Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways
Abstract
1. Introduction
2. Fatty Acids and Lipid Supply for Membrane Formation in Cancer
2.1. De Novo Synthesis of Saturated and Monounsaturated Fatty Acids
2.2. PUFA Intake and Omega-6/Omega-3 Balance for Membrane Fatty Acid-Mediated Signaling
3. The Membrane Fatty Acid-Based Profile in Cancer and the Relevance of Erythrocytes
4. The Study of the Cancer Lipidome and the Discovery of De Novo Pathways: Fatty Acid Positional Isomers as New Biomarkers of Metabolic Shift
5. Link between Obesity and Cancer: When the Lipid Supply Becomes Dangerous
6. Some Considerations of Fatty Acid-Based Membrane Lipidomics and Lipid Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| AT | Adipose tissue |
| ATGL | Adipose triglycerides lipase |
| ATP | Adenosine triphosphate |
| CAAs | Cancer-associated adipocytes |
| DMDS | Dimethyl disulfide |
| EFA | Essential fatty acids |
| ERK | Extracellular signal-regulated kinases |
| EVs | Extracellular vesicles |
| FABP | Fatty acid binding protein |
| FADS | Fatty acid desaturase |
| FAO | Fatty acid oxidase |
| FFA | Free fatty acids |
| FGF2 | Fibroblast growth factor 2 |
| GSK3 | Glycogen synthase kinase 3 beta |
| HGF | Hepatocyte growth factor |
| HSL | Hormone sensitive lipase |
| IGF-1 | Insulin growth factor 1 |
| IL-6 | Interleukin 6 |
| JAK2 | Janus kinases 2 |
| JNK | c-Jun N-terminal kinases |
| LDL | Low density lipoproteins |
| LNCAP | Prostate derived from metastatic site |
| LR | Leptin receptor |
| MAGL | Mono acylglycerol lipase |
| MAPK | Mitogen-activated protein kinases |
| MEFs | Mouse embryonic fibroblasts |
| PC3 | Prostate cancer |
| PI3K | Phosphoinositide 3-kinases |
| PKC | Protein kinase C |
| PPAR | Peroxisome proliferator activated receptor |
| SREBP-1 | Sterol regulatory element- binding protein 1 |
| STAT | Signal transducer and activator of transcription protein |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
References
- Gross, R.W.; Han, X. Lipidomics at the interface of structure and function in systems biology. Chem. Biol. 2011, 18, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2017, 7, 1. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition [published correction appears in Nat Rev Mol Cell Biol. 2019, 20, 715]. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Nakazawa, I.; Goto, Y. A role of the cellular phospholipid in the metastasis into the liver. Cell. Mol. Biol. 1981, 27, 23–26. [Google Scholar]
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Buratta, S.; Urbanelli, L.; Costanzi, E.; Emiliani, C.; Chatgilialoglu, C. The n-10 Fatty Acids Family in the Lipidome of Human Prostatic Adenocarcinoma Cell Membranes and Extracellular Vesicles. Cancers (Basel) 2020, 12, 900. [Google Scholar] [CrossRef]
- Asgari, Y.; Zabihinpour, Z.; Salehzadeh-Yazdi, A.; Schreiber, F.; Masoudi-Nejad, A. Alterations in cancer cell metabolism: The Warburg effect and metabolic adaptation. Genomics 2015, 105, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Rabinowitz, J.D.; Coller, H.A. Partners in the Warburg effect. Elife 2016, 5, e15938. [Google Scholar] [CrossRef]
- Yeung, S.J.; Pan, J.; Lee, M.H. Roles of p53, MYC and HIF-1 in regulating glycolysis—The seventh hallmark of cancer. Cell. Mol. Life Sci. 2008, 65, 3981–3999. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Cardenas, H.; Matei, D. Ovarian Cancer—Why Lipids Matter. Cancers 2019, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target. J. Lipid Res. 2020, 6, 687–695. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V. Cellular Fatty Acid Metabolism and Cancer. Cell Metabol. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lei, J.P.; Wei, G.Q.; Chen, H.; Ma, C.Y.; Jiang, H.Z. Natural fatty acid synthase inhibitors as potent therapeutic agents for cancers: A review. Pharm. Biol. 2016, 54, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Subramanian, C.; Jackowski, S.; Rock, C.O. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J. Biol. Chem. 2012, 287, 14615–14620. [Google Scholar] [CrossRef] [PubMed]
- Marin-Valencia, I.; Yang, C.; Mashimo, T.; Cho, S.; Baek, H.; Yang, X.L.; Rajagopalan, K.N.; Maddie, M.; Vemireddy, V.; Zhao, Z.; et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012, 15, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Comerford, S.A.; Huang, Z.; Du, X.; Wang, Y.; Cai, L.; Witkiewicz, A.K.; Walters, H.; Tantawy, M.N.; Fu, A.; Manning, H.C.; et al. Acetate dependence of tumors. Cell 2014, 159, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Igal, R.A. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim. Biophys. Acta 2016, 1861, 1865–1880. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; De Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Duncan, R.E.; Bazinet, R.P.; Archer, M.C. Fatty acid synthase plays a role in cancer metabolism beyond providing fatty acids for phospholipid synthesis or sustaining elevations in glycolytic activity. Exp. Cell Res. 2014, 320, 302–310. [Google Scholar] [CrossRef]
