Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis
Abstract
1. Introduction
2. Results
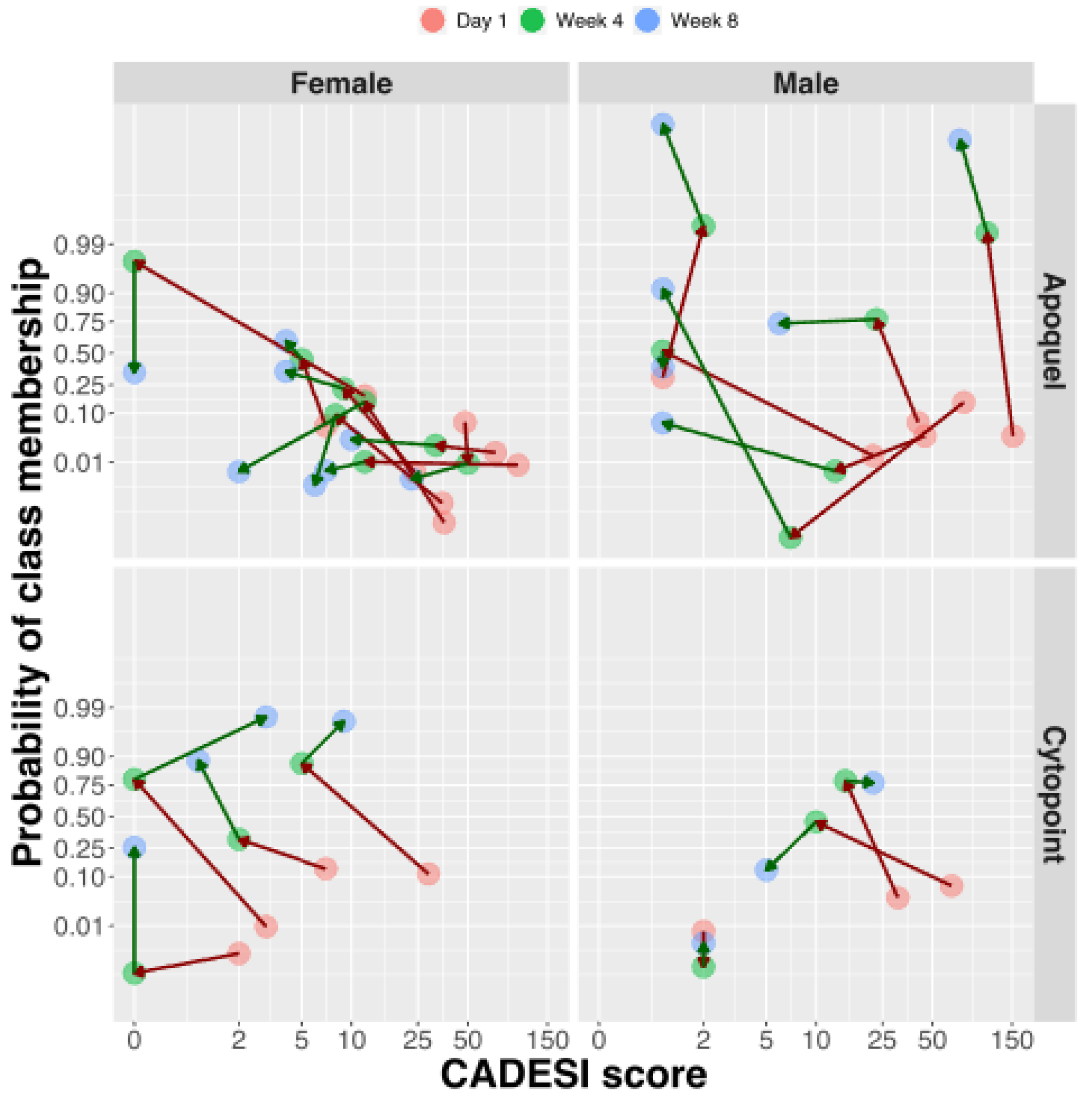
2.1. Dogs and Clinical Response to Treatment
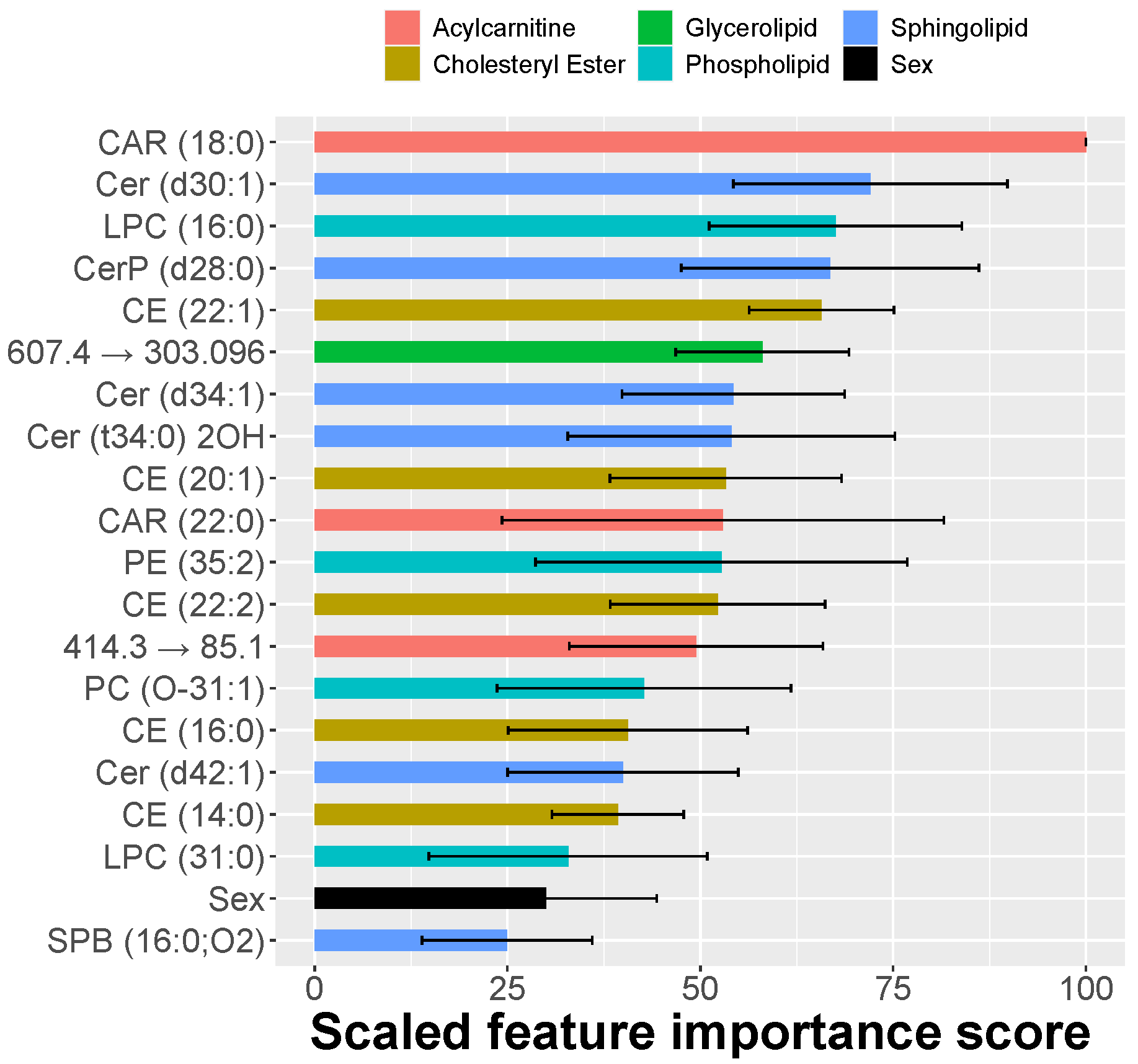
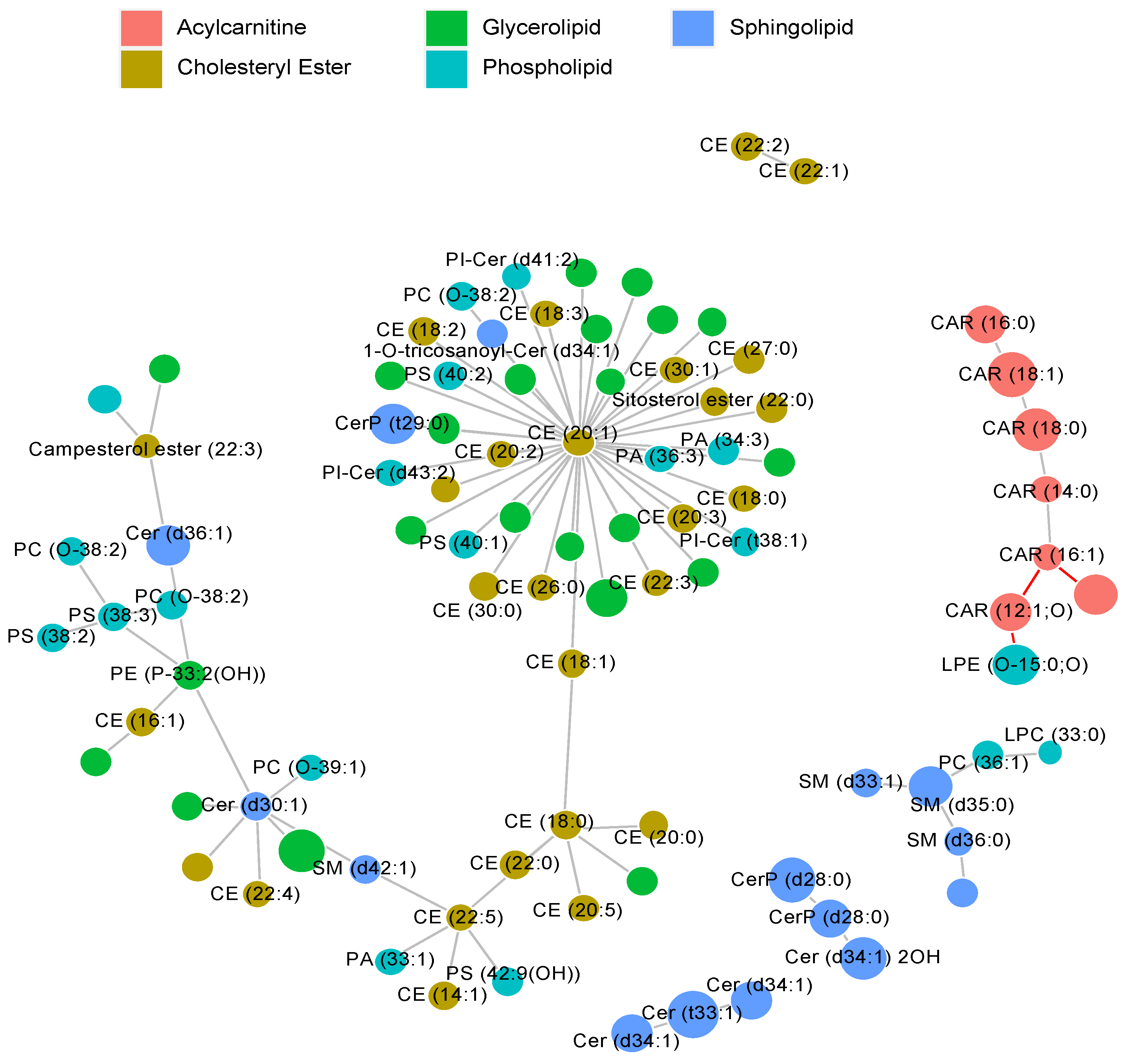
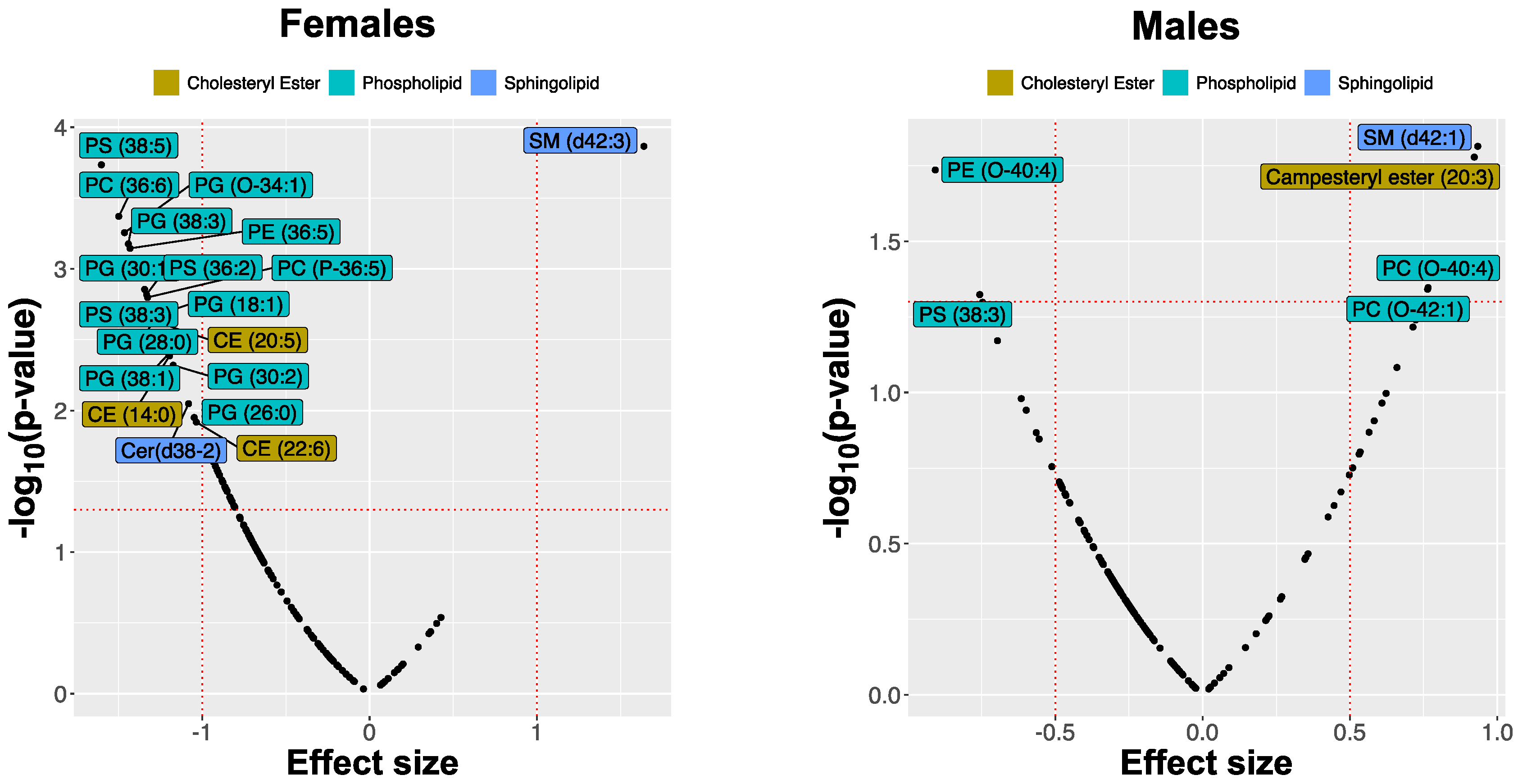
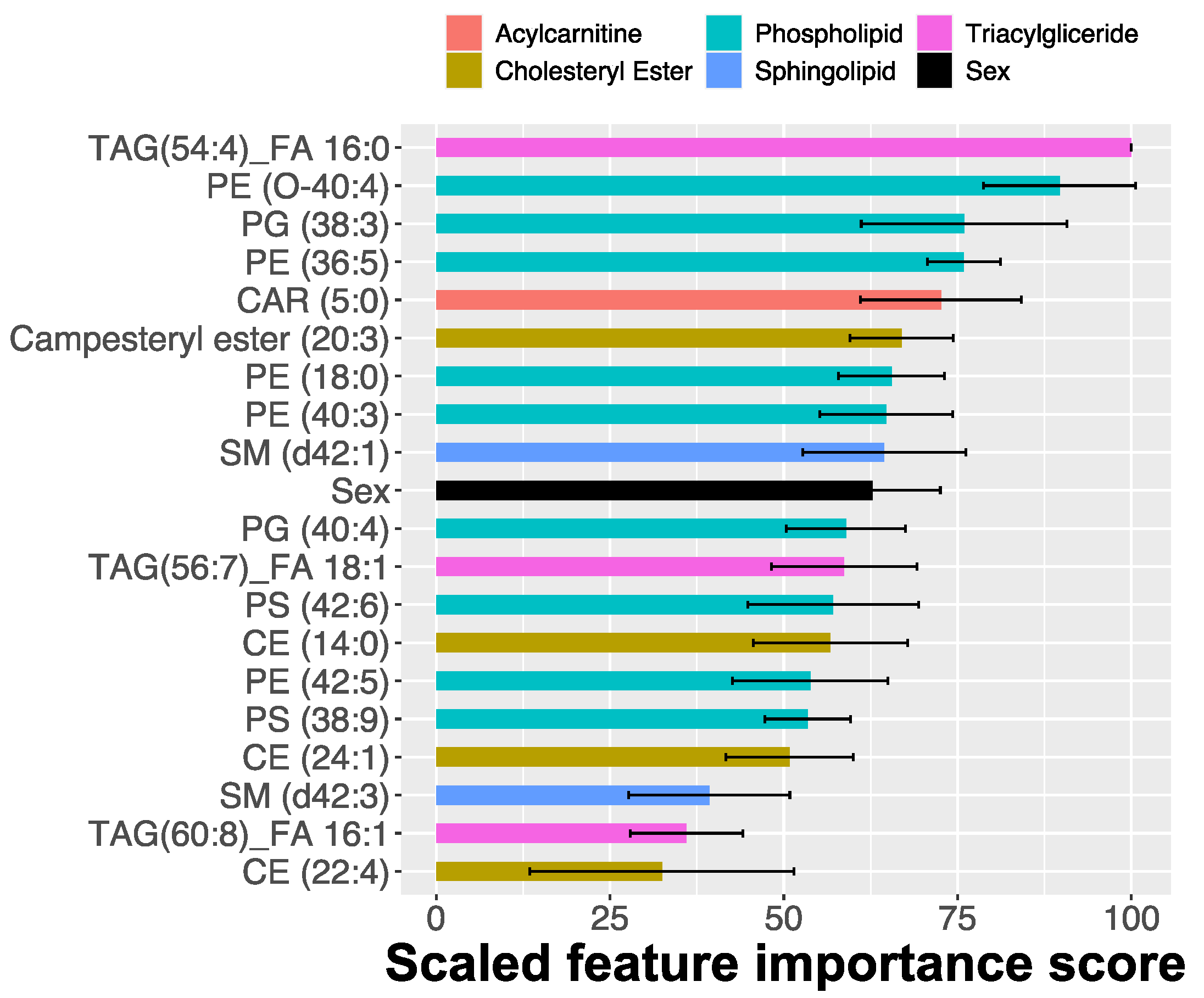
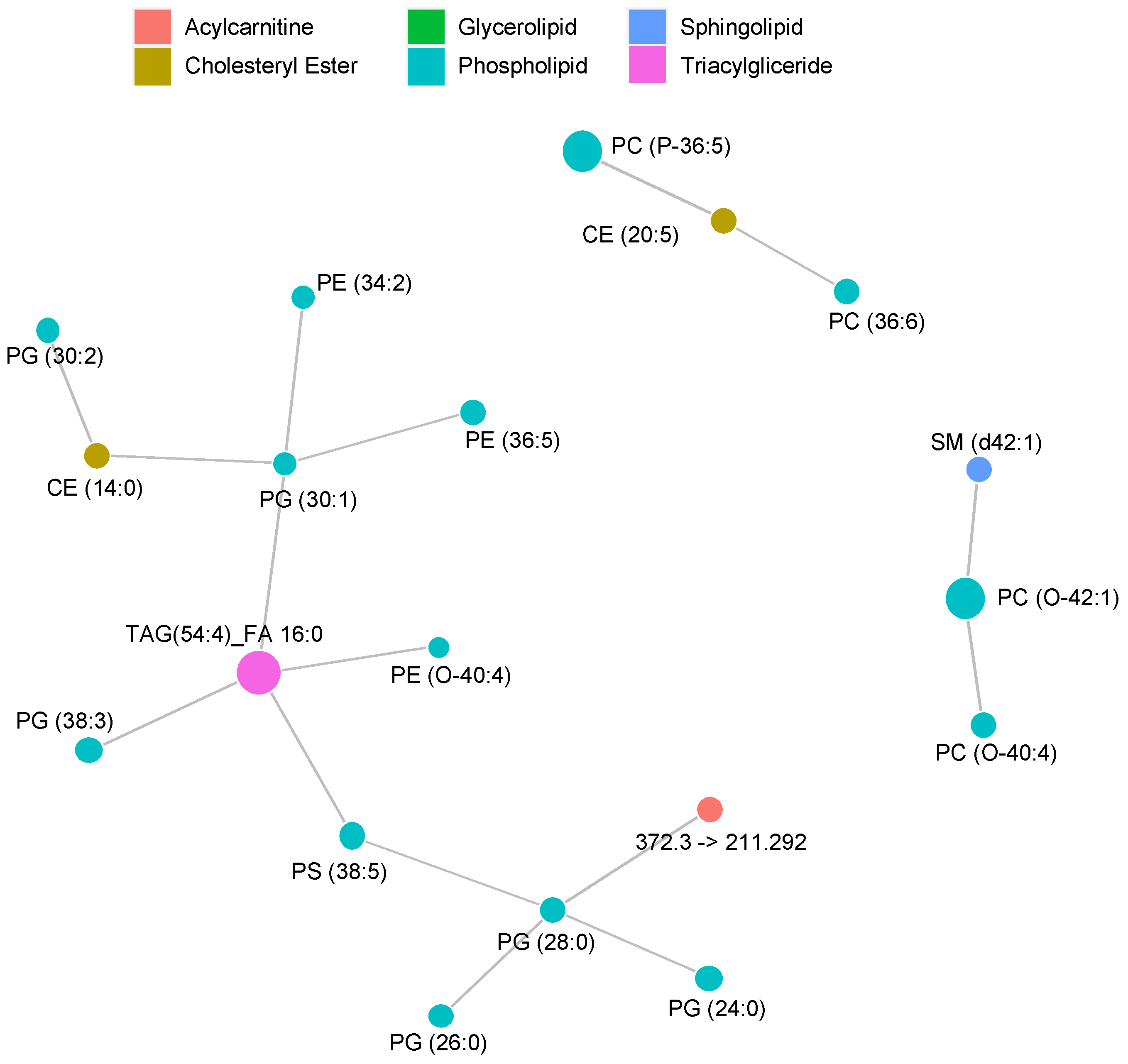
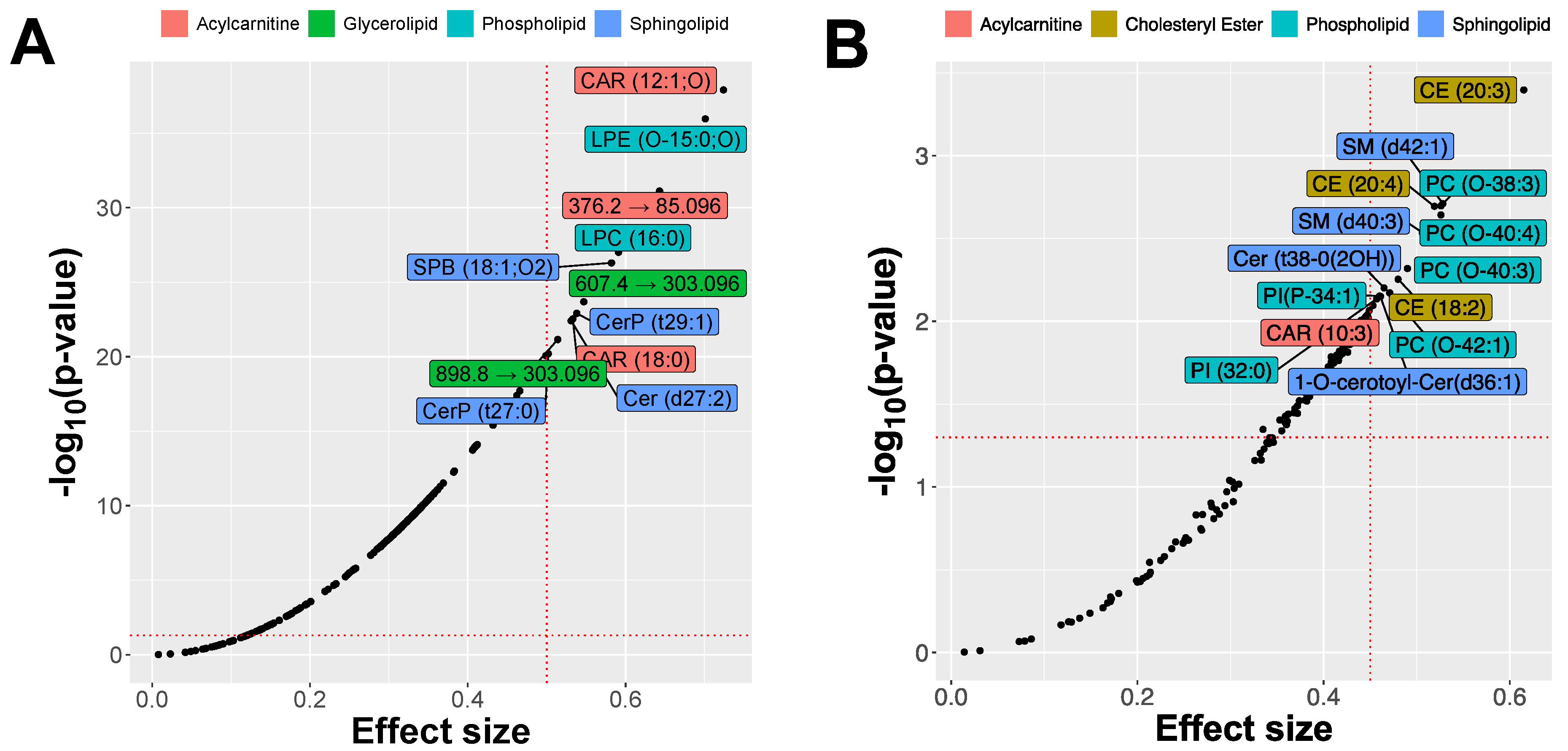
2.2. MRM Profiling of Skin Samples
2.3. MRM Profiling of Blood Samples
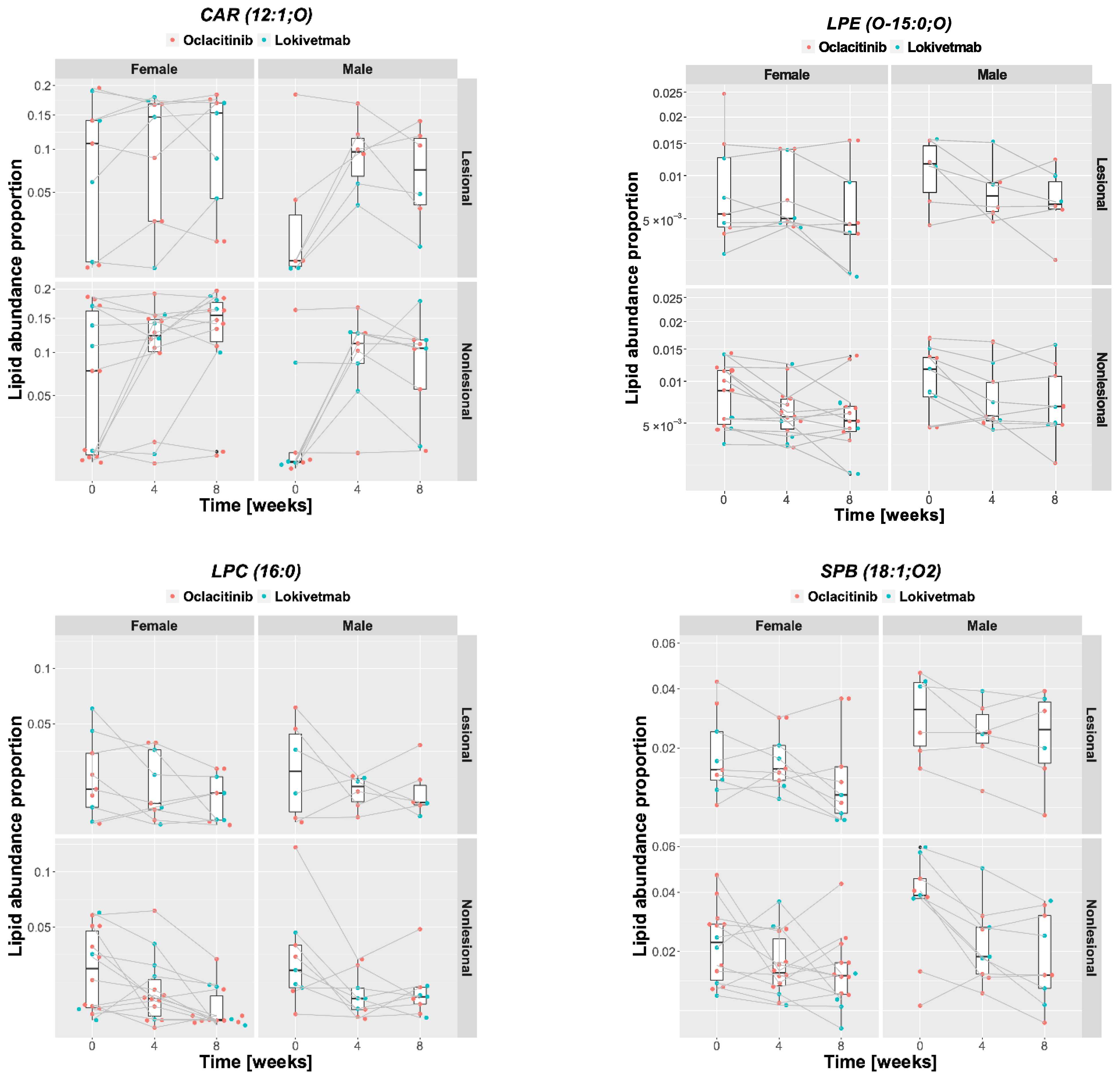
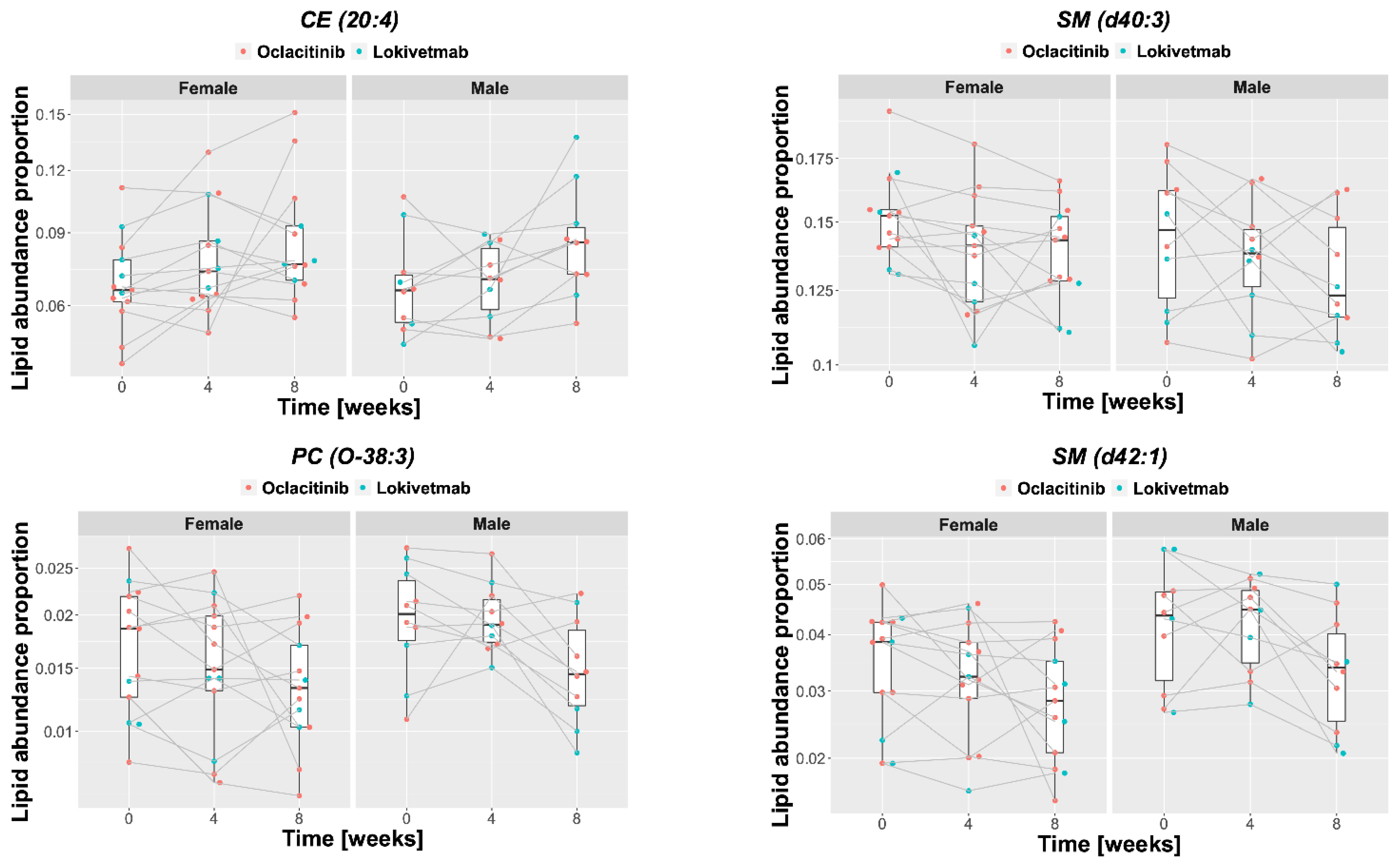
2.4. Lipid Changes during Treatment
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Extraction of Skin Samples
4.3. Extraction of DBS Samples
4.4. MRM Profiling
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Chiesa Fuxench, Z.C.; Block, J.K.; Boguniewicz, M.; Boyle, J.; Fonacier, L.; Gelfand, J.M.; Grayson, M.H.; Margolis, D.J.; Mitchell, L.; Silverberg, J.I.; et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J. Investig. Dermatol. 2019, 139, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Linek, M.; Favrot, C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Noli, C. Assessing quality of life for pets with dermatologic disease and their owners. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 83–93. [Google Scholar] [CrossRef]
- DeBoer, D.J.; Hillier, A. The ACVD task force on canine atopic dermatitis (XV): Fundamental concepts in clinical diagnosis. Vet. Immunol. Immunopathol. 2001, 81, 271–276. [Google Scholar] [CrossRef]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef]
- Marsella, R.; Girolomoni, G. Canine models of atopic dermatitis: A useful tool with untapped potential. J. Investig. Dermatol. 2009, 129, 2351–2357. [Google Scholar] [CrossRef]
- Martel, B.C.; Lovato, P.; Baumer, W.; Olivry, T. Translational animal models of atopic dermatitis for preclinical studies. Yale J. Biol. Med. 2017, 90, 389–402. [Google Scholar]
