Non-Invasive Analysis of Human Liver Metabolism by Magnetic Resonance Spectroscopy
Abstract
1. Introduction
1.1. Observation of Hepatic Metabolites by MRS
1.2. Advances in MRS Instrumentation
1.3. In Vivo 1H MRS of Liver
1.3.1. 1H MRS of Liver Lipids
1.3.2. 1H MRS of Other Hepatic Metabolites
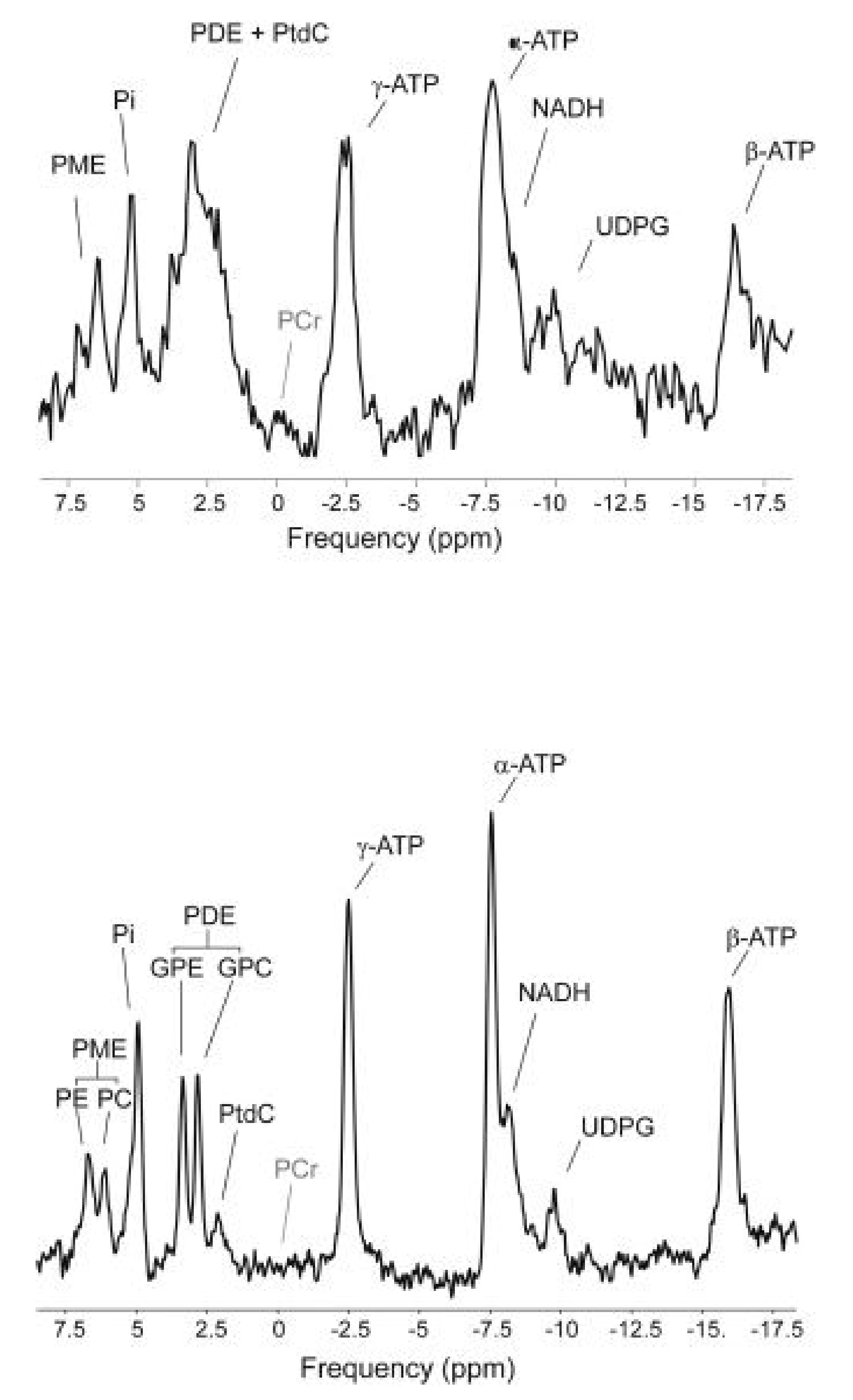
1.4. In Vivo 31P MRS of Liver
1.5. In Vivo 13C MRS of Liver
1.6. In Vivo MRS of Other Nuclei in the Study of Hepatic Metabolism
1.6.1. Deuterium
1.6.2. Fluorine
2. Future Perspectives and Main Conclusions
Main Conclusions
Funding
Conflicts of Interest
References
- Francis, I.R.; Chenevert, T.L.; Gubin, B.; Collomb, L.; Ensminger, W.; Walker-Andrews, S.; Glazer, G.M. Malignant hepatic tumors: P-31 MR spectroscopy with one-dimensional chemical shift imaging. Radiology 1991, 180, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Jue, T.; Rothman, D.L.; Tavitian, B.A.; Shulman, R.G. Natural-abundance 13C NMR study of glycogen repletion in human liver and muscle. Proc. Natl. Acad. Sci. USA 1989, 86, 1439–1442. [Google Scholar] [CrossRef]
- Rothman, D.L.; Magnusson, I.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Quantitation of Hepatic Glycogenolysis and Gluconeogenesis in Fasting Humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Jue, T.; Rothman, D.L.; Lohman, J.A.; Hughes, E.W.; Hanstock, C.C.; Shulman, R.G. Surface coil localization of 31P NMR signals from orthotopic human kidney and liver. Proc. Natl. Acad. Sci. USA 1988, 85, 971–974. [Google Scholar] [CrossRef]
- Fischbach, F.; Bruhn, H. Assessment of in vivo 1H magnetic resonance spectroscopy in the liver: A review. Liver Int. 2008, 28, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, B.; Huang, Y.; Liu, X.; Zhang, S.-W.; Xin, X.-G.; Zheng, J.-Z. 3.0 T proton magnetic resonance spectroscopy of the liver: Quantification of choline. World J. Gastroenterol. 2013, 19, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, F.; Schirmer, T.; Thormann, M.; Freund, T.; Ricke, J.; Bruhn, H. Quantitative proton magnetic resonance spectroscopy of the normal liver and malignant hepatic lesions at 3.0 Tesla. Eur. Radiol. 2008, 18, 2549–2558. [Google Scholar] [CrossRef]
- Munakata, T.; Griffiths, R.D.; Martin, P.A.; Jenkins, S.A.; Shields, R.; Edwards, R.H.T. Anin vivo 31P MRS study of patients with liver cirrhosis: Progress towards a non-invasive assessment of disease severity. NMR Biomed. 1993, 6, 168–172. [Google Scholar] [CrossRef]
- Grant, A.; Metzger, G.J.; Van de Moortele, P.-F.; Adriany, G.; Olman, C.; Zhang, L.; Koopermeiners, J.; Eryaman, Y.; Koeritzer, M.; Adams, M.E.; et al. 10.5 T MRI static field effects on human cognitive, vestibular, and physiological function. Magn. Reson. Imaging 2020, 73, 163–176. [Google Scholar] [CrossRef]
- Budinger, T.F.; Bird, M.D.; Frydman, L.; Long, J.; Mareci, T.; Rooney, W.D.; Rosen, B.; Schenck, J.F.; Schepkin, V.D.; Sherry, D.; et al. Toward 20 T magnetic resonance for human brain studies: Opportunities for discovery and neuroscience rationale. Magma Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 617–639. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef]
- Lopez-Kolkovsky, A.L.; Mériaux, S.; Boumezbeur, F. Metabolite and macromolecule T1 and T2 relaxation times in the rat brain in vivo at 17.2T. Magn. Reson. Med. 2016, 75, 503–514. [Google Scholar] [CrossRef]
- Pollack, M.H.; Jensen, J.E.; Simon, N.M.; Kaufman, R.E.; Renshaw, P.F. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 739–743. [Google Scholar] [CrossRef]
- Deelchand, D.K.; Uğurbil, K.; Henry, P.-G. Investigating brain metabolism at high fields using localized 13C NMR spectroscopy without H-1 decoupling. Magn. Reson. Med. 2006, 55, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Kalleveen, I.; de Castro, C.A.; van Laarhoven, H.; Klom, D.; van Der Kemp, W.; Stoker, J.; Nederveen, A. Inherently decoupled H-1 antennas and P-31 loops for metabolic imaging of liver metastasis at 7 T. NMR Biomed. 2020, 33, e4221. [Google Scholar] [CrossRef]