- Scanferlato, R.; Bortolotti, M.; Sansone, A.; Chatgilialoglu, C.; Polito, L.; De Spirito, M.; Maulucci, G.; Bolognesi, A.; Ferreri, C. Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 832. [Google Scholar] [CrossRef]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell 2017, 32, 807–823. [Google Scholar] [CrossRef]
- Hagiwara, A.; Cornu, M.; Cybulski, N.; Polak, P.; Betz, C.; Trapani, F.; Terracciano, L.; Heim, M.H.; Rüegg, M.A.; Hall, M.N. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012, 15, 725–738. [Google Scholar] [CrossRef]
- Schug, Z.T.; Peck, B.; Jones, D.T.; Zhang, Q.; Grosskurth, S.; Alam, I.S.; Goodwin, L.M.; Smethurst, E.; Mason, S.; Blyth, K.; et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015, 27, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Yue, S.H.; Li, J.J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.Q.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Bidaut, G.; Ouaissi, M.; Servais, S.; Gouirand, V.; Olivares, O.; Lac, S.; Borge, L.; Roques, J.; Gayet, O.; et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Lopez-Vilaro, L.; Nasarre, L.; Perez-Olabarria, M.; Vazquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortes, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef]
- Accioly, M.T.; Pacheco, P.; Maya-Monteiro, C.M.; Carrossini, N.; Robbs, B.K.; Oliveira, S.S.; Kaufmann, C.; Morgado-Diaz, J.A.; Bozza, P.T.; Viola, J.P.B. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E-2 synthesis in colon cancer cells. Cancer Res. 2008, 68, 1732–1740. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.F.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 6. [Google Scholar] [CrossRef]
- Tontonoz, P.; Wang, B. Phospholipid remodeling in physiology and disease. Ann. Rev. Physiol. 2019, 81, 165–188. [Google Scholar]
- Serhan, C.N. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Lands, B. Omega-3 PUFAs lower the propensity for arachidonic acid cascade overreactions. BioMed Res. Int. 2015, 2015, 285135. [Google Scholar] [CrossRef]
- Bodén, S.; Myte, R.; Wennberg, M.; Harlid, S.; Johansson, I.; Shivappa, N.; Hébert, J.R.; Van Guelpen, B.; Nilsson, L.M. The inflammatory potential of diet in determining cancer risk; A prospective investigation of two dietary pattern scores. PLoS ONE 2019, 14, e0214551. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef]
- Ristimaki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002, 62, 632–635. [Google Scholar]
- Xu, Y.; Qian, S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014, 37, 112–119. [Google Scholar]
- Zanoaga, O.; Jurj, A.; Raduly, L.; Cojocneanu-Petric, R.; Fuentes-Mattei, E.; Wu, O.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Implications of dietary omega-3 and omega-6 polyunsaturated fatty acids in breast cancer (Review). Exp. Ther. Med. 2018, 15, 1167–1176. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Ferreri, C.; Chatgilialoglu, C. Lipidomics and Health: An Added Value to Olive Oil. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing, 1st ed.; Shahidi, P., Kiritsakis, P., Eds.; Wiley: Chichester, UK, 2017; Chapter 27; pp. 505–520. [Google Scholar]
- Schley, P.D.; Brindley, D.N.; Field, C.J. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007, 137, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Bisogno, T.; Ligresti, A.; Bifulco, M.; Melck, D.; Di Marzo, V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam. Clin. Pharmacol. 2002, 16, 297–302. [Google Scholar] [CrossRef]
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide Modulates Inflammation-Associated Vascular Endothelial Growth Factor (VEGF) Signaling via the Akt/mTOR Pathway in a Selective Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α)-Dependent Manner. PLoS ONE 2016, 11, e0156198. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis [published correction appears in Nat Chem Biol. 2020 Jan 2]. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Reed, C.F. Phospholipid exchange between plasma and erythrocytes in man and the dog. J. Clin. Investig. 1968, 47, 749–760. [Google Scholar] [CrossRef]