- Marsella, R.; Olivry, T.; Carlotti, D.N. International task force on canine atopic, D. Current evidence of skin barrier dysfunction in human and canine atopic dermatitis. Vet. Dermatol. 2011, 22, 239–248. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochimica Biophys. Acta 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochimica Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef]
- Fischer, C.L.; Blanchette, D.R.; Brogden, K.A.; Dawson, D.V.; Drake, D.R.; Hill, J.R.; Wertz, P.W. The roles of cutaneous lipids in host defense. Biochimica Biophys. Acta 2014, 1841, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yoon, J.S.; Yoshihara, T.; Iwasaki, T.; Nishifuji, K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet. Dermatol. 2009, 20, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Nishifuji, K.; Sasaki, A.; Ide, K.; Ishikawa, J.; Yoshihara, T.; Iwasaki, T. Alteration of stratum corneum ceramide profiles in spontaneous canine model of atopic dermatitis. Exp. Dermatol. 2011, 20, 732–736. [Google Scholar] [CrossRef]
- Popa, I.; Remoue, N.; Osta, B.; Pin, D.; Gatto, H.; Haftek, M.; Portoukalian, J. The lipid alterations in the stratum corneum of dogs with atopic dermatitis are alleviated by topical application of a sphingolipid-containing emulsion. Clin. Exp. Dermatol. 2012, 37, 665–671. [Google Scholar] [CrossRef]
- Angelbeck-Schulze, M.; Mischke, R.; Rohn, K.; Hewicker-Trautwein, M.; Naim, H.Y.; Baumer, W. Canine epidermal lipid sampling by skin scrub revealed variations between different body sites and normal and atopic dogs. BMC Vet. Res. 2014, 10, 152. [Google Scholar] [CrossRef]
- Chermprapai, S.; Broere, F.; Gooris, G.; Schlotter, Y.M.; Rutten, V.; Bouwstra, J.A. Altered lipid properties of the stratum corneum in Canine Atopic Dermatitis. Biochimica Biophys. Acta Biomembr. 2018, 1860, 526–533. [Google Scholar] [CrossRef]
- Thijs, J.L.; Strickland, I.; Bruijnzeel-Koomen, C.; Nierkens, S.; Giovannone, B.; Csomor, E.; Sellman, B.R.; Mustelin, T.; Sleeman, M.A.; de Bruin-Weller, M.S.; et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J. Allergy Clin. Immunol. 2017, 140, 730–737. [Google Scholar] [CrossRef]
- Marsella, R.; De Benedetto, A. Atopic dermatitis in animals and people: An update and comparative review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef]