- Van Houtum, Q.; Welting, D.; Gosselink, W.; Klomp, D.; De Castro, C.A.; Van Der Kemp, W. Low SAR 31P (multi-echo) spectroscopic imaging using an integrated whole-body transmit coil at 7T. NMR Biomed. 2019, 32, e4178. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, J.S.; Seevinck, P.R.; Andreychenko, A.; Raaijmakers, A.J.E.; Luijten, P.R.; Viergever, M.A.; Koopman, M.; Boer, V.O.; Klomp, D.W.J. 19F MRSI of capecitabine in the liver at 7T using broadband transmit-receive antennas and dual-band RF pulses. NMR Biomed. 2015, 28, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging–A clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef]
- Di Martino, M.; Pacifico, L.; Bezzi, M.; Di Miscio, R.; Sacconi, B.; Chiesa, C.; Catalano, C. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J. Gastroenterol. 2016, 22, 8812–8819. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2017, 67, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Roumans, K.H.M.; Lindeboom, L.; Veeraiah, P.; Remie, C.M.E.; Phielix, E.; Havekes, B.; Bruls, Y.; Brouwers, M.C.G.J.; Ståhlman, M.; Alssema, M.; et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 2020, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Gajdosik, M.; Chadzynski, G.L.; Hangel, G.; Mlynarik, V.; Chmelík, M.; Valkovič, L.; Bogner, W.; Pohmann, R.; Scheffler, K.; Trattnig, S.; et al. Ultrashort-TE stimulated echo acquisition mode (STEAM) improves the quantification of lipids and fatty acid chain unsaturation in the human liver at 7 T. NMR Biomed. 2015, 28, 1283–1293. [Google Scholar] [CrossRef]
- Weis, J.; Kullberg, J.; Ahlström, H. Multiple breath-hold proton spectroscopy of human liver at 3T: Relaxation times and concentrations of glycogen, choline, and lipids. J. Magn. Reson. Imaging 2017, 47, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R.; Pettigrew, R.I.; Gharib, A. Liver Metabolite Concentrations Measured with1H MR Spectroscopy. Radiology 2012, 265, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ter Voert, E.; Heijmen, L.; van Asten, J.J.A.; Wright, A.J.; Nagtegaal, I.D.; Punt, C.J.A.; de Wilt, J.H.W.; van Laarhoven, H.W.M.; Heerschap, A. Levels of choline-containing compounds in normal liver and liver metastases of colorectal cancer as recorded by H-1 MRS. NMR Biomed. 2019, 32, e4035. [Google Scholar] [CrossRef]
- Chen, W.; Avison, M.J.; Zhu, X.H.; Shulman, R.G. NMR studies of proton NOEs in glycogen. Biochemistry 1993, 32, 11483–11487. [Google Scholar] [CrossRef]
- Zhou, Y.; van Zijl, P.C.M.; Xu, X.; Xu, J.; Li, Y.; Chen, L.; Yadav, N.N. Magnetic resonance imaging of glycogen using its magnetic coupling with water. Proc. Natl. Acad. Sci. USA 2020, 117, 3144–3149. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.L.; Lanir, A.; Metz, K.R.; Kruskal, J.B.; Lee, R.G.; Balschi, J.; Federman, M.; Khettry, U.; Clouse, M.E. 23Na- and 31P-NMR studies of perfused mouse liver during nitrogen hypoxia. Am. J. Physiol. Liver Physiol. 1992, 262, G636–G644. [Google Scholar] [CrossRef]
- Kitai, T.; Tanaka, A.; Terasaki, M.; Okamoto, R.; Ozawa, K.; Morikawa, S.; Inubushi, T. Energy metabolism of the liver in brain dead dogs assessed by 31P-NMR spectroscopy and arterial ketone body ratio. Life Sci. 1991, 49, 511–518. [Google Scholar] [CrossRef]
- Buchli, R.; Meier, D.; Martin, E.; Boesiger, P. Assessment of absolute metabolite concentrations in human tissue by P-31 MRS in vivo. Part II: Muscle, liver, kidney. Magn. Reson. Med. 1994, 32, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Traussnigg, S.; Kienbacher, C.; Gajdosik, M.; Valkovič, L.; Halilbasic, E.; Stift, J.; Rechling, C.; Hofer, H.; Steindl-Munda, P.; Ferenci, P.; et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017, 37, 1544–1553. [Google Scholar] [CrossRef]
