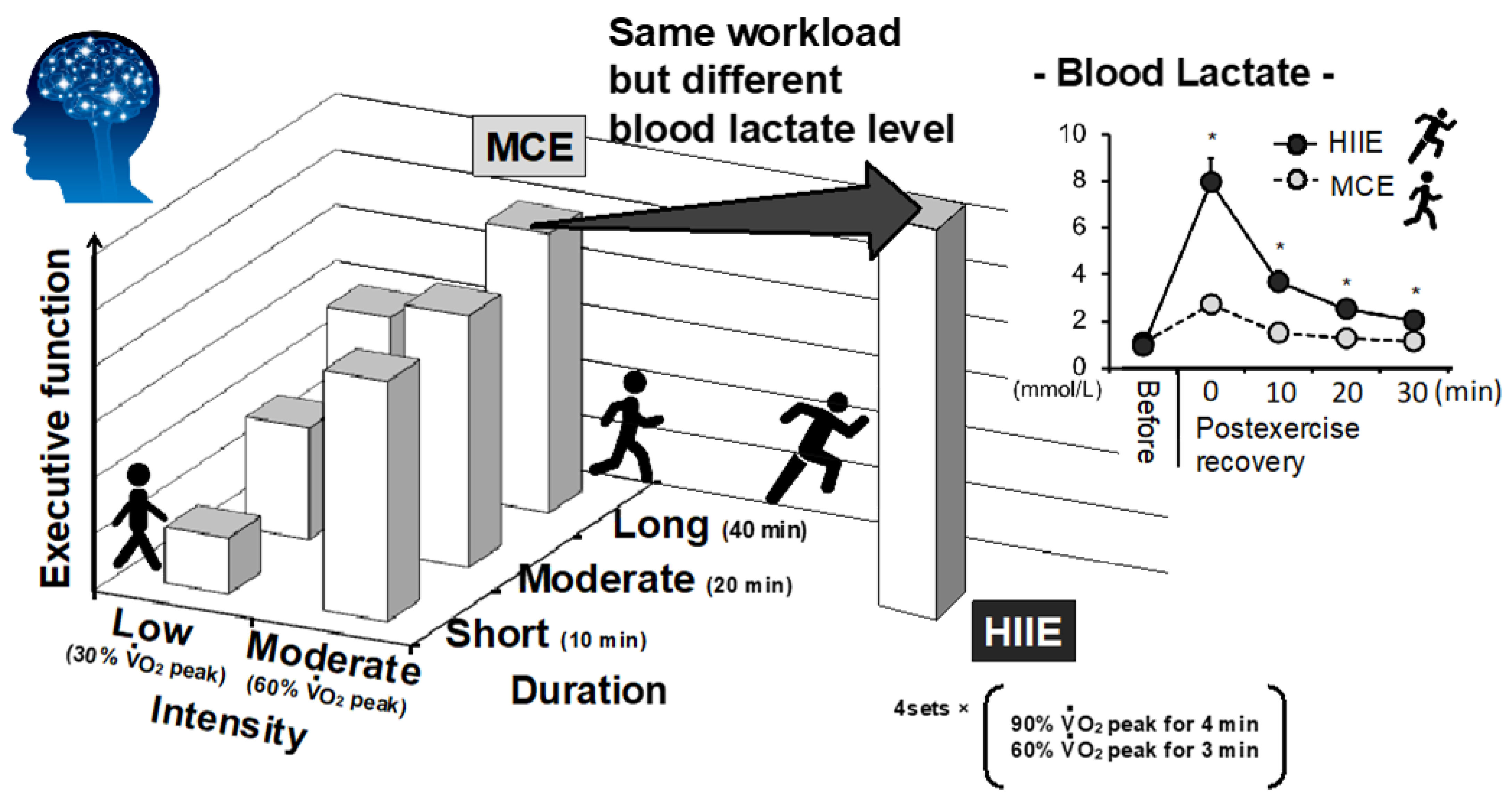
2. Exercise Intensity and Modality for Brain Health Regarding Chronic Exercise Adaptation (Implication of Lactate)
3. Chronic Cognitive and Mental Alterations with Regular Exercise and Its Potential Link to Chronic Exercise-Induced Anatomical and Cerebral Microvasculature Alterations