- Brement, T.; Laly, M.J.; Combarros, D.; Guillemaille, D.; Bourdeau, P.J.; Bruet, V. Reliability of different sets of criteria in diagnosing canine atopic dermatitis applied to a population of 250 dogs seen in a veterinary teaching hospital. Vet. Dermatol. 2019, 30, 188.e59. [Google Scholar] [CrossRef]
- Kendall, A.C.; Koszyczarek, M.M.; Jones, E.A.; Hart, P.J.; Towers, M.; Griffiths, C.E.M.; Morris, M.; Nicolaou, A. Lipidomics for translational skin research: A primer for the uninitiated. Exp. Dermatol. 2018, 27, 721–728. [Google Scholar] [CrossRef]
- Franco, J.; Ferreira, C.; Paschoal Sobreira, T.J.; Sundberg, J.P.; HogenEsch, H. Profiling of epidermal lipids in a mouse model of dermatitis: Identification of potential biomarkers. PLoS ONE 2018, 13, e0196595. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Rajwa, B.; Ferreira, C.R.; Sundberg, J.P.; HogenEsch, H. Lipidomic profiling of the epidermis in a mouse model of dermatitis reveals sexual dimorphism and changes in lipid composition before the onset of clinical disease. Metabolites 2020, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Brunner, P.M.; Silverberg, J.I.; Guttman-Yassky, E.; Paller, A.S.; Kabashima, K.; Amagai, M.; Luger, T.A.; Deleuran, M.; Werfel, T.; Eyerich, K.; et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J. Investig. Dermatol. 2017, 137, 18–25. [Google Scholar] [CrossRef]
- Oliveira, C.; Torres, T. More than skin deep: The systemic nature of atopic dermatitis. Eur. J. Dermatol. 2019, 29, 250–258. [Google Scholar] [CrossRef]
- Little, P.R.; King, V.L.; Davis, K.R.; Cosgrove, S.B.; Stegemann, M.R. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet. Dermatol. 2015, 26, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Moyaert, H.; Van Brussel, L.; Borowski, S.; Escalada, M.; Mahabir, S.P.; Walters, R.R.; Stegemann, M.R. A blinded, randomized clinical trial evaluating the efficacy and safety of lokivetmab compared to ciclosporin in client-owned dogs with atopic dermatitis. Vet. Dermatol. 2017, 28, 593.e145. [Google Scholar] [CrossRef]