- Buehler, T.; Kreis, R.; Boesch, C. Comparison of 31P saturation and inversion magnetization transfer in human liver and skeletal muscle using a clinical MR system and surface coils. NMR Biomed. 2014, 28, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Jones, K.A.; Shulman, R.G. In vivo 31P nuclear magnetic resonance saturation transfer measurements of phosphate exchange reactions in the yeast Saccharomyces cerevisiae. FEBS Lett. 1985, 193, 189–193. [Google Scholar] [CrossRef]
- Alger, J.R.; Hollander, J.A.D.; Shulman, R.G. In vivo phosphorus-31 nuclear magnetic resonance saturation transfer studies of adenosinetriphosphatase kinetics in Saccharomyces cerevisiae. Biochemistry 1982, 21, 2957–2963. [Google Scholar] [CrossRef]
- Laufs, A.; Livingstone, R.; Nowotny, B.; Nowotny, P.; Wickrath, F.; Giani, G.; Bunke, J.; Roden, M.; Hwang, J.-H. Quantitative liver 31 P magnetic resonance spectroscopy at 3T on a clinical scanner. Magn. Reson. Med. 2013, 71, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Cohen, J.S. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992, 5, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.F.; Lyon, R.C.; Faustino, P.J.; Cohen, J.S. Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. J. Biol. Chem. 1987, 262, 14875–14878. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, X.-D.; Dong, S.; Xu, L.-C.; Xiao, X.-S. Evaluation of Early Imaging Response After Chemoembolization of Hepatocellular Carcinoma by Phosphorus-31 Magnetic Resonance Spectroscopy—Initial Experience. J. Vasc. Interv. Radiol. 2011, 22, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, S.D.; Sargentoni, J.; Bell, J.D.; Saeed, N.; Changani, K.K.; Davidson, B.R.; Rolles, K.; Burroughs, A.K.; Hodgson, H.J.F.; Foster, C.S.; et al. In vivo and in vitro hepatic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver Int. 2008, 17, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Cox, I.; Menon, D.K.; Sargentoni, J.; Bryant, D.J.; Collins, A.G.; Coutts, G.A.; Iles, R.A.; Bell, J.D.; Benjamin, I.; Gilbey, S.; et al. Phosphorus-31 magnetic resonance spectroscopy of the human liver using chemical shift imaging techniques. J. Hepatol. 1992, 14, 265–275. [Google Scholar] [CrossRef]
- Bernardo-Seisdedos, G.; Bilbao, J.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; de Juan, V.G.; Bizkarguenaga, M.; Mateos, B.; Fondevila, M.F.; Abril-Fornaguera, J.; Diercks, T.; et al. Metabolic Landscape of the Mouse Liver by Quantitative 31 P Nuclear Magnetic Resonance Analysis of the Phosphorome. Hepatology 2020, 74, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Chmelik, M.; Povazan, M.; Krššák, M.; Gruber, S.; Tkačov, M.; Trattnig, S.; Bogner, W. In vivo 31P magnetic resonance spectroscopy of the human liver at 7 T: An initial experience. NMR Biomed. 2014, 27, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Purvis, L.A.; Clarke, W.; Valkovič, L.; Levick, C.; Pavlides, M.; Barnes, E.; Cobbold, J.F.; Robson, M.D.; Rodgers, C.T. Phosphodiester content measured in human liver by in vivo 31 P MR spectroscopy at 7 tesla. Magn. Reson. Med. 2017, 78, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Valkovič, L.; Chmelík, M.; Krššák, M. In-vivo 31 P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism. Anal. Biochem. 2017, 529, 193–215. [Google Scholar] [CrossRef]
- Bierwagen, A.; Begovatz, P.; Nowotny, P.; Markgraf, D.; Nowotny, B.; Koliaki, C.; Giani, G.; Klüppelholz, B.; Lundbom, J.; Roden, M. Characterization of the peak at 2.06 ppm in 31P magnetic resonance spectroscopy of human liver: Phosphoenolpyruvate or phosphatidylcholine? NMR Biomed. 2015, 28, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Chmelík, M.; Valkovic, L.; Wolf, P.; Bogner, W.; Gajdosik, M.; Halilbasic, E.; Gruber, S.; Trauner, M.; Krebs, M.; Trattnig, S.; et al. Phosphatidylcholine contributes to in vivo 31P MRS signal from the human liver. Eur. Radiol. 2015, 25, 2059–2066. [Google Scholar] [CrossRef]