4. Can Acute Alterations in CBF to Exercise Affect Cognitive Performance?
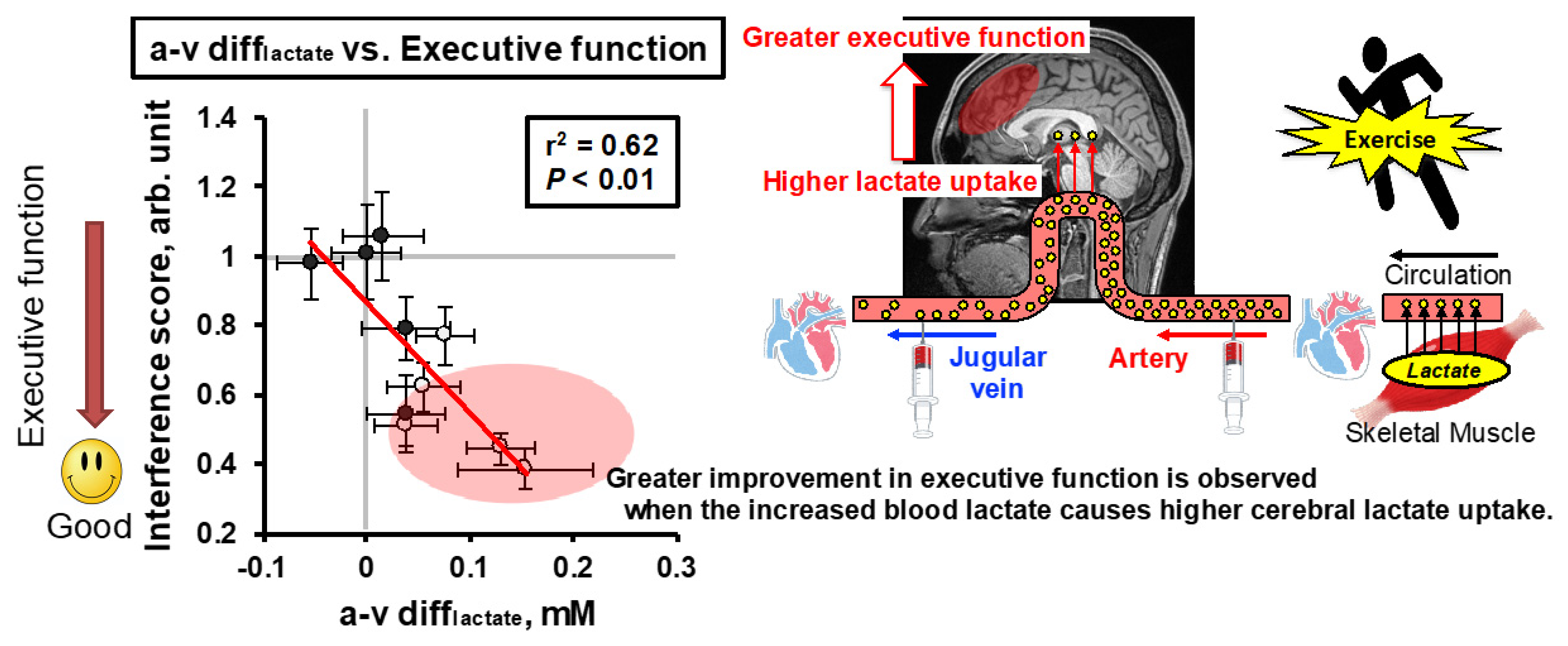
5. Cerebral Lactate Metabolism and Cognitive Performance
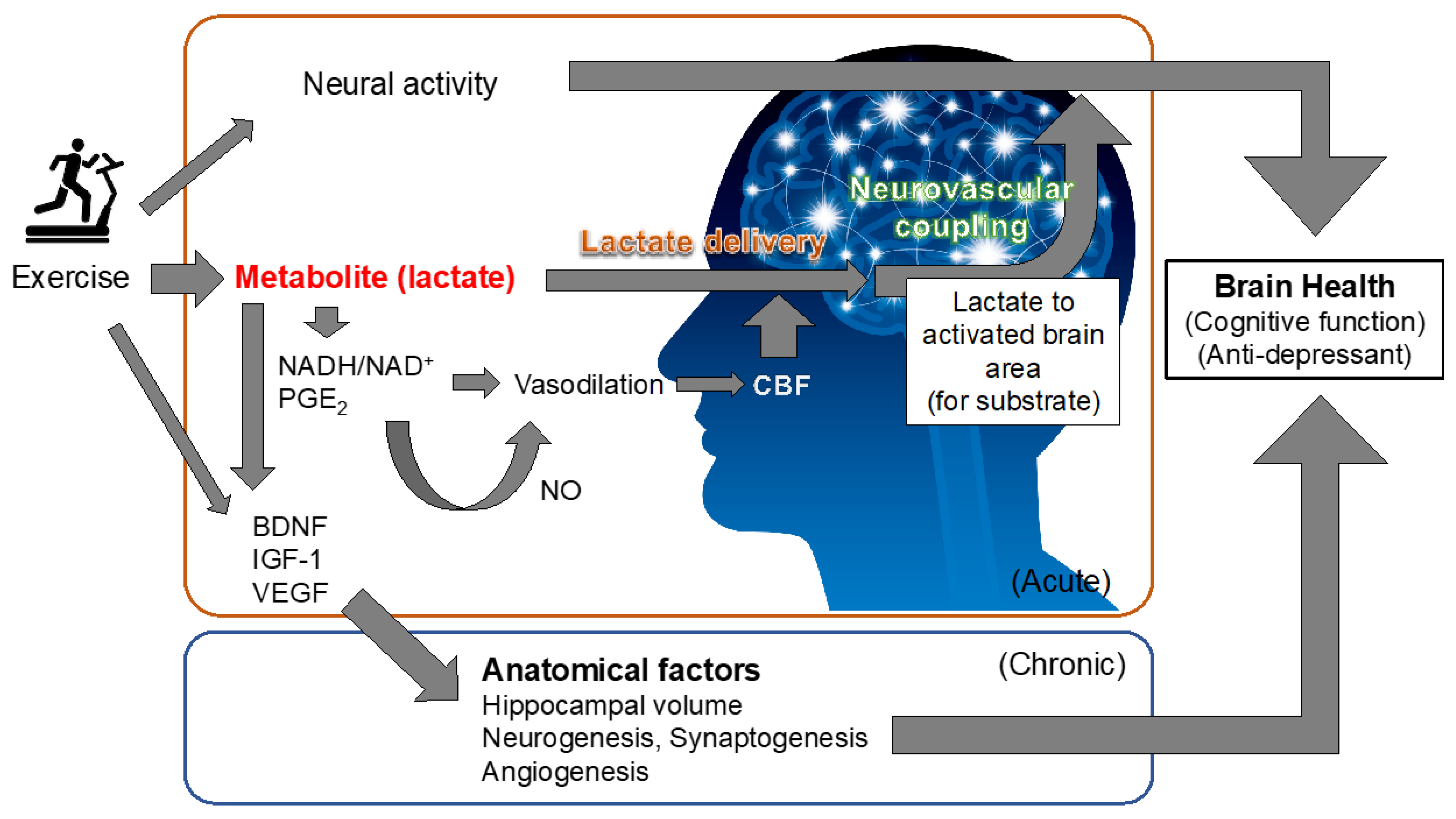
6. Exercise-Induced Improvement in Brain Health Based on Chronic Anatomical and Cerebral Microvasculature Alterations and Its Potential Link to Exercise-Produced Lactate in Active Muscle
