Increased Reliance on Carbohydrates for Aerobic Exercise in Highland Andean Leaf-Eared Mice, but Not in Highland Lima Leaf-Eared Mice
Abstract
:1. Introduction
2. Results
2.1. Resting Metabolism
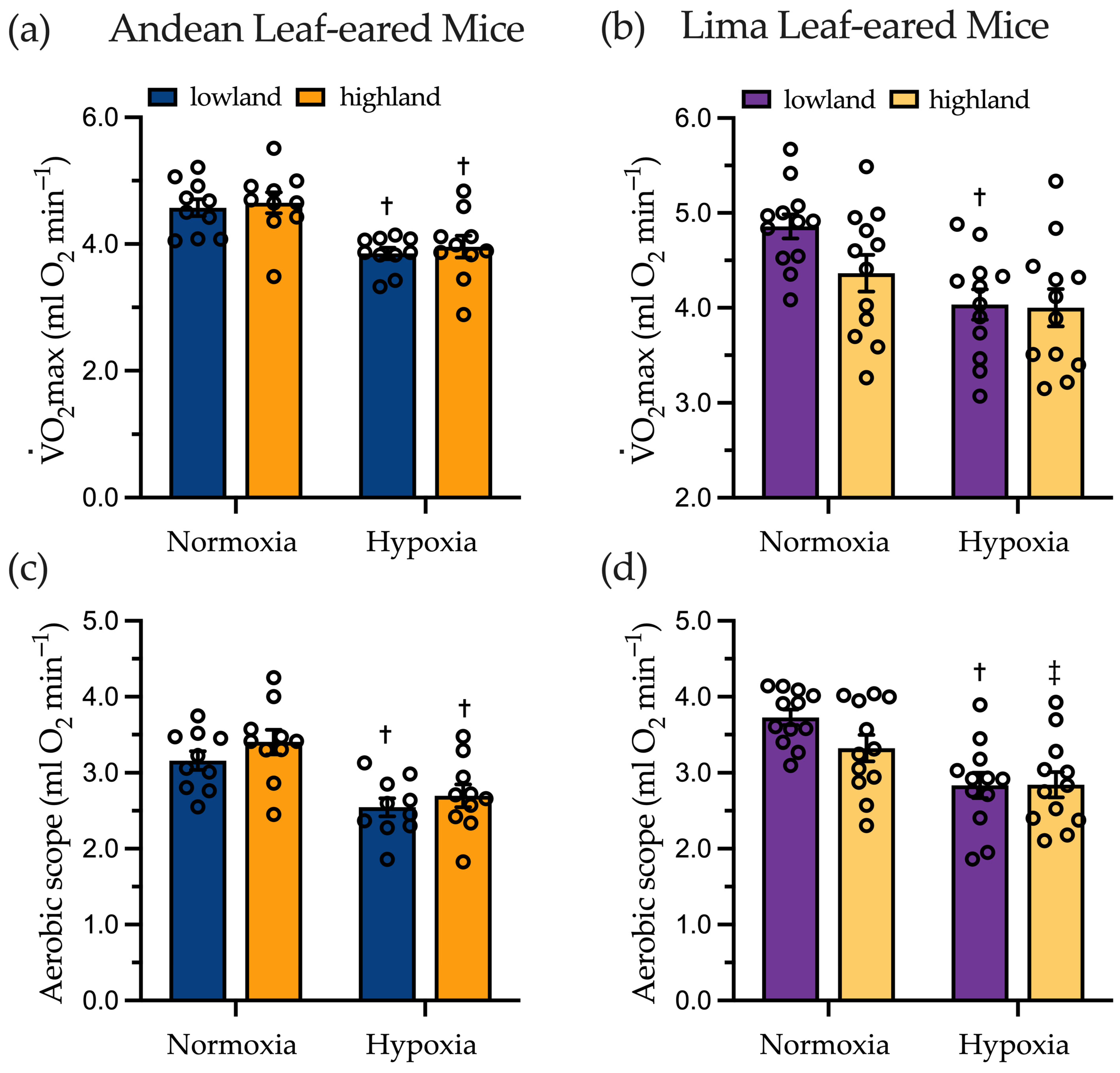
2.2. Maximal Oxygen Consumption and Aerobic Scope
2.3. Submaximal Exercise Metabolism
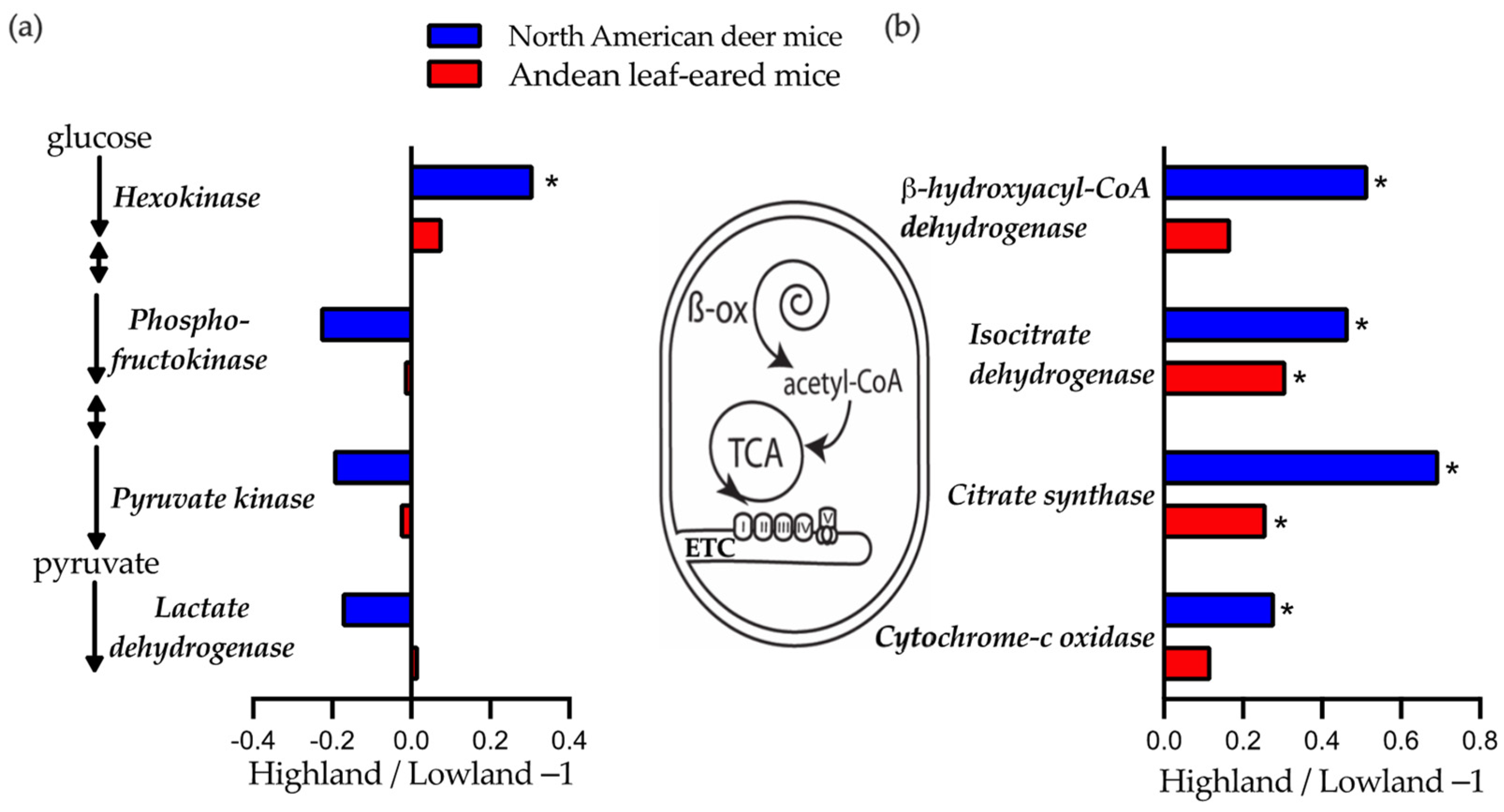
2.4. Skeletal Muscle Enzyme Activities
3. Discussion
3.1. Aerobic Capacity and Aerobic Scope
3.2. Substrate Use at Rest and Submaximal Exercise
3.3. Muscle Enzyme Vmax
3.4. Phenotypic Plasticity and Interpopulation Phenotypic Differences
3.5. Conclusions
4. Materials and Methods
4.1. Study Design
4.2. Respirometry
4.3. Muscle Enzymatic Capacities (Apparent Vmax)
4.4. Calculations and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| - | Enzyme | Lowland | Highland |
|---|---|---|---|
| Glycolytic | HK | 1.97 ± 0.21 | 2.25 ± 0.13 |
| PFK | 35.6 ± 3.9 | 33.2 ± 3.2 | |
| PK | 361.1 ± 25.2 | 413.4 ± 25.4 | |
| LDH | 523.3 ± 12.9 | 557.9 ± 21.3 * | |
| ß-oxidation | HOAD | 15.6 ± 2.0 | 24.5 ± 1.0 |
| Aerobic metabolism | CS | 22.6 ± 1.2 | 24.5 ± 1.4 |
| COX | 12.5 ± 0.9 | 13.3 ± 1.0 |
References
- Lailvaux, S.P.; Husak, J. The Life History of Whole-Organism Performance. Q. Rev. Biol. 2014, 89, 285–318. [Google Scholar] [CrossRef]
- Storz, J.F.; Cheviron, Z.A.; McClelland, G.; Scott, G.R. Evolution of physiological performance capacities and environmental adaptation: Insights from high-elevation deer mice (Peromyscus maniculatus). J. Mammal. 2019, 100, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.-P.; Ramirez, O.; Arana, M.; Pinedo-Bernal, P.; McClelland, G. Increase in Carbohydrate Utilization in High-Altitude Andean Mice. Curr. Biol. 2012, 22, 2350–2354. [Google Scholar] [CrossRef] [Green Version]
- Schippers, M.-P.; LeMoine, C.; McClelland, G.B. Patterns of fuel use during locomotion in mammals revisited: The importance of aerobic scope. J. Exp. Biol. 2014, 217, 3193–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, D.S.; Connaty, A.D.; Mahalingam, S.; Wall, N.; Cheviron, Z.A.; Storz, J.F.; Scott, G.R.; McClelland, G.B. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R400–R411. [Google Scholar] [CrossRef] [Green Version]
- McClelland, G.B.; Lyons, S.A.; Robertson, C.E. Fuel use in mammals: Conserved patterns and evolved strategies for aerobic locomotion and thermogenesis. Integr. Comp. Biol. 2017, 57, 231–239. [Google Scholar] [CrossRef]