- Szczepanik, M.; Wilkolek, P.; Golynski, M.; Sitkowski, W.; Taszkun, I.; Toczek, W. The influence of treatment with lokivetmab on transepidermal water loss (TEWL) in dogs with spontaneously occurring atopic dermatitis. Vet. Dermatol. 2019, 30, 330–393. [Google Scholar] [CrossRef]
- Marsella, R.; Ahrens, K.; Wilkes, R.; Trujillo, A.; Dorr, M. Comparison of various treatment options for canine atopic dermatitis: A blinded, randomized, controlled study in a colony of research atopic beagle dogs. Vet. Dermatol. 2020, 31, 284.e269. [Google Scholar] [CrossRef]
- Angelbeck-Schulze, M.; Stahl, J.; Brodesser, S.; Rohn, K.; Naim, H.; Hewicker-Trautwein, M.; Kietzmann, M.; Baumer, W.; Mischke, R. Comparison of three different sampling methods for canine skin lipids. Vet. Dermatol. 2013, 24, 233–251. [Google Scholar] [CrossRef]
- Amat, F.; Soria, A.; Tallon, P.; Bourgoin-Heck, M.; Lambert, N.; Deschildre, A.; Just, J. New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 919–934. [Google Scholar] [CrossRef]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef]
- Rosario, C.S.; Cardozo, C.A.; Neto, H.J.C.; Filho, N.A.R. Do gender and puberty influence allergic diseases? Allergol. Immunopathol. 2021, 49, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.; Einhorn, L.; Panakova, L. Gender aspects in allergies of pets—A secondary publication and update. World Allergy Organ. J. 2017, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Anturaniemi, J.; Uusitalo, L.; Hielm-Bjorkman, A. Environmental and phenotype-related risk factors for owner-reported allergic/atopic skin symptoms and for canine atopic dermatitis verified by veterinarian in a Finnish dog population. PLoS ONE 2017, 12, e0178771. [Google Scholar] [CrossRef]
- Reiter, L.V.; Torres, S.M.; Wertz, P.W. Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet. Dermatol. 2009, 20, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Inman, A.O.; Olivry, T.; Dunston, S.M.; Monteiro-Riviere, N.A.; Gatto, H. Electron microscopic observations of stratum corneum intercellular lipids in normal and atopic dogs. Vet. Pathol. 