- Kupriyanova, Y.; Zaharia, O.P.; Bobrov, P.; Karusheva, Y.; Burkart, V.; Szendroedi, J.; Hwang, J.-H.; Roden, M.; Al-Hasani, H.; Buyken, A.; et al. Early changes in hepatic energy metabolism and lipid content in recent-onset type 1 and 2 diabetes mellitus. J. Hepatol. 2020, 74, 1028–1037. [Google Scholar] [CrossRef]
- Ms, L.P.; Gajdosik, M.; Wolf, P.; Smajis, S.; Fellinger, P.; Kuehne, A.; Krumpolec, P.; Trattnig, S.; Winhofer, Y.; Krebs, M.; et al. Absolute Quantification of Phosphor-Containing Metabolites in the Liver Using 31P MRSI and Hepatic Lipid Volume Correction at 7T Suggests No Dependence on Body Mass Index or Age. J. Magn. Reson. Imaging 2018, 49, 597–607. [Google Scholar] [CrossRef]
- Hakkarainen, A.; Lundbom, J.; Tuominen, E.K.; Taskinen, M.-R.; Pietiläinen, K.H.; Lundbom, N. Measuring short-term liver metabolism non-invasively: Postprandial and post-exercise 1H and 31P MR spectroscopy. Magma Magn. Reson. Mater. Phys. Biol. Med. 2014, 28, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bawden, S.; Stephenson, M.; Ciampi, E.; Hunter, K.; Marciani, L.; Macdonald, I.; Aithal, G.; Morris, P.; Gowland, P. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A 31P MRS study. Clin. Nutr. 2015, 35, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Elsing, C.; Wegmüller, H.; Felblinger, J.; Vock, P.; Reichen, J. Effect of ethanol and fructose on liver metabolism: A dynamic (31) phosphorus magnetic resonance spectroscopy study in normal volunteers. Magn. Reson. Imaging 1997, 15, 1067–1077. [Google Scholar] [CrossRef]
- Sevastianova, K.; Hakkarainen, A.; Kotronen, A.; Cornér, A.; Arkkila, P.; Arola, J.; Westerbacka, J.; Bergholm, R.; Lundbom, J.; Lundbom, N.; et al. Nonalcoholic Fatty Liver Disease: Detection of Elevated Nicotinamide Adenine Dinucleotide Phosphate with in Vivo 3.0-T31P MR Spectroscopy with Proton Decoupling. Radiology 2010, 256, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Changani, K.K. Evidence for altered hepatic gluconeogenesis in patients with cirrhosis using in vivo 31-phosphorus magnetic resonance spectroscopy. Gut 2001, 49, 557–564. [Google Scholar] [CrossRef]
- Chu, W.C.W.; Lam, W.W.M.; Lee, K.-H.; Yeung, D.K.W.; Sihoe, J.; Yeung, C.-K. Phosphorus-31 MR Spectroscopy in Pediatric Liver Transplant Recipients: A Noninvasive Assessment of Graft Status with Correlation with Liver Function Tests and Liver Biopsy. Am. J. Roentgenol. 2005, 184, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Leij-Halfwerk, S.; Agteresch, H.J.; Sijens, P.E.; Dagnelie, P.C. Adenosine triphosphate infusion increases liver energy status in advanced lung cancer patients: Anin vivo31P magnetic resonance spectroscopy study. Hepatology 2002, 35, 421–424. [Google Scholar] [CrossRef]
- Petersen, K.F.; West, A.B.; Reuben, A.; Rothman, D.L.; Shulman, G.I. Noninvasive assessment of hepatic triglyceride content in humans with 13C nuclear magnetic resonance spectroscopy. Hepatology 1996, 24, 114–117. [Google Scholar] [CrossRef]
- Stender, S.; Zaha, V.; Malloy, C.R.; Sudderth, J.; DeBerardinis, R.J.; Park, J.M. Assessment of Rapid Hepatic Glycogen Synthesis in Humans Using Dynamic 13 C Magnetic Resonance Spectroscopy. Hepatol. Commun. 2020, 4, 425–433. [Google Scholar] [CrossRef]
- Skamarauskas, J.T.; Oakley, F.; Smith, F.E.; Bawn, C.; Dunn, M.; Vidler, D.S.; Clemence, M.; Blain, P.G.; Taylor, R.; Gamcsik, M.P.; et al. Noninvasivein vivomagnetic resonance measures of glutathione synthesis in human and rat liver as an oxidative stress biomarker. Hepatology 2013, 59, 2321–2330. [Google Scholar] [CrossRef]
- Petersen, K.F.; Cline, G.W.; Gerard, D.P.; Magnusson, I.; Rothman, D.L.; Shulman, G. Contribution of net hepatic glycogen synthesis to disposal of an oral glucose load in humans. Metabolism 2001, 50, 598–601. [Google Scholar] [CrossRef]