- Weber, J.-M. Metabolic fuels: Regulating fluxes to select mix. J. Exp. Biol. 2011, 214, 286–294. [Google Scholar] [CrossRef] [Green Version]
- McClelland, G.B.; Scott, G.R. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol. 2019, 81, 561–583. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Human Physiology at Extreme Altitudes on Mount Everest. Science 1984, 223, 784–788. [Google Scholar] [CrossRef]
- Brutsaert, T.D. Do high-altitude natives have enhanced exercise performance at altitude? Appl. Physiol. Nutr. Metab. 2008, 33, 582–592. [Google Scholar] [CrossRef]
- Chappell, M.A.; Snyder, L.R. Biochemical and physiological correlates of deer mouse alpha-chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. USA 1984, 81, 5484–5488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, M.A.; Mahalingam, S.; Patel, P.; Connaty, A.D.; Ivy, C.M.; Cheviron, Z.A.; Storz, J.F.; McClelland, G.; Scott, G.R. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R779–R791. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W. Exercise limitations at high altitude: The metabolic problem and search for its solution. In Circulation, Respiration and Metabolism; Gilles, R., Ed.; Springer: Berlin, Germany, 1985; pp. 240–249. [Google Scholar]
- Brooks, G.A.; Butterfield, G.E.; Wolfe, R.R.; Groves, B.M.; Mazzeo, R.S.; Sutton, J.R.; Wolfel, E.E.; Reeves, J.T. Increased dependence on blood glucose after acclimatization to 4300 m. J. Appl. Physiol. 1991, 70, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005, 33, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Daut, J.; Elzinga, G. Substrate dependence of energy metabolism in isolated guinea-pig cardiac muscle: A microcalorimetric study. J. Physiol. 1989, 413, 379–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, K.C., Jr.; Altshuler, D.L.; Suarez, R.K. Oxygen consumption rates in hovering hummingbirds reflect substrate-dependent differences in P/O ratios: Carbohydrate as a ‘premium fuel’. J. Exp. Biol. 2007, 210, 2146–2153. [Google Scholar] [CrossRef] [Green Version]
- Beall, C.M. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, M.A.; Hayes, J.P.; Snyder, L.R.G. Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): Physiology of β-globin variants and α-globin recombinants. Evolution 1988, 42, 681–688. [Google Scholar]