7. Can Cerebral Blood Flow Regulation That Determines Brain Function Be Modified by Lactate?
8. Therapeutic Example of Exercise Modification to Consider the Interaction of Lactate
9. Summary and Future Perspective
Funding
Conflicts of Interest
References
- Gomez-Pinilla, F.; Hillman, C. The influence of exercise on cognitive abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef] [Green Version]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Krustrup, P.; Mohr, M.; Steensberg, A.; Bencke, J.; Kjaer, M.; Bangsbo, J. Muscle and blood metabolites during a soccer game: Implications for sprint performance. Med. Sci. Sports Exerc. 2006, 38, 1165–1174. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol. Behav. 2016, 155, 224–230. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Takenaka, S.; Suga, T.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Impact of Exercise Intensity and Duration on Postexercise Executive Function. Med. Sci. Sports Exerc. 2016, 49, 774–784, Erratum in 2017, 49, 774–784. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- ACSM. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med. Sci. Sports Exerc. 1998, 30, 975–991. [Google Scholar]
- Gormley, S.E.; Swain, D.P.; High, R.; Spina, R.J.; Dowling, E.A.; Kotipalli, U.S.; Gandrakota, R. Effect of intensity of aerobic training on VO2max. Med. Sci. Sports Exerc. 2008, 40, 1336–1343. [Google Scholar] [CrossRef]
- Swain, D.P.; Franklin, B.A. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am. J. Cardiol. 2006, 97, 141–147. [Google Scholar] [CrossRef]
- Helgerud, J.; Hoydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Hood, M.S.; Little, J.P.; Tarnopolsky, M.A.; Myslik, F.; Gibala, M.J. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med. Sci. Sports Exerc. 2011, 43, 1849–1856. [Google Scholar] [CrossRef]
- Talanian, J.L.; Galloway, S.D.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. (1985) 2007, 102, 1439–1447. [Google Scholar] [CrossRef]
- Mekari, S.; Earle, M.; Martins, R.; Drisdelle, S.; Killen, M.; Bouffard-Levasseur, V.; Dupuy, O. Effect of High Intensity Interval Training Compared to Continuous Training on Cognitive Performance in Young Healthy Adults: A Pilot Study. Brain Sci. 2020, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Korman, N.; Armour, M.; Chapman, J.; Rosenbaum, S.; Kisely, S.; Suetani, S.; Firth, J.; Siskind, D. High Intensity Interval training (HIIT) for people with severe mental illness: A systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020, 284, 112601. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Ogoh, S.; Pilatus, U.; Hashimoto, T. Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol. Behav. 2016, 160. [Google Scholar] [CrossRef] [Green Version]
- Matura, S.; Fleckenstein, J.; Deichmann, R.; Engeroff, T.; Fuzeki, E.; Hattingen, E.; Hellweg, R.; Lienerth, B.; Pilatus, U.; Schwarz, S.; et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: Results of the randomised controlled SMART trial. Transl. Psychiatry 2017, 7, e1172. [Google Scholar] [CrossRef] [Green Version]
- Tyndall, A.V.; Clark, C.M.; Anderson, T.J.; Hogan, D.B.; Hill, M.D.; Longman, R.S.; Poulin, M.J. Protective Effects of Exercise on Cognition and Brain Health in Older Adults. Exerc. Sport Sci. Rev. 2018, 46, 215–223. [Google Scholar] [CrossRef]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yücel, M. Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Front. Hum. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef]
- Pajonk, F.G.; Wobrock, T.; Gruber, O.; Scherk, H.; Berner, D.; Kaizl, I.; Kierer, A.; Müller, S.; Oest, M.; Meyer, T.; et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarumi, T.; Zhang, R. The Role of Exercise-Induced Cardiovascular Adaptation in Brain Health. Exerc. Sport Sci. Rev. 2015, 43, 181–189. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2016, 8, 336. [Google Scholar] [CrossRef]