2001, 38, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Pin, D.; Remoue, N.; Osta, B.; Callejon, S.; Videmont, E.; Gatto, H.; Portoukalian, J.; Haftek, M. Analysis of epidermal lipids in normal and atopic dogs, before and after administration of an oral omega-6/omega-3 fatty acid feed supplement. A pilot study. Vet. Res. Commun. 2011, 35, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J. Dermatol. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Ottas, A.; Fishman, D.; Okas, T.L.; Pussa, T.; Toomik, P.; Martson, A.; Kingo, K.; Soomets, U. Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PLoS ONE 2017, 12, e0188580. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines—Old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, G.; Liu, X.; Shao, Y.; Gao, P.; Xin, C.; Cui, Z.; Zhao, X.; Xu, G. Serum metabolomics study and eicosanoid analysis of childhood atopic dermatitis based on liquid chromatography-mass spectrometry. J. Proteome Res. 2014, 13, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Blakeborough, L.; Harries, M.; Haslam, I.S. Cholesterol homeostasis: Links to hair follicle biology and hair disorders. Exp. Dermatol. 2020, 29, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N.; Sato, W.J.; Kelly, A.; Ganguli-Indra, G.; Indra, A.K. Epidermal Lipids: Key Mediators of Atopic Dermatitis Pathogenesis. Trends Mol. Med. 2019, 25, 551–562. [Google Scholar] [CrossRef]
- Piotto, S.; Trapani, A.; Bianchino, E.; Ibarguren, M.; Lopez, D.J.; Busquets, X.; Concilio, S. The effect of hydroxylated fatty acid-containing phospholipids in the remodeling of lipid membranes. Biochimica Biophys. Acta 2014, 1838, 1509–1517. [Google Scholar] [CrossRef]
- Catala, A. Lipid peroxidation modifies the assembly of biological membranes “The Lipid Whisker Model”. Front. Physiol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Serbulea, V.; DeWeese, D.; Leitinger, N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free Radic. Biol. Med. 2017, 111, 156–168. [Google Scholar] [CrossRef]