- Storz, J.F. High-Altitude Adaptation: Mechanistic Insights from Integrated Genomics and Physiology. Mol. Biol. Evol. 2021, 38, 2677–2691. [Google Scholar] [CrossRef]
- Mahalingam, S.; McClelland, G.B.; Scott, G.R. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J. Physiol. 2017, 595, 4785–4801. [Google Scholar] [CrossRef]
- Storz, J.F.; Scott, G.R. Life Ascending: Mechanism and Process in Physiological Adaptation to High-Altitude Hypoxia. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 503–526. [Google Scholar] [CrossRef] [Green Version]
- Tate, K.B.; Ivy, C.M.; Velotta, J.P.; Storz, J.F.; McClelland, G.; Cheviron, Z.A.; Scott, G.R. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. J. Exp. Biol. 2017, 220, 3616–3620. [Google Scholar] [CrossRef] [Green Version]
- West, C.M.; Ivy, C.M.; Husnudinov, R.; Scott, G.R. Evolution and developmental plasticity of lung structure in high-altitude deer mice. J. Comp. Physiol. B 2021, 191, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Bozinovic, F.; Nespolo, R.F. Effect of ambient temperature and energy demands on digestive functions in leaf-eared mice (Phyllotis darwini) from central Chile. Int. J. Biometeorol. 1997, 41, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, K.; Sabat, P.; Piriz, G.; Bogdanovich, J.M.; Nespolo, R.F.; Bozinovic, F. Is Maximum Food Intake in Endotherms Constrained by Net or Factorial Aerobic Scope? Lessons from the Leaf-Eared Mouse. Front. Physiol. 2016, 7, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steppan, S.J.; Ramirez-Baca, O. Genus Phyllotis. In Mammals of South America; Patton, J.L., Pardiñas, U.F.J., D’Elia, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; pp. 535–555. [Google Scholar]
- Storz, J.F.; Quiroga-Carmona, M.; Opazo, J.C.; Bowen, T.; Farson, M.; Steppan, S.J.; D’Elía, G. Discovery of the world’s highest-dwelling mammal. Proc. Natl. Acad. Sci. USA 2020, 117, 18169–18171. [Google Scholar] [CrossRef]
- Gilbert-Kawai, E.T.; Milledge, J.S.; Grocott, M.; Martin, D.S. King of the Mountains: Tibetan and Sherpa Physiological Adaptations for Life at High Altitude. Physiology 2014, 29, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Cheviron, Z.A.; Bachman, G.C.; Connaty, A.D.; McClelland, G.; Storz, J.F. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc. Natl. Acad. Sci. USA 2012, 109, 8635–8640. [Google Scholar] [CrossRef] [Green Version]