- Befroy, D.E.; Perry, R.J.; Jain, N.; Dufour, S.; Cline, G.W.; Trimmer, J.K.; Brosnan, J.; Rothman, D.L.; Petersen, K.F.; Shulman, G.I. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat. Med. 2013, 20, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, L.; De Graaf, R.A.; Nabuurs, C.I.; Van Ewijk, P.A.; Hesselink, M.K.; Wildberger, J.E.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Quantum coherence spectroscopy to measure dietary fat retention in the liver. JCI Insight 2016, 1, e84671. [Google Scholar] [CrossRef][Green Version]
- De Graaf, R.A. Theoretical and experimental evaluation of broadband decoupling techniques for in vivo nuclear magnetic resonance spectroscopy. Magn. Reson. Med. 2005, 53, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Jones, J.; Schmid, A.I.; Barosa, C.; Lettner, A.; Kacerovsky-Bielesz, G.; Szendroedi, J.; Chmelik, M.; Nowotny, P.; Chandramouli, V.; et al. Postprandial and Fasting Hepatic Glucose Fluxes in Long-Standing Type 1 Diabetes. Diabetes 2011, 60, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Matyka, K.; Dixon, R.M.; Mohn, A.; Rajagopalan, B.; Shmueli, E.; Styles, P.; Dunger, D.B. Daytime liver glycogen accumulation, measured by C-13 magnetic resonance spectroscopy, in young children with Type 1 diabetes mellitus. Diabet. Med. 2001, 18, 659–662. [Google Scholar] [CrossRef]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.H.; Nowotny, P.; Man, C.D.; Cobelli, C.; Cline, G.W.; Shulman, G.; Waldhäusl, W.; et al. Alterations in Postprandial Hepatic Glycogen Metabolism in Type 2 Diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Sarabhai, T.; Kahl, S.; Szendroedi, J.; Markgraf, D.F.; Zaharia, O.-P.; Barosa, C.; Herder, C.; Wickrath, F.; Bobrov, P.; Hwang, J.-H.; et al. Monounsaturated fat rapidly induces hepatic gluconeogenesis and whole-body insulin resistance. JCI Insight 2020, 5, 4520. [Google Scholar] [CrossRef] [PubMed]
- Krššák, M. 13C MRS in Human Tissue. eMagRes 2016, 17, 1027–1038. [Google Scholar] [CrossRef]
- Bischof, M.G.; Bernroider, E.; Krssak, M.; Krebs, M.; Stingl, H.; Nowotny, P.; Yu, C.; Shulman, G.; Waldhäusl, W.; Roden, M. Hepatic Glycogen Metabolism in Type 1 Diabetes After Long-Term Near Normoglycemia. Diabetes 2002, 51, 49–54. [Google Scholar] [CrossRef]
- Bischof, M.G.; Krssak, M.; Krebs, M.; Bernroider, E.; Stingl, H.; Waldhäusl, W.; Roden, M. Effects of Short-Term Improvement of Insulin Treatment and Glycemia on Hepatic Glycogen Metabolism in Type 1 Diabetes. Diabetes 2001, 50, 392–398. [Google Scholar] [CrossRef]
- Hernández, E.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Jonkers, R.A.M.; Groen, A.K.; Nicolay, K.; van Loon, L.J.; Prompers, J.J. Effects of acute exercise on lipid content and dietary lipid uptake in liver and skeletal muscle of lean and diabetic rats. Am. J. Physiol. Metab. 2015, 309, E874–E883. [Google Scholar] [CrossRef] [PubMed]
- Veeraiah, P.; Brouwers, K.; Wildberger, J.E.; Schrauwen-Hinderling, V.B.; Lindeboom, L. Application of a BIlinear Rotation Decoupling (BIRD) filter in combination with J-difference editing for indirect 13 C measurements in the human liver. Magn. Reson. Med. 2020, 84, 2911–2917. [Google Scholar] [CrossRef]
- Keshari, K.; Wilson, D.M. Chemistry and biochemistry of13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2013, 43, 1627–1659. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.J.; Nakano, H.; Sugai, S.; Tomohiro, M.; Kase, Y.; Uchio, Y.; Yamaguchi, T.; Matsuo, Y.; Naganuma, T.; Takeda, N.; et al. Hyperpolarized 13 C Magnetic Resonance Imaging of Fumarate Metabolism by Parahydrogen-induced Polarization: A Proof-of-Concept in vivo Study. ChemPhysChem 2021, 22, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2018, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Thomas, M.A.; Behar, K.L.; de Graaf, R.A. NMR visibility of deuterium-labeled liver glycogen in vivo. Magn. Reson. Med. 2021, 86, 62–68. [Google Scholar] [CrossRef]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; de Graaf, R.A. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Thomas, M.A.; Behar, K.L.; De Feyter, H.M. Characterization of Kinetic Isotope Effects and Label Loss in Deuterium-Based Isotopic Labeling Studies. ACS Chem. Neurosci. 2020, 12, 234–243. [Google Scholar] [CrossRef]