- Schauberger, E.; Peinhaupt, M.; Cazares, T.; Lindsley, A.W. Lipid mediators of allergic disease: Pathways, treatments, and emerging therapeutic targets. Curr. Allergy Asthma Rep. 2016, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R. Advances in our understanding of canine atopic dermatitis. Vet. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Saevik, B.K.; Thoresen, S.I.; Taugbol, O. Fatty acid composition of serum lipids in atopic and healthy dogs. Res. Vet. Sci. 2002, 73, 153–158. [Google Scholar] [CrossRef]
- Fuhrmann, H.; Zimmermann, A.; Guck, T.; Oechtering, G. Erythrocyte and plasma fatty acid patterns in dogs with atopic dermatitis and healthy dogs in the same household. Can. J. Vet. Res. 2006, 70, 191–196. [Google Scholar] [PubMed]
- Zhou, K.; Blom, T. Trafficking and functions of bioactive sphingolipids: Lessons from cells and model membranes. Lipid Insights 2015, 8, 11–20. [Google Scholar] [CrossRef]
- Futamura, M.; Leshem, Y.A.; Thomas, K.S.; Nankervis, H.; Williams, H.C.; Simpson, E.L. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: Many options, no standards. J. Am. Acad. Dermatol. 2016, 74, 288–294. [Google Scholar] [CrossRef]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017, 177, 1316–1321. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Fonacier, L.; Boguniewicz, M.; Schwartz, L.B.; Simpson, E.; Grayson, M.H.; Ong, P.Y.; Fuxench, Z.C.C. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann. Allergy Asthma Immunol. 2018, 121, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.L.; de Bruin-Weller, M.S.; Hijnen, D. Current and future biomarkers in atopic dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.L.; Nierkens, S.; Herath, A.; Bruijnzeel-Koomen, C.A.; Knol, E.F.; Giovannone, B.; de Bruin-Weller, M.S.; Hijnen, D. A panel of biomarkers for disease severity in atopic dermatitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 698–701. [Google Scholar] [CrossRef]
- Willemse, T. Atopic skin disease: A review and a reconsideration of diagnostic criteria. J. Small An. Pract. 1986, 27, 771–778. [Google Scholar] [CrossRef]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B.; International Committe on Allergic Diseases of Animals (ICADA). Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Dermatol. 2014, 25, 77–85. [Google Scholar] [CrossRef]