- Cheviron, Z.A.; Connaty, A.D.; McClelland, G.; Storz, J.F. Functional Genomics of Adaptation to Hypoxic Cold-Stress in High-Altitude Deer Mice: Transcriptomic Plasticity and Thermogenic Performance. Evolution 2013, 68, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.B.; Essén-Gustavsson, B.; Hoppeler, H.; Jones, J.H.; Kayar, S.R.; Laughlin, M.H.; Lindholm, A.; Longworth, K.E.; Taylor, C.R.; Weibel, E.R. O2 delivery at VO2max and oxidative capacity in muscles of standardbred horses. J. Appl. Physiol. 1992, 73, 2274–2282. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Kang, L.; Ayala, J.E.; Fueger, P.T.; Lee, R. The physiological regulation of glucose flux into muscle in vivo. J. Exp. Biol. 2011, 214, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, R.K.; Darveau, C.-A.; Hochachka, P.W. Roles of hierarchical and metabolic regulation in the allometric scaling of metabolism in Panamanian orchid bees. J. Exp. Biol. 2005, 208, 3603–3607. [Google Scholar] [CrossRef] [Green Version]
- Glancy, B.; Balaban, R.S. Energy metabolism design of the striated muscle cell. Physiol. Rev. 2021, 101, 1561–1607. [Google Scholar] [CrossRef] [PubMed]
- Le Moine, C.M.; Morash, A.J.; McClelland, G.B. Changes in HIF-1α protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1098–R1104. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W.; Mommsen, T.P. Protons and Anaerobiosis. Science 1983, 219, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W. Muscle Enzymatic Composition and Metabolic Regulation in High Altitude Adapted Natives. Int. J. Sports Med. 1992, 13, S89–S91. [Google Scholar] [CrossRef] [PubMed]
- Mensen de Silva, E.; Cazorla, A. Lactate, -GP, and Krebs cycle in sea-level and high-altitude native guinea pigs. Am. J. Physiol. 1973, 224, 669–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson, S.Z.; Robertson, C.E.; Mahalingam, S.; McClelland, G.B. Plasticity of non-shivering thermogenesis and brown adipose tissue in high-altitude deer mice. J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef]
- Lyons, S.A.; Tate, K.B.; Welch, K.C., Jr.; McClelland, G.B. Lipid oxidation during thermogenesis in high-altitude deer mice (Peromyscus maniculatus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R735–R746. [Google Scholar] [CrossRef]
- Robertson, C.E.; McClelland, G.B. Ancestral and developmental cold alter brown adipose tissue function and adult thermal acclimation in Peromyscus. J. Comp. Physiol. B 2021, 191, 589–601. [Google Scholar] [CrossRef]