- Dushianthan, A.; Cusack, R.; Koster, G.; Grocott, M.P.W.; Postle, A.D. Insight into erythrocyte phospholipid molecular flux in healthy humans and in patients with acute respiratory distress syndrome. PLoS ONE 2019, 14, e0221595. [Google Scholar] [CrossRef]
- Ferreri, C.; Chatgilialoglu, C. Membrane Lipidomics for Personalized Health; Wiley: Chichester, UK, 2015. [Google Scholar]
- Giacometti, G.; Ferreri, C.; Sansone, A.; Chatgilialoglu, C.; Marzetti, C.; Spyratou, E.; Georgakilas, A.G.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; et al. High predictive values of rbc membrane-based diagnostics by biophotonics in an integrated approach for autism spectrum disorders. Sci. Rep. 2017, 7, 9854. [Google Scholar] [CrossRef]
- Sansone, A.; Tolika, E.; Louka, M.; Sunda, V.; Deplano, S.; Melchiorre, M.; Anagnostopoulos, D.; Chatgilialoglu, C.; Formisano, C.; Di Micco, R.; et al. Hexadecenoic fatty acid isomers in human blood lipids and their relevance for the interpretation of lipidomic profiles. PLoS ONE 2016, 11, e0152378. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Verrastro, O.; Iacona, C.; Agostini, F.; Pazzeschi, C.; Sasdelli, A.S.; Melchiorre, M.; Ferreri, C. Functional lipidomics in patients on home parenteral nutrition: Effect of lipid emulsions. World J. Gastroenterol. 2017, 23, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.; Riordan, H.D.; Jackson, J.A.; Wong, K.; Miranda-Massari, J.R.; Gonzalez, M.J. Erythrocyte membrane fatty acid composition in cancer patients. P. R. Health Sci. J. 2004, 23, 107–113. [Google Scholar]
- Okunoi, M.; Hamazaki, K.; Ogura, T.; Kitade, H.; Matsuura, T.; Yoshida, R.; Hijikawa, T.; Kwon, M.; Arita, S.; Itomura, M.; et al. Abnormalities in Fatty Acids in Plasma, Erythrocytes and Adipose Tissue in Japanese Patients with Colorectal Cancer. In Vivo 2013, 27, 203–210. [Google Scholar]
- Pala, V.; Krogh, V.; Muti, P.; Chajes, V.; Riboli, E.; Micheli, A.; Saadatian, M.; Sieri, S.; Berrino, F. Erythrocyte membrane fatty acids and subsequent breast cancer: A prospective Italian study. J. Natl. Cancer Inst. 2001, 93, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Coviello, G.; Tutino, V.; Notarnicola, M.; Caruso, M.G. Erythrocyte Membrane Fatty Acids Profile in Colorectal Cancer Patients: A Preliminary Study. Anticancer Res. 2014, 34, 4775–4779. [Google Scholar] [PubMed]
- Amézaga, J.; Arranz, S.; Urruticoechea, A.; Ugartemendia, G.; Larraioz, A.; Louka, M.; Uriarte, M.; Ferreri, C.; Tueros, I. Altered red blood cell membrane fatty acid profile in cancer patients. Nutrients 2018, 10, 1853. [Google Scholar] [CrossRef] [PubMed]
- Cottet, V.; Collin, M.; Gross, A.S.; Boutron-Ruault, M.C.; Morois, S.; Clavel-Chapelon, F.; Chajes, V. Erythrocyte membrane phospholipid fatty acid concentrations and risk of colorectal adenomas: A case-control nested in the French E3N-EPIC cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Rahrovani, F.; Javanbakht, M.H.; Ehsani, A.H.; Esrafili, A.; Mohammadi, H.; Ghaedi, E.; Zarei, M.; Djalali, M. Erythrocyte membrane saturated fatty acids profile in newly diagnosed Basal Cell Carcinoma patients. Clin. Nutr. Espen 2018, 23, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Rahrovani, F.; Javanbakht, M.H.; Ghaedi, E.; Mohammadi, H.; Ehsani, A.H.; Esrafili, A.; Djalali, M. Erythrocyte Membrane Unsaturated (Mono and Poly) Fatty Acids Profile in Newly Diagnosed Basal Cell Carcinoma Patients. Clin. Nutr. Res. 2018, 7, 21–30. [Google Scholar] [CrossRef]
- Kuriki, K.; Wakai, K.; Hirose, K.; Matsuo, K.; Ito, H.; Suzuki, T.; Saito, T.; Kanemitsu, Y.; Hirai, T.; Kato, T.; et al. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.; King, I.B.; Moshofsky, R.; Lampe, J.W.; Gao, D.L.; Ray, R.M.; Thomas, D.B. Erythrocyte fatty acids and breast cancer risk: A case-control study in Shanghai, China. Am. J. Clin. Nutr. 2007, 85, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.; O’Malley, J.; Mori, M.; Garzotto, M.; Palma, A.J.; King, I.B. Erythrocyte fatty acids and prostate cancer risk: A comparison of methods. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 161–169. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, P.; Rodriguez, M.C.; Sanchez-Yague, J. Identification of potential erythrocyte phospholipid fatty acid biomarkers of advanced lung adenocarcinoma, squamous cell lung carcinoma, and small cell lung cancer. Tumor Biol. 2015, 36, 5687–5698. [Google Scholar] [CrossRef]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic signaling in the cancer genome atlas. Cell 2018, 173, 321–337. [Google Scholar] [CrossRef]