- Rose, I.A.; Oconnell, E.L. Intramolecular Hydrogen Transfer in Phosphoglucose Isomerase Reaction. J. Biol. Chem. 1961, 236, 3086. [Google Scholar] [CrossRef]
- Wolf, W.; Albright, M.J.; Silver, M.S.; Weber, H.; Reichardt, U.; Sauer, R. Fluorine-19 NMR spectroscopic studies of the metabolism of 5-fluorouracil in the liver of patients undergoing chemotherapy. Magn. Reson. Imaging 1987, 5, 165–169. [Google Scholar] [CrossRef]
- Peters, G.J.; Lankelma, J.; Kok, R.M.; Noordhuis, P.; Van Groeningen, C.J.; Van Der Wilt, C.L.; Meyer, S.; Pinedo, H.M. Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother. Pharmacol. 1993, 31, 269–276. [Google Scholar] [CrossRef]
- Li, C.-W.; Gonen, O. Simultaneous 3D NMR spectroscopy of fluorine and phosphorus in human liver during 5-fluorouracil chemotherapy. Magn. Reson. Med. 1996, 35, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Boesch, J.; He, C.-W.L.L.; Padavic-Shaller, K.A.; Negendank, W.; Brown, T.R. Proton-decoupled 19F spectroscopy of 5-FU catabolites in human liver. Magn. Reson. Med. 1997, 37, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.; Waluch, V.; Presant, C.A. Non-invasive 19F-NMRS of 5-fluorouracil in pharmacokinetics and pharmacodynamic studies. NMR Biomed. 1998, 11, 380–387. [Google Scholar] [CrossRef]
- Mohankrishnan, P.; Hutchins, L.; Nauke, S.; Sprigg, J.; Cardwell, D.; Williamson, M.R.; Komoroski, R.A.; Jagannathan, N.R. Metabolism of 5-fluorouracil in human liver: An in vivo F-19 NMR study. Curr. Sci. 1999, 76, 677–680. [Google Scholar]
- Schlemmer, H.-P.; Bachert, P.; Semmler, W.; Hohenberger, P.; Schlag, P.; Lorenz, W.J.; van Kaick, G. Drug monitoring of 5-fluorouracil: In vivo 19F NMR study during 5-FU chemotherapy in patients with metastases of colorectal adenocarcinoma. Magn. Reson. Imaging 1994, 12, 497–511. [Google Scholar] [CrossRef]
- Findlay, M.P.N.; Leach, M.O.; Cunningham, D.; Collins, D.; Payne, G.S.; Glaholm, J.; Mansi, J.L.; McCready, V.R. The non-invasive monitoring of low dose, infusional 5-fluorouracil and its modulation by interferon-α using in vivo 19F magnetic resonance spectroscopy in patients with colorectal cancer: A pilot study. Ann. Oncol. 1993, 4, 597–602. [Google Scholar] [CrossRef]
- Harada, M.; Nishitani, H.; Koga, K.; Miura, I.; Kimura, A. Comparative Studies on the Metabolism of New Fluorinated Pyrimidine Drugs in the Liver by in vivo 19F Magnetic Resonance Spectroscopic Observation. Jpn. J. Cancer Res. 1993, 84, 197–202. [Google Scholar] [CrossRef]
- Klomp, D.; Van Laarhoven, H.; Scheenen, T.; Kamm, Y.; Heerschap, A. Quantitative 19F MR spectroscopy at 3 T to detect heterogeneous capecitabine metabolism in human liver. NMR Biomed. 2006, 20, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.S.; Collins, D.J.; Loynds, P.; Mould, G.; Murphy, P.S.; Dzik-Jurasz, A.S.K.; Kessar, P.; Haque, N.; Yamaguchi, M.; Atarashi, S.; et al. Quantitative assessment of the hepatic pharmacokinetics of the antimicrobial sitafloxacin in humans using in vivo 19F magnetic resonance spectroscopy. Br. J. Clin. Pharmacol. 2005, 59, 244–248. [Google Scholar] [CrossRef]
- Bilecen, D.; Schulte, A.-C.; Kaspar, A.; Küstermann, E.; Seelig, J.; von Elverfeldt, D.; Scheffler, K. Detection of the non-steroidal anti-inflammatory drug niflumic acid in humans: A combined 19F-MRSin vivoandin vitrostudy. NMR Biomed. 2003, 16, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Marco-Rius, I.; Wright, A.J.; Hu, D.-E.; Savic, D.; Miller, J.J.; Timm, K.N.; Tyler, D.; Brindle, K.M.; Comment, A. Probing hepatic metabolism of [2-13C]dihydroxyacetone in vivo with 1H-decoupled hyperpolarized 13C-MR. Magma Magn. Reson. Mater. Physics Biol. Med. 2020, 34, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Moreno, K.X.; Satapati, S.; DeBerardinis, R.J.; Burgess, S.C.; Malloy, C.; Merritt, M.E. Real-time Detection of Hepatic Gluconeogenic and Glycogenolytic States Using Hyperpolarized [2-13C]Dihydroxyacetone. J. Biol. Chem. 2014, 289, 35859–35867. [Google Scholar] [CrossRef]