- Salzano, F.; Callegari-Jacques, S.M. South American Indians: A Case Study in Evolution; Clarendon Press: Oxford, UK, 1988. [Google Scholar]
- Storz, J.F.; Dubach, J.M. Natural selection drives altitudinal divergence at the albumin locus in deer mice, Peromyscus maniculatus. Evolution 2004, 58, 1342–1352. [Google Scholar] [CrossRef] [Green Version]
- Goymann, W.; Schwabl, H. The tyranny of phylogeny—A plea for a less dogmatic stance on two-species comparisons. BioEssays 2021, 2100071. [Google Scholar] [CrossRef] [PubMed]
- Rengifo, E.M.; Pacheco, V.M. Taxonomic revision of the Andean leaf-eared mouse, Phyllotis andium Thomas 1912 (Rodentia: Cricetidae), with the description of a new species. Zootaxa 2015, 4018, 349–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arana, M.; Ramírez, O.; María, S.S.; Kunimoto, C.; Velarde, R.; De La Cruz, C.; Ruíz, M.L. Population density and reproduction of two Peruvian leaf-eared mice (Phyllotis spp.). Rev. Chil. Hist. Nat. 2002, 75, 751–756. [Google Scholar] [CrossRef]
- Pearson, O.P.; Ralph, C.P. The diversity and abundance of vertebrates along an altitudinal gradient in Peru. Mem. Del Mus. De Hist. Nat. Javier Prado 1978, 18, 1–97. [Google Scholar]
- White, C.R.; Portugal, S.J.; Martin, G.R.; Butler, P.J. Respirometry: Anhydrous Drierite Equilibrates with Carbon Dioxide and Increases Washout Times. Physiol. Biochem. Zool. 2006, 79, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Egan, B.; Zierath, J. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.E.; McClelland, G.B. Developmental delay in shivering limits thermogenic capacity in juvenile high-altitude deer mice (Peromyscus maniculatus). J. Exp. Biol. 2019, 222, jeb.210963. [Google Scholar] [CrossRef]
- Withers, P. Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J. Appl. Physiol. 1977, 42, 120–123. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Rennie, M.J.; Edwards, R.H.T.; Krywawych, S.; Davies, C.T.M.; Halliday, D.; Waterlow, J.C.; Millward, D.J. Effect of Exercise on Protein Turnover in Man. Clin. Sci. 1981, 61, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Lowland | Highland | Lowland | Highland | |
| Mass (g) | 43.5 ± 1.9 | 45.7 ± 3.3 | 43.3 ± 2.1 | 46.5 ± 3.4 |
| Resting | ||||
| O2 (mL min−1) | 1.41 ± 0.12 | 1.25 ± 0.09 | 1.31 ± 0.09 | 1.26 ± 0.05 |
| RER | 0.788 ± 0.012 | 0.787 ± 0.010 | 0.772 ± 0.008 | 0.821 ± 0.013 * |