- Stromberg, L.R.; Lilley, L.M.; Mukundan, H. Advances in Lipidomics for Cancer Biomarker Discovery. In Proteomic and Metabolomic Approaches to Biomarker Discovery; Academic Press: Cambridge, MA, USA, 2020; pp. 421–436. [Google Scholar] [CrossRef]
- Sansone, A.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C. Hexadecenoic Fatty Acid Isomers: A Chemical Biology Approach for Human Plasma Biomarker Development. Chem. Res. Toxicol. 2013, 26, 1703–1709. [Google Scholar] [CrossRef]
- Ge, L.; Gordon, J.S.; Hsuan, C.; Stenn, K.; Prouty, S.M. Identification of the Delta-6 desaturase of human sebaceous glands: Expression and enzyme activity. J. Invest. Dermatol. 2003, 120, 707–714. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl-CoA desaturase-1: A novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis 2010, 31, 1509–1515. [Google Scholar] [CrossRef]
- Pappas, A.; Anthonavage, M.; Gordon, J.S. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J. Invest. Dermatol. 2002, 118, 164–171. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.D.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, R.; Langari, H.; Fathinezhad, Z.; Bahari Sani, Z.; Avan, A.; Mobarhan, M.G.; Rezayi, M. Exosomes: New insights into cancer mechanisms. J. Cell. Biochem. 2020, 121, 7–16. [Google Scholar] [CrossRef]
- Lattka, E.; Illig, T.; Koletzko, B.; Heinrich, J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Nishiumi, S.; Kono, S.; Takao, S.; Azuma, T.; Yoshida, M. Differences in elongation of very long chain fatty acids and fatty acid metabolism between triple-negative and hormone receptor-positive breast cancer. BMC Cancer 2017, 17, 589. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bengtsson, A.; Asadi, A.; Gao, H.; Dahlman-Wright, K.; Jacobsson, A. Estrogen Enhances the Expression of the Polyunsaturated Fatty Acid Elongase Elovl2 via ERa in Breast Cancer Cells. PLoS ONE 2016, 11, e0164241. [Google Scholar] [CrossRef]
- Tamura, K.; Makino, A.; Hullin-Matsuda, F.; Kobayashi, T.; Furihata, M.; Chung, S.; Ashida, S.; Miki, T.; Fujioka, T.; Shuin, T.; et al. Novel Lipogenic Enzyme ELOVL7 Is Involved in Prostate Cancer Growth through Saturated Long-Chain Fatty Acid Metabolism. Cancer Res. 2009, 69, 8133–8140. [Google Scholar] [CrossRef]
- Triki, M.; Rinaldi, G.; Planque, M.; Broekaert, D.; Winkelkotte, A.M.; Maier, C.R.; Raman, S.J.; Vandekeere, A.; Van Elsen, J.; Orth, M.F.; et al. mTOR Signaling and SREBP Activity Increase FADS2 Expression and Can Activate Sapienate Biosynthesis. Cell Rep. 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Park, H.G.; Kothapalli, K.S.D.; Park, W.J.; DeAllie, C.; Liu, L.; Liang, A.; Lawrence, P.; Brenna, J.T. Palmitic acid (16:0) competes with omega-6 linoleic and omega-3 alpha-linolenic acids for FADS2 mediated Delta 6-desaturation. BBA. Mol. Cell Biol. Lipids 2016, 1861, 91–97. [Google Scholar] [CrossRef]
- Park, H.G.; Engel, M.G.; Vogt-Lowell, K.; Lawrence, P.; Kothapalli, K.S.; Brenna, J.T. The role of fatty acid desaturase (FADS) genes in oleic acid metabolism: FADS1 Delta 7 desaturates 11-20:1 to 7,11-20:2. Prostaglandins Leukot. Essent. Fatty Acids 2018, 128, 21–25. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Wollins, D. American Society of Clinical Oncology Obesity Initiative: Rationale, Progress, and Future Directions. J. Clin. Oncol. 2016, 34, 4256–4260. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Salmeron, M.; Chocarro-Calvo, A.; Garcia-Martinez, J.M.; De la Vieja, A.; Garcia-Jimenez, C. Epidemiological bases and molecular mechanisms linking obesity, diabetes, and cancer. Endocrinol. Diabetes Y Nutr. 2017, 64, 109–117. [Google Scholar] [CrossRef]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity and Endometrial Cancer. In Obesity and Cancer. Recent Results in Cancer Research; Pischon, T., Nimptsch, K., Eds.; Springer: Cham, Switzerland, 2016; Volume 208. [Google Scholar] [CrossRef]
- Feng, Y.H. The association between obesity and gynecological cancer. Gynecol. Minim. Invasive Ther. Gmit 2015, 4, 102–105. [Google Scholar] [CrossRef]
- Hoyo, C.; Cook, M.B.; Kamangar, F.; Freedman, N.D.; Whiteman, D.C.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.M.; Sharp, L.; et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 2012, 41, 1706–1718. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Dreifuss, N.H.; Patti, M.G. Obesity and esophageal cancer: GERD, Barrett´s esophagus, and molecular carcinogenic pathways. Exp. Rev. Gastroent. Hepatol. 2020, 14, 425–433. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.X.; Wang, X.L.; Wang, J.H.; Yan, Z.P.; Cheng, J.M.; Gong, G.Q.; Li, G.P. Body Mass Index and Risk of Gastric Cancer: A Meta-analysis of a Population with More Than Ten Million from 24 Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1395–1408. [Google Scholar] [CrossRef]