- Plant, J.D.; Gortel, K.; Kovalik, M.; Polissar, N.L.; Neradilek, M.B. Development and validation of the Canine Atopic Dermatitis Lesion Index, a scale for the rapid scoring of lesion severity in canine atopic dermatitis. Vet. Dermatol. 2012, 23, 515.e103. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Singh, S.K.; Kumari, P.; Kanwal, S.; Soman, S.P.; Choudhury, S.; Garg, S.K. Alterations in circulating concentrations of IL-17, IL-31 and total IgE in dogs with atopic dermatitis. Vet. Dermatol. 2019, 30, 383.e114. [Google Scholar] [CrossRef] [PubMed]
- Asahina, R.; Ueda, K.; Oshima, Y.; Kanei, T.; Kato, M.; Furue, M.; Tsukui, T.; Nagata, M.; Maeda, S. Serum canine thymus and activation-regulated chemokine (TARC/CCL17) concentrations correlate with disease severity and therapeutic responses in dogs with atopic dermatitis. Vet. Dermatol. 2020, 31, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Fischer, N.; Rostaher, A.; Olivry, T. Evaluation of plasma C-reactive protein as a biomarker in dogs with atopic -dermatitis receiving allergen-specific immunotherapy: A pilot study. Schweiz Arch Tierheilkd 2021, 163, 67–72. [Google Scholar] [CrossRef]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Jiang, Y.J. The mechanisms by which lipids coordinately regulate the formation of the protein and lipid domains of the stratum corneum: Role of fatty acids, oxysterols, cholesterol sulfate and ceramides as signaling molecules. Dermatoendocrinology 2011, 3, 113–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yin, C.; Wang, S.; Xiao, Y. JAK-STAT in lipid metabolism of adipocytes. JAKSTAT 2013, 2, e27203. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; Stephens, J.M. The role of JAK-STAT signaling in adipose tissue function. Biochimica Biophys. Acta 2014, 1842, 431–439. [Google Scholar] [CrossRef]
- Zajac, M.; Szczepanik, M.P.; Wilkolek, P.M.; Adamek, L.R.; Pomorski, Z.J.; Sitkowski, W.; Golynski, M.G. Assessment of the relationship between transepidermal water loss (TEWL) and severity of clinical signs (CADESI-03) in atopic dogs. Vet. Dermatol. 2014, 25, 503–506. [Google Scholar] [CrossRef]