| Submaximal exercise | ||||
| O2 (mL min−1) | 3.17 ± 0.09 | 3.57 ± 0.13 * | 2.79 ± 0.15 † | 2.89 ± 0.14 † |
| RER | 0.888 ± 0.015 | 0.919 ± 0.010 | 0.853 ± 0.012 | 0.921 ± 0.033 * |
| Lipid oxidation (mL min−1) | 1.22 ± 0.19 | 0.96 ± 0.12 | 1.44 ± 0.13 | 0.90 ± 0.23 |
| Lipid oxidation (% O2) | 37.9 ± 5.2 | 27.5 ± 3.6 | 49.9 ± 4.0 | 32.4 ± 7.9 ‡ |
| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Lowland | Highland | Lowland | Highland | |
| Mass (g) | 45.6 ± 1.9 | 39.7 ± 2.3 | 46.2 ± 2.0 | 40.8 ± 2.3 |
| Resting | ||||
| O2 (mL min−1) | 1.13 ± 0.07 | 1.04 ± 0.07 | 1.20 ± 0.04 | 1.16 ± 0.06 |
| RER | 0.795 ± 0.008 | 0.776 ± 0.007 | 0.763 ± 0.008 † | 0.761 ± 0.006 |
| Submaximal exercise | ||||
| O2 (mL min−1) | 3.70 ± 0.13 | 3.17 ± 0.09 | 3.01 ± 0.12 † | 3.01 ± 0.14 |
| RER | 0.869 ± 0.008 | 0.878 ± 0.018 | 0.850 ± 0.011 | 0.860 ± 0.009 |
| Lipid oxidation (mL min−1) | 1.64 ± 0.11 | 1.25 ± 0.19 | 1.51 ± 0.10 | 1.40 ± 0.07 |
| Lipid oxidation (% O2) | 44.3 ± 2.8 | 41.3 ± 6.1 | 50.9 ± 3.6 | 47.5 ± 3.1 |
| - | Lowland | Highland | |
|---|---|---|---|
| Glycolytic | HK | 1.85 ± 0.13 | 2.00 ± 0.14 |
| PFK | 42.8 ± 2.8 | 41.8 ± 2.9 | |
| PK | 385.1 ± 22.3 | 371.9 ± 15.6 | |
| LDH | 756.5 ± 33.9 | 775.3 ± 41.8 | |
| ß-oxidation | HOAD | 19.9 ± 1.0 | 22.3 ± 1.5 |
| Aerobic metabolism | CS | 27.4 ± 2.0 | 35.9 ± 2.3 * |
| IDH | 14.6 ± 1.1 | 18.4 ± 1.4 * | |
| COX | 11.8 ± 1.9 | 13.8 ± 2.2 | |
| CS/LDH | 0.037 ± 0.003 | 0.047 ± 0.003 * | |
| PK/LDH | 0.51 ± 0.02 | 0.49 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schippers, M.-P.; Ramirez, O.; Arana, M.; McClelland, G.B. Increased Reliance on Carbohydrates for Aerobic Exercise in Highland Andean Leaf-Eared Mice, but Not in Highland Lima Leaf-Eared Mice. Metabolites 2021, 11, 750. https://doi.org/10.3390/metabo11110750
Schippers M-P, Ramirez O, Arana M, McClelland GB. Increased Reliance on Carbohydrates for Aerobic Exercise in Highland Andean Leaf-Eared Mice, but Not in Highland Lima Leaf-Eared Mice. Metabolites. 2021; 11(11):750. https://doi.org/10.3390/metabo11110750
Chicago/Turabian StyleSchippers, Marie-Pierre, Oswaldo Ramirez, Margarita Arana, and Grant B. McClelland. 2021. "Increased Reliance on Carbohydrates for Aerobic Exercise in Highland Andean Leaf-Eared Mice, but Not in Highland Lima Leaf-Eared Mice" Metabolites 11, no. 11: 750. https://doi.org/10.3390/metabo11110750