- Du, X.; Hidayat, K.; Shi, B.M. Abdominal obesity and gastroesophageal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, BSR20160474. [Google Scholar] [CrossRef]
- Olefson, S.; Moss, S.F. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015, 18, 23–32. [Google Scholar] [CrossRef]
- Saitta, C.; Pollicino, T.; Raimondo, G. Obesity and liver cancer. Ann. Hepatol. 2019, 18, 810–815. [Google Scholar] [CrossRef]
- Rahmani, J.; Varkaneh, H.K.; Kontogiannis, V.; Ryan, P.M.; Bawadi, H.; Fatahi, S.; Zhang, Y. Waist Circumference and Risk of Liver Cancer: A Systematic Review and Meta-Analysis of over 2 Million Cohort Study Participants. Liver Cancer 2020, 9, 6–14. [Google Scholar] [CrossRef]
- Wilson, K.M.; Cho, E. Obesity and Kidney Cancer Recent Results. Cancer Res. 2016, 208, 81–93. [Google Scholar]
- Sanfilippo, K.M.; McTigue, K.M.; Fidler, C.J.; Neaton, J.D.; Chang, Y.F.; Fried, L.F.; Liu, S.M.; Kuller, L.H. Hypertension and Obesity and the Risk of Kidney Cancer in 2 Large Cohorts of US Men and Women. Hypertension 2014, 63, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.R.; Xu, Y.H. Body mass index and risk of renal cell cancer: A dose-response meta-analysis of published cohort studies. Int. J. Cancer 2014, 135, 1673–1686. [Google Scholar] [CrossRef]
- Thordardottir, M.; Lindqvist, E.K.; Lund, S.H.; Costello, R.; Burton, D.; Korde, N.; Mailankody, S.; Eiriksdottir, G.; Launer, L.J.; Gudnason, V.; et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: A population-based study. Blood Adv. 2017, 1, 2186–2192. [Google Scholar] [CrossRef]
- Wallin, A.; Larsson, S.C. Body mass index and risk of multiple myeloma: A meta-analysis of prospective studies. Eur. J. Cancer 2011, 47, 1606–1615. [Google Scholar] [CrossRef]
- Bullwinkle, E.M.; Parker, M.D.; Bonan, N.F.; Falkenberg, L.G.; Davison, S.P.; DeCicco-Skinner, K.L. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Lett. 2016, 380, 114–121. [Google Scholar] [CrossRef]
- Niedermaier, T.; Behrens, G.; Schmid, D.; Schlecht, I.; Fischer, B.; Leitzmann, M.F. Body mass index, physical activity, and risk of adult meningioma and glioma. A meta-analysis. Neurology 2015, 85, 1342–1350. [Google Scholar] [CrossRef]
- Rutkowski, R.; Reszec, J.; Hermanowicz, A.; Chrzanowski, R.; Lyson, T.; Mariak, Z.; Chyczewski, L. Correlation of leptin receptor expression with BMI in differential grades of human meningiomas. Oncol. Lett. 2016, 11, 2515–2519. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bernstein, L.; Van den Brandt, P.A.; Calle, E.E.; English, D.R.; Folsom, A.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer 2011, 129, 1708–1717. [Google Scholar] [CrossRef]
- Xu, M.; Jung, X.M.; Hines, O.J.; Eibl, G.; Chen, Y.J. Obesity and pancreatic cancer overview of epidemiology and potential prevention by weight loss. Pancreas 2018, 47, 158–162. [Google Scholar] [CrossRef]
- Liu, P.H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.Y.; He, X.S.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, K.; Gill, R.S.; Birch, D.W.; Karmali, S. The Association between Obesity and Colorectal Cancer. Gastroenterol. Res. Pract. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Useros, J.; Garcia-Foncillas, J. Obesity and colorectal cancer: Molecular features of adipose tissue. J. Transl. Med. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Newton, C.C.; Kitahara, C.M.; Patel, A.V.; Hartge, P.; Koshiol, J.; McGlynn, K.A.; Adami, H.O.; De Gonzalez, A.B.; Freeman, L.E.B.; et al. Body Size Indicators and Risk of Gallbladder Cancer: Pooled Analysis of Individual-Level Data from 19 Prospective Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.M.; Brown-Glabeman, U.; Soares, H.; Belmonte, J.; Liem, B.; Nir, I.; Phuoc, V.; Gullapalli, R.R. Gallbladder cancer: Review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018, 18, 665. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention. CA Cancer J. Clin. 2017, 67, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, leptin and breast cancer: Epidemiological evidence and proposed mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, obesity, and postmenopausal invasive breast cancer risk. A secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Agurs-Collins, T.; Ross, S.A.; Dunn, B.K. The many faces of obesity and its influence on breast cancer risk. Front. Oncol. 2019, 9, 765. [Google Scholar] [CrossRef]