- Ellison-Wright, I.; Bullmore, E. Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr. Res. 2010, 117, 1–12. [Google Scholar] [CrossRef]
- Schmaal, L.S.; Veltman, D.J.; van Erp, T.G.; Sämann, P.G.; Frodl, T.; Jahanshad, N.; Loehrer, E.; Tiemeier, H.; Hofman, A.; Niessen, W.J.; et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 2016, 21, 806–812. [Google Scholar] [CrossRef]
- Besteher, B.; Gaser, C.; Nenadić, I. Brain Structure and Subclinical Symptoms: A Dimensional Perspective of Psychopathology in the Depression and Anxiety Spectrum. Neuropsychobiology 2020, 79, 270–283. [Google Scholar] [CrossRef]
- Ogoh, S. Relationship between cognitive function and regulation of cerebral blood flow. J. Physiol. Sci. JPS 2017, 67, 345–351. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Rother, J.; Knab, R.; Hamzei, F.; Fiehler, J.; Reichenbach, J.R.; Buchel, C.; Weiller, C. Negative dip in BOLD fMRI is caused by blood flow--oxygen consumption uncoupling in humans. NeuroImage 2002, 15, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Hock, C.; Villringer, K.; Muller-Spahn, F.; Wenzel, R.; Heekeren, H.; Schuh-Hofer, S.; Hofmann, M.; Minoshima, S.; Schwaiger, M.; Dirnagl, U.; et al. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer’s disease monitored by means of near-infrared spectroscopy (NIRS)--correlation with simultaneous rCBF-PET measurements. Brain Res 1997, 755, 293–303. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Mentis, M.J.; Horwitz, B.; Grady, C.L.; Alexander, G.E.; VanMeter, J.W.; Maisog, J.M.; Pietrini, P.; Schapiro, M.B.; Rapoport, S.I. Visual cortical dysfunction in Alzheimer’s disease evaluated with a temporally graded “stress test” during PET. Am. J. Psychiatry 1996, 153, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, S.; Passant, U. Functional imaging of the frontal lobes in organic dementia. Regional cerebral blood flow findings in normals, in patients with frontotemporal dementia and in patients with Alzheimer’s disease, performing a word fluency test. Dement. Geriatr. Cogn. Disord. 1997, 8, 105–109. [Google Scholar] [CrossRef]
- Ide, K.; Schmalbruch, I.K.; Quistorff, B.; Horn, A.; Secher, N.H. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J. Physiol. 2000, 522, 159–164. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N. Cerebral blood flow during exercise: Mechanisms of regulation. J. Appl. Physiol. (1985) 2009, 107, 1370–1380. [Google Scholar] [CrossRef] [Green Version]
- Ogoh, S.; Ainslie, P.N. Regulatory mechanisms of cerebral blood flow during exercise: New concepts. Exerc. Sport Sci. Rev. 2009, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Brothers, R.M.; Barnes, Q.; Eubank, W.L.; Hawkins, M.N.; Purkayastha, S.; O-Yurvati, A.; Raven, P.B. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J. Physiol. 2005, 569, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Dalsgaard, M.K.; Yoshiga, C.C.; Dawson, E.A.; Keller, D.M.; Raven, P.B.; Secher, N.H. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1461–H1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Ogoh, S.; Hirasawa, A.; Oue, A.; Sadamoto, T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J. Physiol. 2011, 589, 2847–2856. [Google Scholar] [CrossRef]
- Brisswalter, J.; Collardeau, M.; Rene, A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 2002, 32, 555–566. [Google Scholar] [CrossRef]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Grego, F.; Vallier, J.M.; Collardeau, M.; Rousseu, C.; Cremieux, J.; Brisswalter, J. Influence of exercise duration and hydration status on cognitive function during prolonged cycling exercise. Int. J. Sports Med. 2005, 26, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Tsukamoto, H.; Hirasawa, A.; Hasegawa, H.; Hirose, N.; Hashimoto, T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol. Rep. 2014, 2, e12163. [Google Scholar] [CrossRef]