- Holm, E.A.; Wulf, H.C.; Thomassen, L.; Jemec, G.B. Instrumental assessment of atopic eczema: Validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J. Am. Acad. Dermatol. 2006, 55, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Cornegliani, L.; Vercelli, A.; Sala, E.; Marsella, R. Transepidermal water loss in healthy and atopic dogs, treated and untreated: A comparative preliminary study. Vet. Dermatol. 2012, 23, 41–44. [Google Scholar] [CrossRef]
- Aitchison, J. Principal component analysis of compositional data. Biometrika 1983, 70, 57–65. [Google Scholar] [CrossRef]
- Quinn, T.P.; Erb, I.; Gloor, G.; Notredame, C.; Richardson, M.F.; Crowley, T.M. A field guide for the compositional analysis of any-omics data. GigaScience 2019, 8, giz107. [Google Scholar] [CrossRef] [PubMed]
- Tsagris, M.; Preston, S.; Wood, A.T.A. Improved classification for compositional data using the α-transformation. J. Classif. 2016, 33, 243–261. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
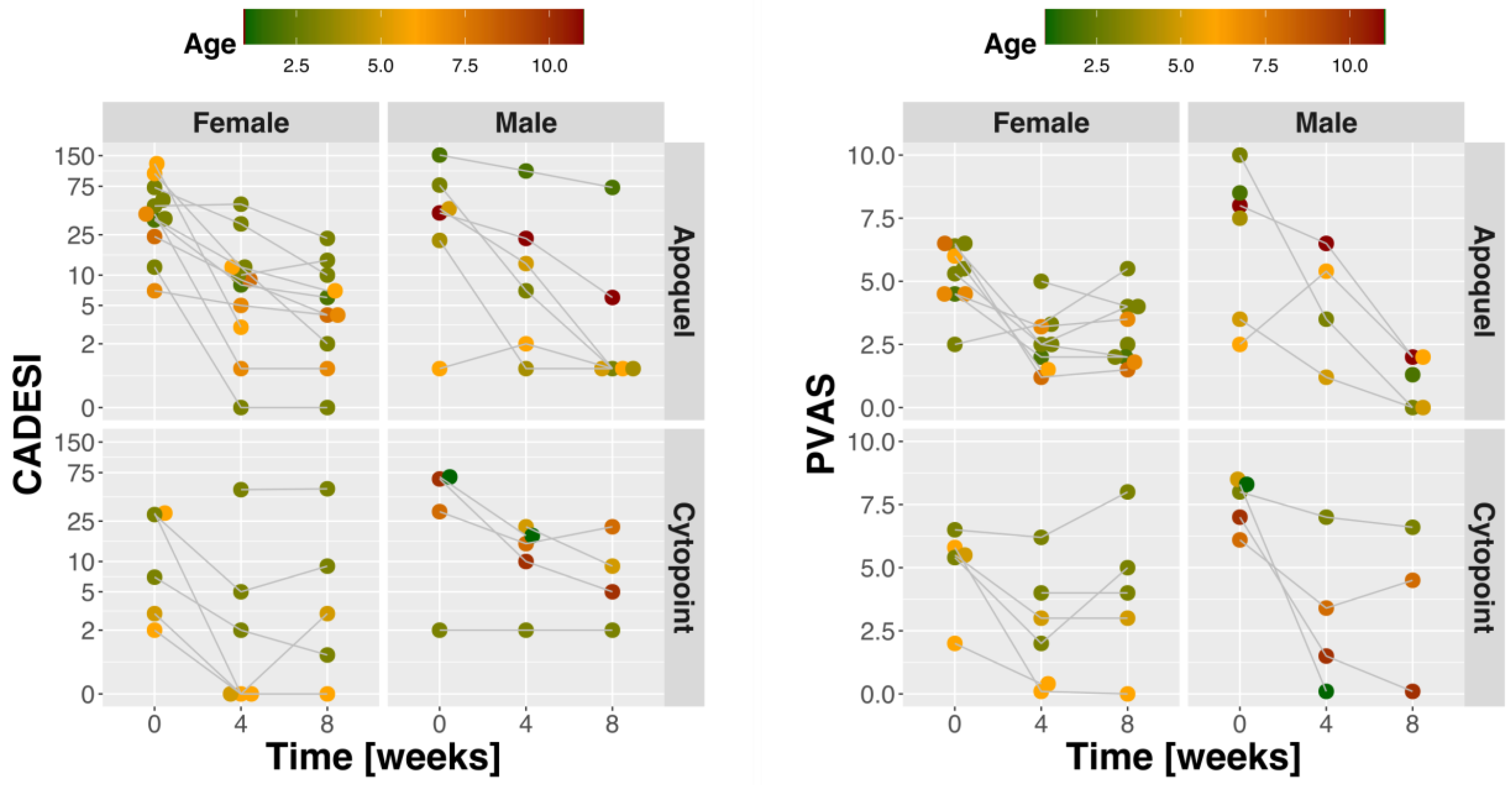
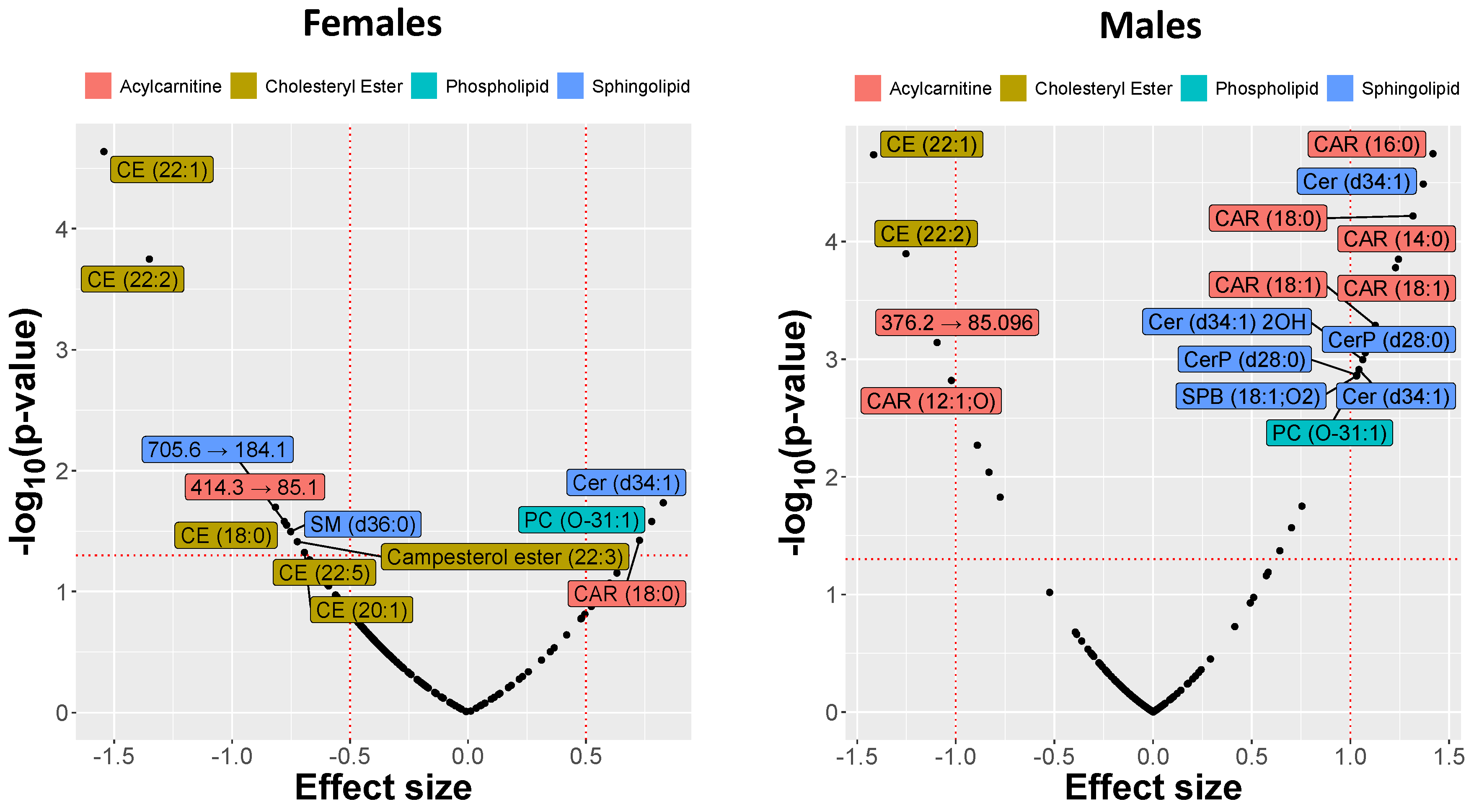
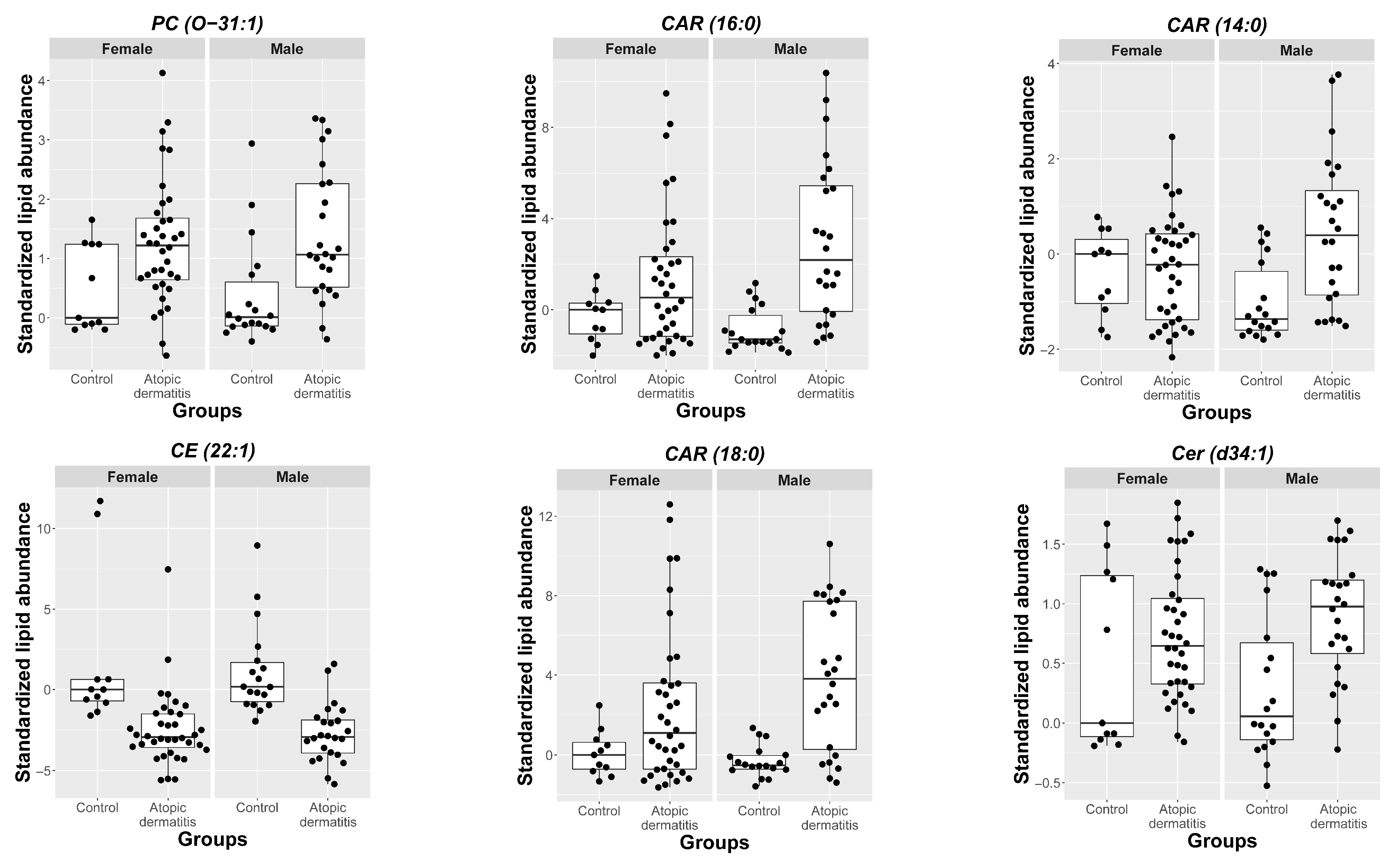
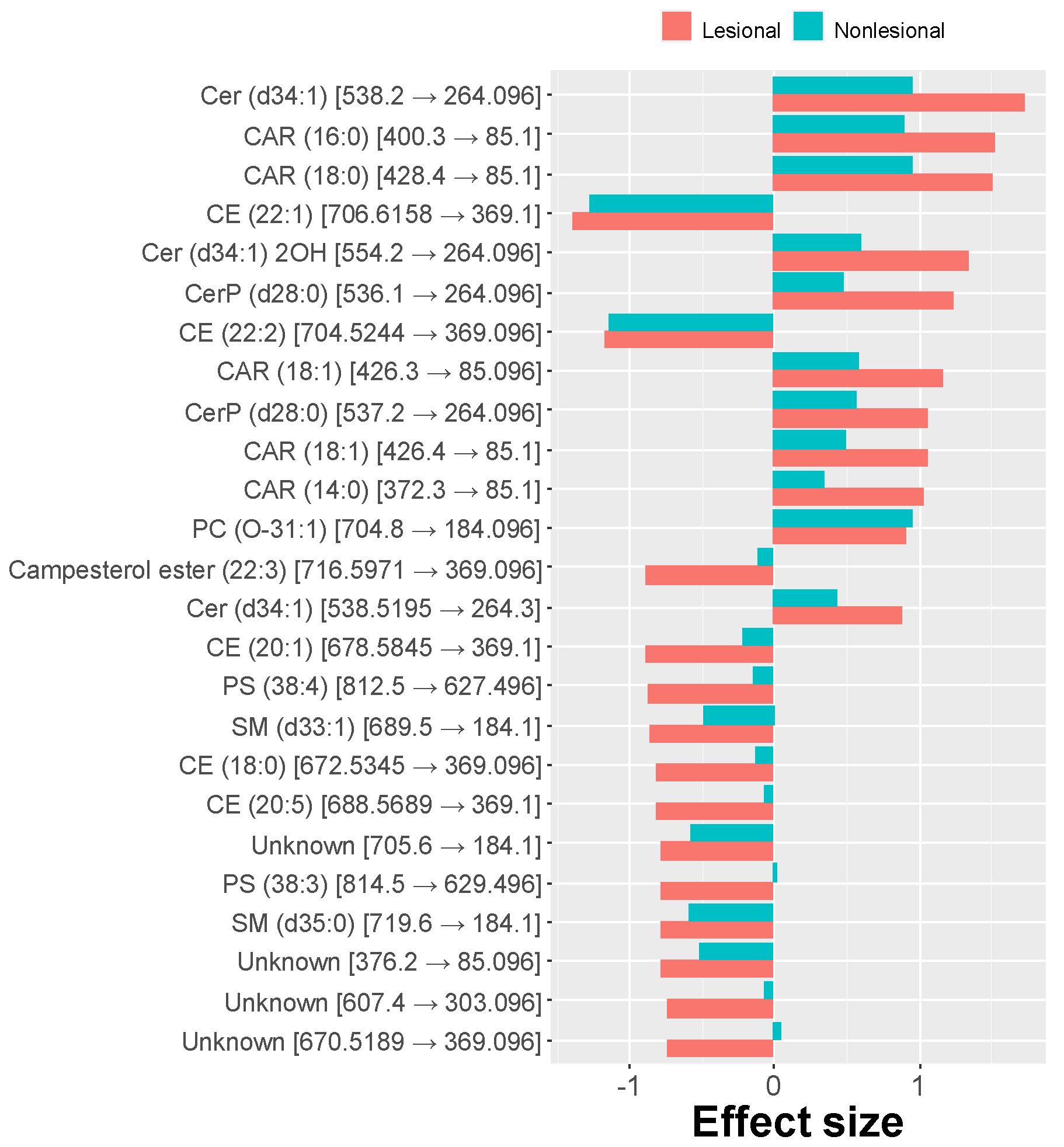
| Status | Sex | Mean Age | SD | Median Age | MAD |
|---|---|---|---|---|---|
| Atopic | Female | 4.63 | 1.93 | 4.00 | 2.22 |
| Male | 5.27 | 3.23 | 5.00 | 2.97 | |
| Healthy | Female | 6.60 | 2.99 | 5.50 | 2.22 |
| Male | 6.37 | 3.40 | 5.00 | 1.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, J.; Rajwa, B.; Gomes, P.; HogenEsch, H. Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis. Metabolites 2021, 11, 670. https://doi.org/10.3390/metabo11100670
Franco J, Rajwa B, Gomes P, HogenEsch H. Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis. Metabolites. 2021; 11(10):670. https://doi.org/10.3390/metabo11100670
Chicago/Turabian StyleFranco, Jackeline, Bartek Rajwa, Paulo Gomes, and Harm HogenEsch. 2021. "Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis" Metabolites 11, no. 10: 670. https://doi.org/10.3390/metabo11100670
APA StyleFranco, J., Rajwa, B., Gomes, P., & HogenEsch, H. (2021). Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis. Metabolites, 11(10), 670. https://doi.org/10.3390/metabo11100670