- Beral, V.; Hermon, C.; Peto, R.; Reeves, G.; Brinton, L.; Marchbanks, P.; Negri, E.; Ness, R.; Peeters, P.H.M.; Vessey, M.; et al. Ovarian cancer and body size: Individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012, 9, e1001200. [Google Scholar] [CrossRef]
- Kitahara, C.M.; McCullough, M.L.; Franceschi, S.; Rinaldi, S.; Wolk, A.; Neta, G.; Adami, H.O.; Anderson, K.; Andreotti, G.; Freeman, L.E.B.; et al. Anthropometric factors and thyroid cancer risk by histological subtype: Pooled analysis of 22 prospective studies. Thyroid 2016, 26, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.T.; Jia, X.M.; Fan, X.J.; Zhao, L.; Pang, P.; Wang, Y.J.; Luo, Y.K.; Wang, F.L.; Yang, G.Q.; Wang, X.L.; et al. Association of obesity with the clinicopathological features of thyroid cancer in a large, operative population A retrospective case-control study. Medicine 2019, 98, e18213. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Ricci, C.; Behrens, G.; Leitzmann, M.F. Adiposity and risk of thyroid cancer: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Le Gonidec, S.; Escourrou, G.; Valet, P.; Muller, C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to early breast cancer invasion in vitro and in vivo. EJC Suppl. 2010, 8, 126. [Google Scholar] [CrossRef]
- Rybinska, I.; Agresti, R.; Trapani, A.; Tagliabue, E.; Triulzi, T. Adipocytes in breast cancer, the thick and the thin. Cells 2020, 9, 560. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuede, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer associated fibroblasts: The dark side of the coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. BBA. Mol. Cell Biol. Lipids 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and Cancer: Local and Systemic Mechanisms. Annu. Rev. Med. 2015, 66, 297–309. [Google Scholar] [CrossRef]
- Wauman, J.; Tavernier, J. Leptin receptor signaling: Pathways to leptin resistance. Front. Biosci. 2011, 16, 2771–2793. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Gung, J.; Lineberger, C.G.; Hursting, S.D. Abstract SY28-04: Obesity-associated leptin signaling promotes chemotherapy resistance in triple-negative breast cancer: The role of tumor-initiating cell enrichment. Tumor Biol. 2019. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, F.; Perez-Perez, A.; De la Cruz-Merino, L.; Sanchez-Margalet, V. Obesity and breast cancer: Role of leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Nowak, A.; Kobierzycki, C.; Dziegiel, P. The role of leptin in pathogenesis of obesity-related cancers. Postepy Biol. Komorki 2015, 42, 309–328. [Google Scholar]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer-mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose tissue, obesity and adiponectin: Role in endocrine cancer risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef]
- Aronis, K.N.; Siatis, K.E.; Giannopoulou, E.; Kalofonos, H.P. Adiponectin promotes autophagy and apoptosis in endometrial cancer cell lines. Clin. Oncol. 2019, 4, 1–5. [Google Scholar]
- Zhang, L.Z.; Wen, K.; Han, X.X.; Liu, R.; Qu, Q.X. Adiponectin mediates antiproliferative and apoptotic responses in endometrial carcinoma by the AdipoRs/AMPK pathway. Gynecol. Oncol. 2015, 137, 311–320. [Google Scholar] [CrossRef]
- Jiang, J.H.; Fan, Y.C.; Zhang, W.; Sheng, Y.L.; Liu, T.T.; Yao, M.; Gu, J.R.; Tu, H.; Gan, Y. Adiponectin suppresses human pancreatic cancer growth through attenuating the beta-catenin signaling pathway. Int. J. Biol. Sci. 2019, 15, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Cleary, M.P. The balance between leptin and adiponectin in the control of carcinogenesis -focus on mammary tumorigenesis. Biochimie 2012, 94, 2164–2171. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, V.; Passaretti, F.; Hammarstedt, A.; Liguoro, D.; Terracciano, D.; Molea, G.; Canta, L.; Miele, C.; Smith, U.; Beguinot, F.; et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 2012, 55, 2811–2822. [Google Scholar] [CrossRef]
- Louie, S.M.; Roberts, L.S.; Nomura, D.K. Mechanisms linking obesity and cancer. BBA. Mol. Cell Biol. Lipids 2013, 1831, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Du, J.X.; Zhao, J.; Shi, M.T.; Jin, M.W.; Liu, H. Adipocytes promote tumor progression and induce PD-L1 expression via TNF-alpha/IL-6 signaling. Cancer Cell Int. 2020, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I.C. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef]
- Ma, Y.B.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.Q.; Wang, W.; Wang, X.Y.; Fan, X.J. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; Van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef]