- Ragavan, M.; McLeod, M.; Giacalone, A.; Merritt, M. Hyperpolarized Dihydroxyacetone Is a Sensitive Probe of Hepatic Gluconeogenic State. Metabolites 2021, 11, 441. [Google Scholar] [CrossRef]
- Merritt, M.E.; Harrison, C.; Sherry, D.; Malloy, C.; Burgess, S.C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc. Natl. Acad. Sci. USA 2011, 108, 19084–19089. [Google Scholar] [CrossRef]
- Chen, J.; Hackett, E.P.; Kovacs, Z.; Malloy, C.R.; Park, J.M. Assessment of hepatic pyruvate carboxylase activity using hyperpolarized [1-13 C]-l-lactate. Magn. Reson. Med. 2020, 85, 1175–1182. [Google Scholar] [CrossRef]
- Høyer, K.F.; Laustsen, C.; Ringgaard, S.; Qi, H.; Mariager, C.; Nielsen, T.S.; Sundekilde, U.; Treebak, J.T.; Jessen, N.; Stødkilde-Jørgensen, H. Assessment of mouse liver [1-13C]pyruvate metabolism by dynamic hyperpolarized MRS. J. Endocrinol. 2019, 242, 251–260. [Google Scholar] [CrossRef]
- Moreno, K.X.; Harrison, C.E.; Merritt, M.E.; Kovacs, Z.; Malloy, C.R.; Sherry, A.D. Hyperpolarized delta- 1-C-13 gluconolac-tone as a probe of the pentose phosphate pathway. NMR Biomed. 2017, 30, e3713. [Google Scholar] [CrossRef]
- Wibowo, A.; Park, J.M.; Liu, S.-C.; Khosla, C.; Spielman, D.M. Real-Time in Vivo Detection of H2O2 Using Hyperpolarized 13C-Thiourea. ACS Chem. Biol. 2017, 12, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Khemtong, C.; Liu, S.; Hurd, R.E.; Spielman, D.M. In vivo assessment of intracellular redox state in rat liver using hyperpolarized [1-13 C]Alanine. Magn. Reson. Med. 2017, 77, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Rardin, M.J.; He, W.; Nishida, Y.; Newman, J.C.; Carrico, C.; Danielson, S.R.; Guo, A.; Gut, P.; Sahu, A.K.; Li, B.; et al. SIRT5 Regulates the Mitochondrial Lysine Succinylome and Metabolic Networks. Cell Metab. 2013, 18, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Vreken, P.; Boer, M.E.J.D.; Wijburg, F.A.; Van Gennip, A.H.; Ijlst, L. Disorders of mitochondrial fatty acyl-CoA β-oxidation. J. Inherit. Metab. Dis. 1999, 22, 442–487. [Google Scholar] [CrossRef] [PubMed]
- Von Morze, C.; Engelbach, J.A.; Reed, G.D.; Chen, A.P.; Quirk, J.D.; Blazey, T.; Mahar, R.; Malloy, C.R.; Garbow, J.R.; Merritt, M.E. 15 N-carnitine, a novel endogenous hyperpolarized MRI probe with long signal lifetime. Magn. Reson. Med. 2020, 85, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Von Morze, C.; Allu, P.K.R.; Chang, G.Y.; Marco-Rius, I.; Milshteyn, E.; Wang, Z.J.; Ohliger, M.A.; Gleason, C.E.; Kurhanewicz, J.; Vigneron, D.B.; et al. Non-invasive detection of divergent metabolic signals in insulin deficiency vs. insulin resistance in vivo. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [CrossRef] [PubMed]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Serra, S.C.; Miragoli, L.; Karlsson, M.; Cabella, C.; Poggi, L.; Venturi, L.; Tedoldi, F.; Lerche, M.H. Hyperpolarized [1,3-13C2]ethyl acetoacetate is a novel diagnostic metabolic marker of liver cancer. Int. J. Cancer 2014, 136, E117–E126. [Google Scholar] [CrossRef] [PubMed]
- Maptue, N.; Jiang, W.; Harrison, C.; Funk, A.M.; Sharma, G.; Malloy, C.R.; Sherry, D.; Khemtong, C. Esterase-Catalyzed Production of Hyperpolarized 13C-Enriched Carbon Dioxide in Tissues for Measuring pH. ACS Sens. 2018, 3, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Perkons, N.R.; Kiefer, R.M.; Noji, M.C.; Pourfathi, M.; Ackerman, D.; Siddiqui, S.; Tischfield, D.; Profka, E.; Johnson, O.; Pickup, S.; et al. Hyperpolarized Metabolic Imaging Detects Latent Hepatocellular Carcinoma Domains Surviving Locoregional Therapy. Hepatology 2019, 72, 140–154. [Google Scholar] [CrossRef] [PubMed]