- Lucas, S.J.; Ainslie, P.N.; Murrell, C.J.; Thomas, K.N.; Franz, E.A.; Cotter, J.D. Effect of age on exercise-induced alterations in cognitive executive function: Relationship to cerebral perfusion. Exp. Gerontol. 2012, 47, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Smale, B.A.; Northey, J.M.; Smee, D.J.; Versey, N.G.; Rattray, B. Compression garments and cerebral blood flow: Influence on cognitive and exercise performance. Eur. J. Sport Sci. 2018, 18, 315–322. [Google Scholar] [CrossRef]
- Ogoh, S.; Sato, K.; Okazaki, K.; Miyamoto, T.; Hirasawa, A.; Shibasaki, M. Hyperthermia modulates regional differences in cerebral blood flow to changes in CO2. J. Appl. Physiol. (1985) 2014, 117, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The effects of exercise under hypoxia on cognitive function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, T.; Horiuchi, M.; Ichikawa, D.; Sato, K.; Tanaka, N.; Bailey, D.M.; Ogoh, S. Kinetics of exercise-induced neural activation; interpretive dilemma of altered cerebral perfusion. Exp. Physiol. 2012, 97, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Quistorff, B.; Secher, N.H.; Van Lieshout, J.J. Lactate fuels the human brain during exercise. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G.; Stromstad, M.; Rasmussen, P.; Jans, O.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Hirsch, J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage 2005, 24, 539–547. [Google Scholar] [CrossRef]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Dalsgaard, M.K.; Ide, K.; Cai, Y.; Quistorff, B.; Secher, N.H. The intent to exercise influences the cerebral O(2)/carbohydrate uptake ratio in humans. J. Physiol. 2002, 540, 681–689. [Google Scholar] [CrossRef]
- Kemppainen, J.; Aalto, S.; Fujimoto, T.; Kalliokoski, K.K.; Langsjo, J.; Oikonen, V.; Rinne, J.; Nuutila, P.; Knuuti, J. High intensity exercise decreases global brain glucose uptake in humans. J. Physiol. 2005, 568, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.F. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013, 36, 396–404. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef]
- Smith, D.; Pernet, A.; Hallett, W.A.; Bingham, E.; Marsden, P.K.; Amiel, S.A. Lactate: A preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Holloway, R.; Zhou, Z.; Harvey, H.B.; Levasseur, J.E.; Rice, A.C.; Sun, D.; Hamm, R.J.; Bullock, M.R. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. 2007, 149, 919–927; discussion 927. [Google Scholar] [CrossRef]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef]
- Schurr, A.; West, C.A.; Rigor, B.M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 1988, 240, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sorensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Chmura, J.; Nazar, K.; Kaciuba-Uscilko, H. Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int. J. Sports Med. 1994, 15, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Sudo, M.; Komiyama, T.; Aoyagi, R.; Nagamatsu, T.; Higaki, Y.; Ando, S. Executive function after exhaustive exercise. Eur. J. Appl. Physiol. 2017, 117, 2029–2038. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Banschbach, S.; Stransky, E.; Bosch, S.; Straten, G.; Machann, J.; Fritsche, A.; Hipp, A.; Niess, A.; Eschweiler, G.W. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int. J. Neuropsychopharmacol. 2010, 13, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Struder, H.K. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Whitehurst, M.; Fico, B.G.; Dodge, K.M.; Ferrandi, P.J.; Pena, G.; Adelman, A.; Huang, C.J. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. (Maywood N.J.) 2018, 243, 1153–1160. [Google Scholar] [CrossRef]