- Zeng, J.; Sauter, E.R.; Li, B. FABP4: A New Player in Obesity-Associated Breast Cancer. Trends Mol. Med. 2020, 26, 437–440. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Attane, C.; Carrie, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; Le Gonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Xu, M.Q.; Li, X.X.; Su, X.D.; Xiao, X.; Keating, A.; Zhao, R.C. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J. Hematol. Oncol. 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Euhus, D.M.; Scherer, P.E. Paracrine and Endocrine Effects of adipose tissue on cancer development and progression. Endocr. Rev. 2011, 32, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Mulkey, F.; Geyer, S.; Kolitz, J.E.; Blum, W.; Powell, B.L.; George, S.L.; Larson, R.A.; Stone, R.M. Relationship between obesity and clinical outcome in adults with acute myeloid leukemia: A pooled analysis from four CALGB (alliance) clinical trials. Am. J. Hematol. 2016, 91, 199–204. [Google Scholar] [CrossRef]
- Cascetta, P.; Cavaliere, A.; Piro, G.; Torroni, L.; Santoro, R.; Tortora, G.; Melisi, D.; Carbone, C. Pancreatic cancer and obesity: Molecular mechanisms of cell transformation and chemoresistance. Int. J. Mol. Sci. 2018, 19, 3331. [Google Scholar] [CrossRef]
- Mentoor, I.; Engelbrecht, A.M.; Nell, T. Fatty acids: Adiposity and breast cancer chemotherapy, a bad synergy? Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 18–33. [Google Scholar] [CrossRef]
- Kounakis, K.; Chaniotakis, M.; Markaki, M.; Tavernarakis, N. Emerging roles of lipophagy in health and disease. Front. Cell Dev. Biol. 2019, 7, 185. [Google Scholar] [CrossRef]
- Longo, V.D.; Fontana, L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010, 31, 89–98. [Google Scholar] [CrossRef]
- Caccialanza, R.; Aprile, G.; Cereda, E.; Pedrazzoli, P. Fasting in oncology: A word of caution. Nat. Rev. Cancer 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Lovik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Stensrud, M.J.; Valberg, M. Inequality in genetic cancer risk suggests bad genes rather than bad luck. Nat. Commun. 2017, 8, 1165. [Google Scholar] [CrossRef][Green Version]
- Tomasetti, C.; Vogelstein, B. Cancer risk: Role of environment—Response. Science 2015, 347, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Roche, B.; Ujvari, B. Intrinsic versus extrinsic cancer risks: The debate continues. Trends Cancer 2016, 2, 68–69. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. The bad luck of cancer. Science 2015, 347, 12. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Fuentes, N.R.; Wright, R.C.; Chapkin, R.S. Functional link between plasma membrane spatiotemporal dynamics, cancer biology, and dietary membrane-altering agents. Cancer Metastasis Rev. 2018, 37, 519–544. [Google Scholar] [CrossRef]
- Chapkin, R.S.; DeClercq, V.; Kim, E.; Fuentes, N.R.; Fan, Y.Y. Mechanisms by Which Pleiotropic Amphiphilic n-3 PUFA Reduce Colon Cancer Risk. Curr. Colorectal Cancer Rep. 2014, 10, 442–452. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. BBA Biomembr. 2014, 1838, 1657–1679. [Google Scholar] [CrossRef]
- Peláez, R.; Pariente, A.; Pérez-Sala, Á.; Larráyoz, I.M. Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases. Cells 2020, 9, 140. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Vander Heiden, M.G. A framework for examining how diet impacts tumour metabolism. Nat. Rev. Cancer 2019, 19, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Kenny, F.S.; Pinder, S.E.; Elis, I.O.; Gee, J.M.W.; Nicholson, R.I.; Bryce, R.P.; Robertson, J.F.R. Gamma linolenic acid with tamoxifen as primary therapy in breast cancer. Int. J. Cancer 2000, 85, 643–648. [Google Scholar] [CrossRef]

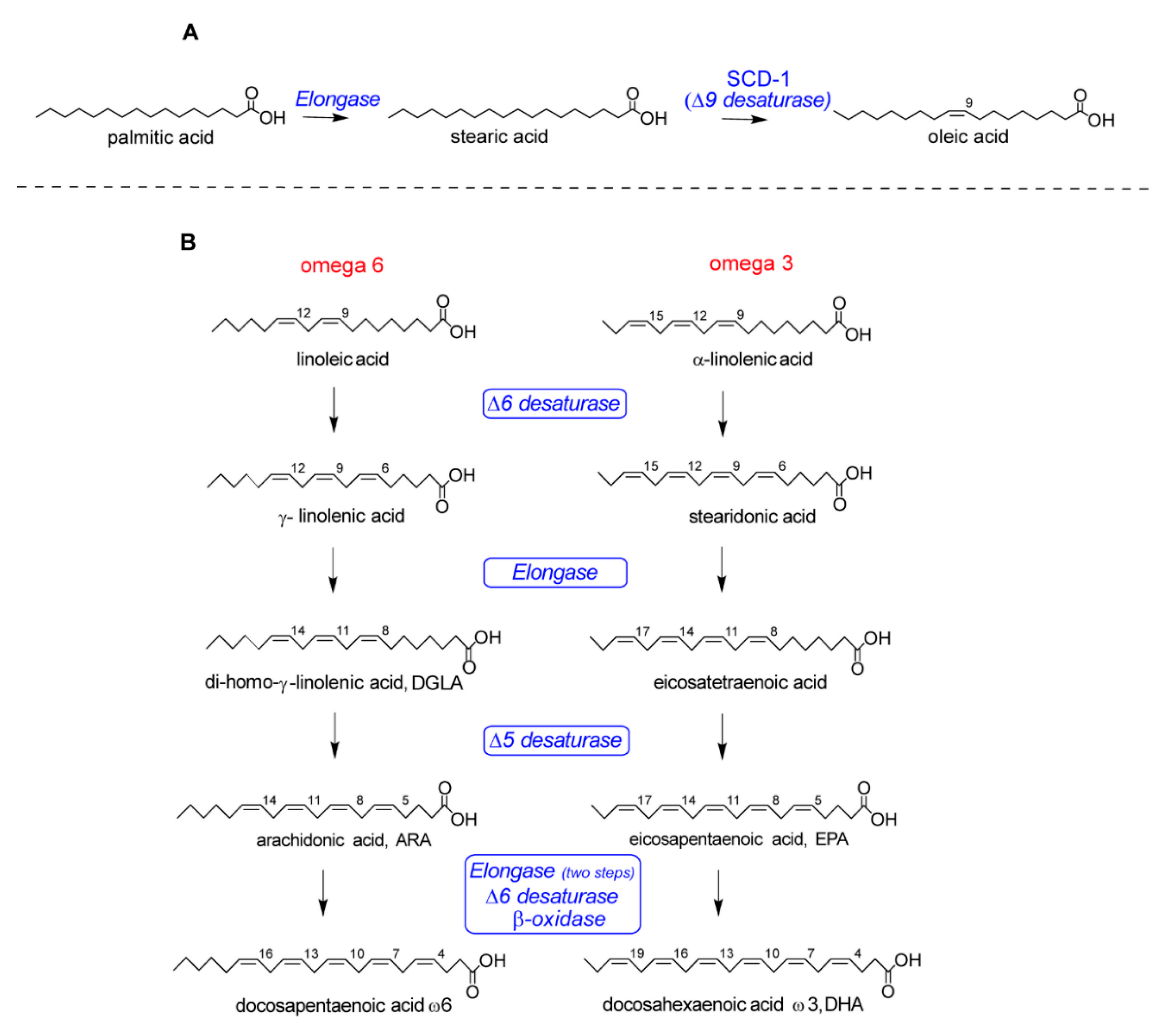

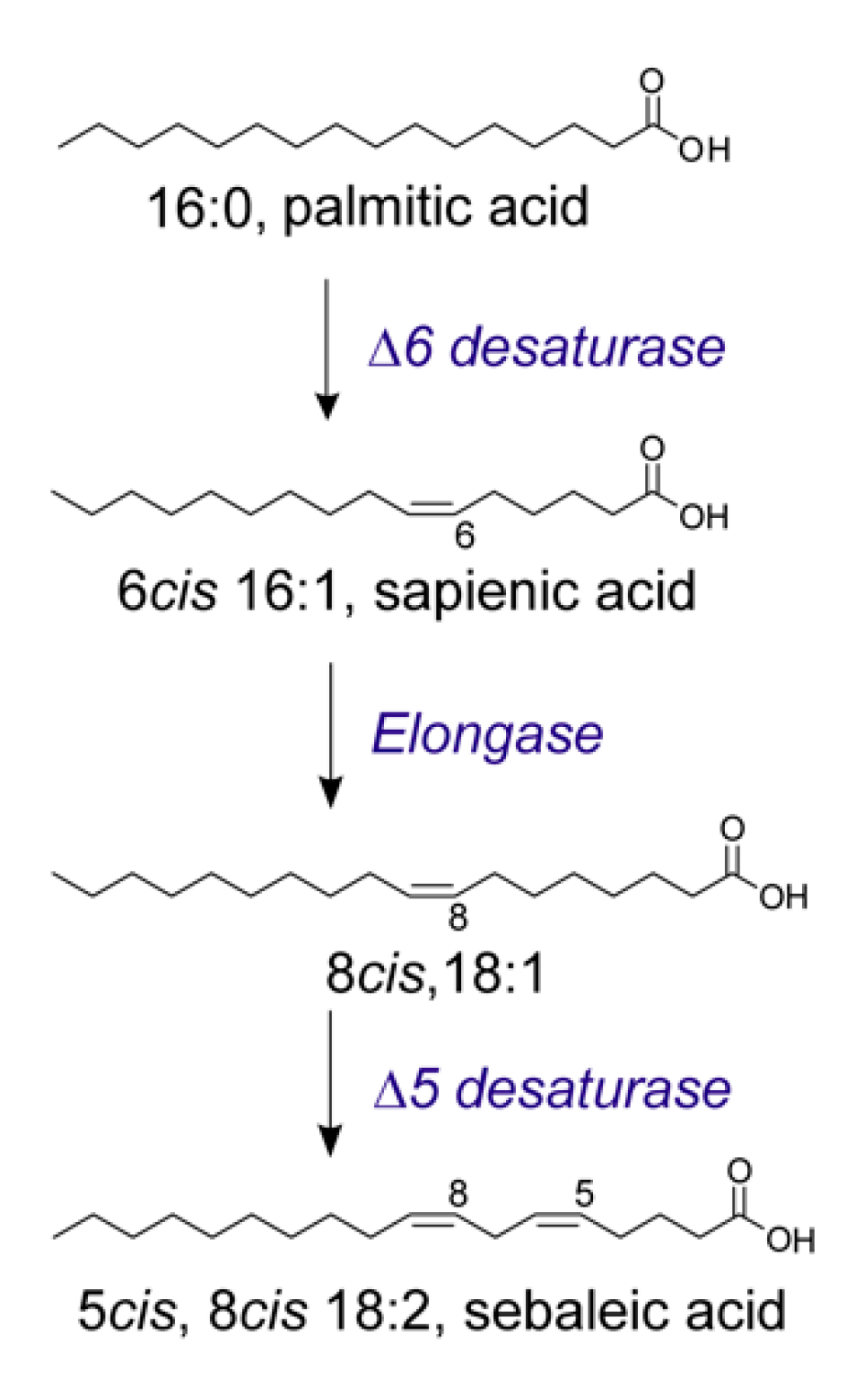

| Entry | Implicated Mechanism | Biological Effects | Lit |
|---|---|---|---|
| 1 | Desaturation from 16:0 to 6c-16:1 (sapienic acid) | Support of membrane biosynthesis during proliferation | [29,30] |
| 2 | mTORC2 regulation of lipid metabolism | Glycolysis and lipogenesis activation | [31,32] |
| 3 | Acetyl CoA synthetase 2 promotion of acetate utilization | Maintaining cancer cell growth under hypoxia and metabolic stress | [33] |
| 4 | Adipokines mediation of ovarian cancer metastasis | Induction of lipolysis and β-oxidation to provide energy | [34] |