| Study Description | Main Findings | Field Strength (T) | Reference |
|---|---|---|---|
| Effects of a lipid-rich breakfast meal followed by exercise on hepatic ATP and lipid levels for healthy subjects. | Liver fat increased postprandially and continued to increase during exercise. Liver ATP did not change from fasting to postprandial state, but significantly decreased after exercise. | 3.0 | [51] |
| Effect of a oral fructose challenge on hepatic ATP reserves in healthy subjects. Baseline liver glycogen was also measured by 13C NMR | Hepatic ATP levels dropped by ~20% from baseline and reached a minimum value 50 min after the load. The time to reach minimum ATP levels was inversely correlated with subject BMI. ATP recovery rate was inversely correlated with baseline glycogen levels. | 3.0 | [52] |
| Effects of acute fructose ingestion with and without an accompanying load of ethanol on liver P-metabolite dynamics in healthy subjects. | Over a 40 min interval post load, P-metabolites were measured with 5 min time resolution. While ethanol had no effects on rates of phosphomonester (PME) formation and ATP depletion resulting from fructose metabolism, it significantly slowed down the rate of PME degradation. | 1.5 | [53] |
| Characterization of P-metabolites and ATP fluxes and correlation with lipid levels determined by 1H MR and biopsy evaluation in subjects with NAFLD and NASH | Several PME and PDE 31P signals were resolved and quantified as well as those from NADPH and UDPG. Significant differences in relative abundances of PME phosphoethanolamine (PE) and ATP between NAFLD and NASH. Significantly lower rates of ATP synthesis fluxes in NASH compared to NAFLD subjects [33]. In another 31P MRS study performed at 3 T, levels of NADPH, a marker of inflammation and fibrosis, were elevated in NASH patients compared to healthy controls [54]. | 7.0, 3.0 (31P) 3.0 (1H) | [33,54] |
| Characterization of PME profile in fasted subjects with compensated and decompensated cirrhosis following infusion with a gluconeogenic substrate—L-alanine. | At baseline, PME levels of both compensated and decompensated cirrhotic subjects were elevated compared to healthy controls. After L-alanine infusion, PME levels of healthy controls were significantly increased, consistent with gluconeogenic activity. This increase was significantly smaller for patients with compensated cirrhosis and was absent in patients with decompensated cirrhosis. | 1.5 | [55] |
| Characterization of P-metabolites in pediatric liver transplant patients with different outcomes of graft function | Patients with impaired graft function had elevated PME/total phosphate compared to those with good graft function and to healthy controls. | 1.5 | [56] |
| Effects of intravenous ATP infusion for 22–24 h on liver energy status in advanced lung cancer patients. | Liver ATP levels were significantly increased following ATP infusion to levels that were similar to those of healthy subjects. This effect was greatest for patients that were undergoing weight loss and who had the lowest baseline ATP liver levels | 1.5 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, J.G. Non-Invasive Analysis of Human Liver Metabolism by Magnetic Resonance Spectroscopy. Metabolites 2021, 11, 751. https://doi.org/10.3390/metabo11110751
Jones JG. Non-Invasive Analysis of Human Liver Metabolism by Magnetic Resonance Spectroscopy. Metabolites. 2021; 11(11):751. https://doi.org/10.3390/metabo11110751
Chicago/Turabian StyleJones, John G. 2021. "Non-Invasive Analysis of Human Liver Metabolism by Magnetic Resonance Spectroscopy" Metabolites 11, no. 11: 751. https://doi.org/10.3390/metabo11110751
APA StyleJones, J. G. (2021). Non-Invasive Analysis of Human Liver Metabolism by Magnetic Resonance Spectroscopy. Metabolites, 11(11), 751. https://doi.org/10.3390/metabo11110751