- Kujach, S.; Olek, R.A.; Byun, K.; Suwabe, K.; Sitek, E.J.; Ziemann, E.; Laskowski, R.; Soya, H. Acute Sprint Interval Exercise Increases Both Cognitive Functions and Peripheral Neurotrophic Factors in Humans: The Possible Involvement of Lactate. Front. Neurosci. 2019, 13, 1455. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, O.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Lucas, S.J.; Cotter, J.D.; Brassard, P.; Bailey, D.M. High-intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J. Cereb. Blood Flow Metab. 2015, 35, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Cassé, F.; Carron, C.; Burlet-Godinot, S.; Toni, N.; Magistretti, P.J.; Martin, J.L. Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Margineanu, M.B.; Mahmood, H.; Fiumelli, H.; Magistretti, P.J. L-Lactate Regulates the Expression of Synaptic Plasticity and Neuroprotection Genes in Cortical Neurons: A Transcriptome Analysis. Front. Mol. Neurosci. 2018, 11, 375. [Google Scholar] [CrossRef]
- Gordon, G.R.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Mintun, M.A.; Vlassenko, A.G.; Rundle, M.M.; Raichle, M.E. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc. Natl. Acad. Sci. USA 2004, 101, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Hollyer, T.R.; Bordoni, L.; Kousholt, B.S.; van Luijk, J.; Ritskes-Hoitinga, M.; Østergaard, L. The evidence for the physiological effects of lactate on the cerebral microcirculation: A systematic review. J. Neurochem. 2019, 148, 712–730. [Google Scholar] [CrossRef]
- Carteron, L.; Solari, D.; Patet, C.; Quintard, H.; Miroz, J.P.; Bloch, J.; Daniel, R.T.; Hirt, L.; Eckert, P.; Magistretti, P.J.; et al. Hypertonic Lactate to Improve Cerebral Perfusion and Glucose Availability After Acute Brain Injury. Crit. Care Med. 2018, 46, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Martin, N.A. Cerebral metabolism following traumatic brain injury: New discoveries with implications for treatment. Front. Neurosci. 2014, 8, 408. [Google Scholar] [CrossRef]
- Bisri, T.; Utomo, B.A.; Fuadi, I. Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian J. Neurosurg. 2016, 11, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Winett, R.A.; Carpinelli, R.N. Potential health-related benefits of resistance training. Prev. Med. 2001, 33, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Takeuchi, T.; Tanaka, D.; Hamaoka, T.; Hashimoto, T.; Isaka, T. An acute bout of localized resistance exercise can rapidly improve inhibitory control. PLoS ONE 2017, 12, e0184075. [Google Scholar] [CrossRef] [PubMed]
- Dora, K.; Suga, T.; Tomoo, K.; Sugimoto, T.; Mok, E.; Tsukamoto, H.; Takada, S.; Hashimoto, T.; Isaka, T. Effect of very low-intensity resistance exercise with slow movement and tonic force generation on post-exercise inhibitory control. Heliyon 2021, 7, e06261. [Google Scholar] [CrossRef] [PubMed]
- Dora, K.; Suga, T.; Tomoo, K.; Sugimoto, T.; Mok, E.; Tsukamoto, H.; Takada, S.; Hashimoto, T.; Isaka, T. Similar improvements in cognitive inhibitory control following low-intensity resistance exercise with slow movement and tonic force generation and high-intensity resistance exercise in healthy young adults: A preliminary study. J. Physiol. Sci. JPS 2021, 71, 22. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, T.; Tsukamoto, H.; Ando, S.; Ogoh, S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites 2021, 11, 813. https://doi.org/10.3390/metabo11120813
Hashimoto T, Tsukamoto H, Ando S, Ogoh S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites. 2021; 11(12):813. https://doi.org/10.3390/metabo11120813
Chicago/Turabian StyleHashimoto, Takeshi, Hayato Tsukamoto, Soichi Ando, and Shigehiko Ogoh. 2021. "Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine" Metabolites 11, no. 12: 813. https://doi.org/10.3390/metabo11120813
APA StyleHashimoto, T., Tsukamoto, H., Ando, S., & Ogoh, S. (2021). Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites, 11(12), 813. https://doi.org/10.3390/metabo11120813