| 5 | Enhanced uptake of exogenous lipoproteins | (a) Cholesteryl ester accumulation, induced by PTEN loss and PI3K/AKT activation, to sustain cancer aggressiveness (b) Increased amount of cholesterol and overexpression of low-density lipoprotein receptor to boost proliferation (c) Sustaining proliferation and aggressive potential of breast cancer tumors | [35] [36] [37] |
| 6 | Increase in lipid droplets in tumor cells | Increased COX-2 expression and storage in droplets, with effects on proliferation | [38] |
| 7 | Stearoyl CoA desaturase essentiality for cancer cell survival | Inhibition of FA desaturation, blocking the synthesis of lipids and impairing cell survival | [39] |
| Cancer Type | Country | Human Cohort Size | Outcomes | Reference |
|---|---|---|---|---|
| Breast/Prostate/Liver/Pancreas/Colon/Lung | Puerto Rico | 255 cancer patients 2800 non cancer patients 34 healthy volunteers | Lower levels of stearic acid and increased content of oleic acid. EPA and DHA/ALA ratio to estimate PUFA imbalances in cancer patients. | [65] |
| Colorectal | Japan | 61 cases 42 controls | Less EPA and linoleic acid and high levels of arachidonic acid in cancer patients. | [66] |
| Breast | Italy | 71 cases 141 controls | High oleic acid and low stearic acid in patients. Oleic acid and MUFA positively associated with breast cancer risk. Saturation index (stearic/oleic acids ratio) inversely correlated. | [67] |
| Colorectal | Italy | 13 cancer patients 13 patients with no malignant diseases | Lower levels of n-3 PUFAs and higher n-6/n-3 PUFA ratio in cancer patients. | [68] |
| Breast/Colon/Lung | Spain | 54 cases 34 controls | Less SFA (C16:0 and C18:0), high MUFA (9c-C18:1 and 11c-C18:1) compared to controls. In the PUFA families, increase in n-6 C18:2 and C20:3 (15.7% and 22.2%, respectively). | [69] |
| Colorectal | France | 328 cases 619 controls | High levels of pentadecanoic and heptadecanoic acids; oleic acid and linoleic acid associated with the risk of advanced adenomas. EPA and DHA negatively associated with the risk of advanced adenomas. | [70] |
| Basal Cell Carcinoma | Iran | 40 cases, 40 controls | Low palmitic and high oleic acid levels in cancer patients. Saturation index (stearic/oleic acids ratio) lower in cancer patients. | [71] |
| Basal Cell Carcinoma | Iran | 40 cases, 40 controls | Higher AA, total omega-6 and LA in cancer patients, lower omega-3. | [72] |
| Colorectal | Japan | 74 cases, 221 controls | Risk of colorectal cancer inversely associated with DHA, AA and PUFAs and positively associated with palmitic acid, SFAs and SFA/PUFA. | [73] |
| Breast | China | 322 cases, 1030 controls | Significant direct association among palmitic, γ-linolenic, palmitoleic and vaccenic acids and risk of breast cancer. Total n-3 fatty acids, EPA and 16:0/16:1 saturation index associated with significantly lower risk of breast cancer. | [74] |
| Prostate | USA | 127 cases, 183 controls | MUFA and α-linolenic/EPA ratio associated with reduced risk of prostate cancer. | [75] |
| Advanced squamous cell lung carcinoma (SCC), lung adenocarcinoma (ADC) and small cell lung cancer (SCLC) | Spain | 63 patients, 50 controls | AA, EPA, palmitic, oleic acids biomarkers in diagnosis and in other aspects related to clinical disease management of cancer. | [76] |
| Cancer Type | Increased Risk (OW/OB vs. Lean) | References |
|---|---|---|
| Endometrial | 150–200% | [96,97] |
| Esophageal | 200–400% | [98,99] |
| Gastric cardia | 168–188% | [100,101] |
| Liver | 17–89% | [102,103,104] |
| Kidney | 200% | [105,106,107] |
| Multiple myeloma | 10–20% | [108,109,110] |
| Meningioma | 10–20% | [111,112] |
| Pancreatic | 50–60% | [113,114] |
| Colorectal | 30–60% | [115,116,117] |
| Gallbladder | 20–60% | [118,119] |
| Breast | 20–40% | [120,121,122,123] |
| Ovarian | 10–30% | [97,124] |
| Thyroid | 10–30% | [125,126,127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreri, C.; Sansone, A.; Ferreri, R.; Amézaga, J.; Tueros, I. Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites 2020, 10, 345. https://doi.org/10.3390/metabo10090345
Ferreri C, Sansone A, Ferreri R, Amézaga J, Tueros I. Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites. 2020; 10(9):345. https://doi.org/10.3390/metabo10090345
Chicago/Turabian StyleFerreri, Carla, Anna Sansone, Rosaria Ferreri, Javier Amézaga, and Itziar Tueros. 2020. "Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways" Metabolites 10, no. 9: 345. https://doi.org/10.3390/metabo10090345
APA StyleFerreri, C., Sansone, A., Ferreri, R., Amézaga, J., & Tueros, I. (2020). Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites, 10(9), 345. https://doi.org/10.3390/metabo10090345









