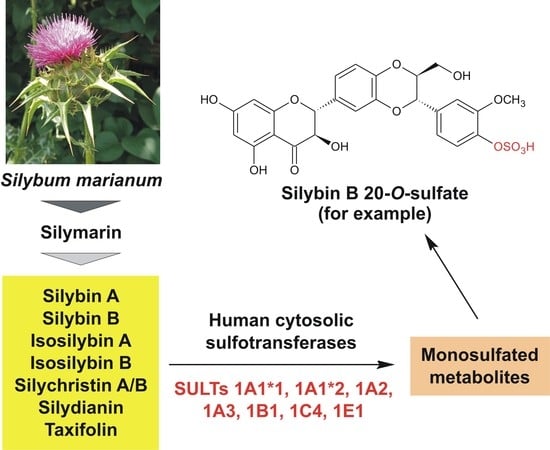
Identification of Human Sulfotransferases Active towards Silymarin Flavonolignans and Taxifolin
Abstract
1. Introduction
2. Results
2.1. Sulfation of Flavonolignans and Taxifolin by Human Tissue Cytosols
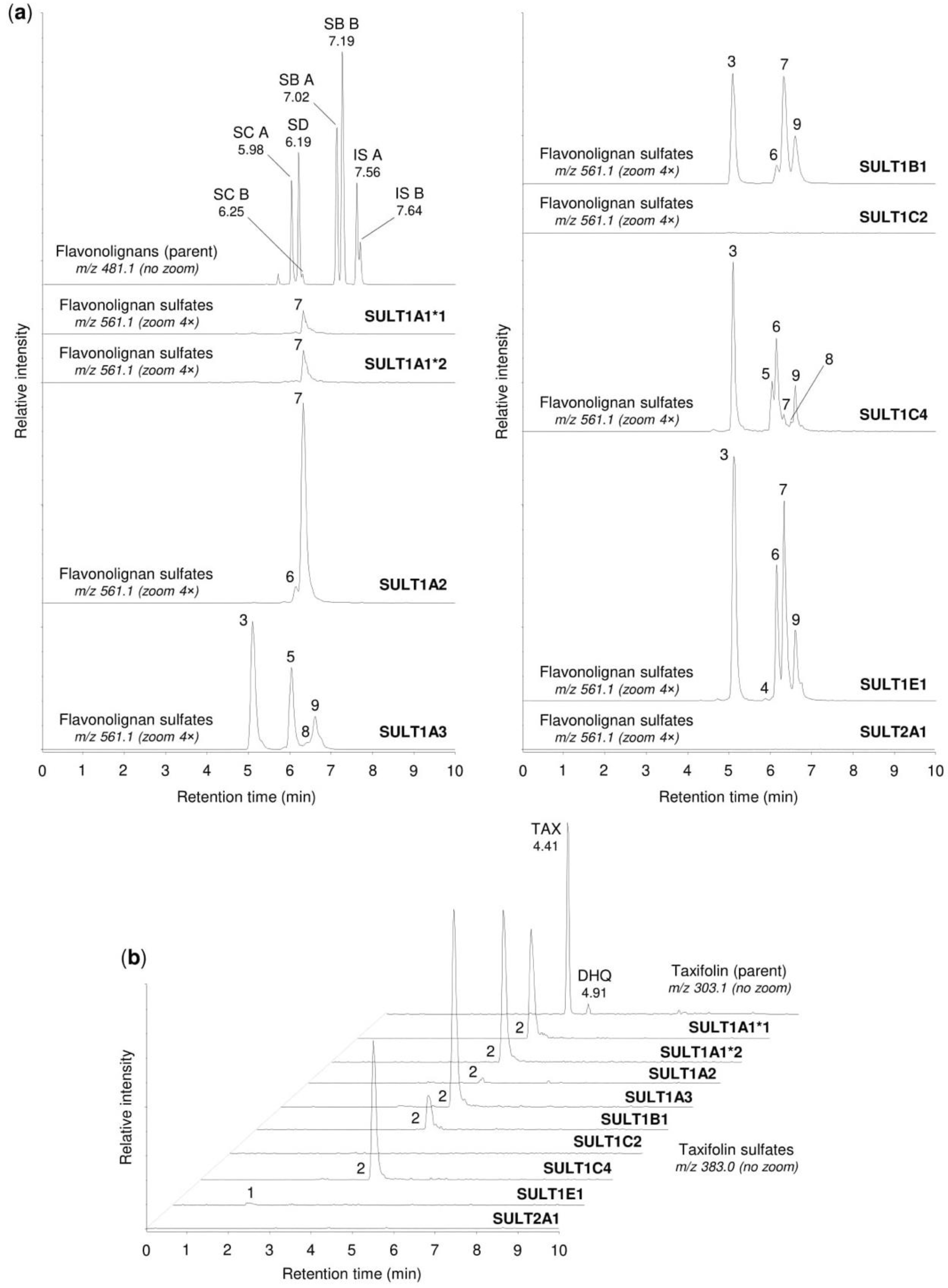
2.2. Sulfation of Flavonolignans and Taxifolin by Human Sulfotransferases
2.3. Sulfation of Silymarin Mixture by Human Sulfotransferases
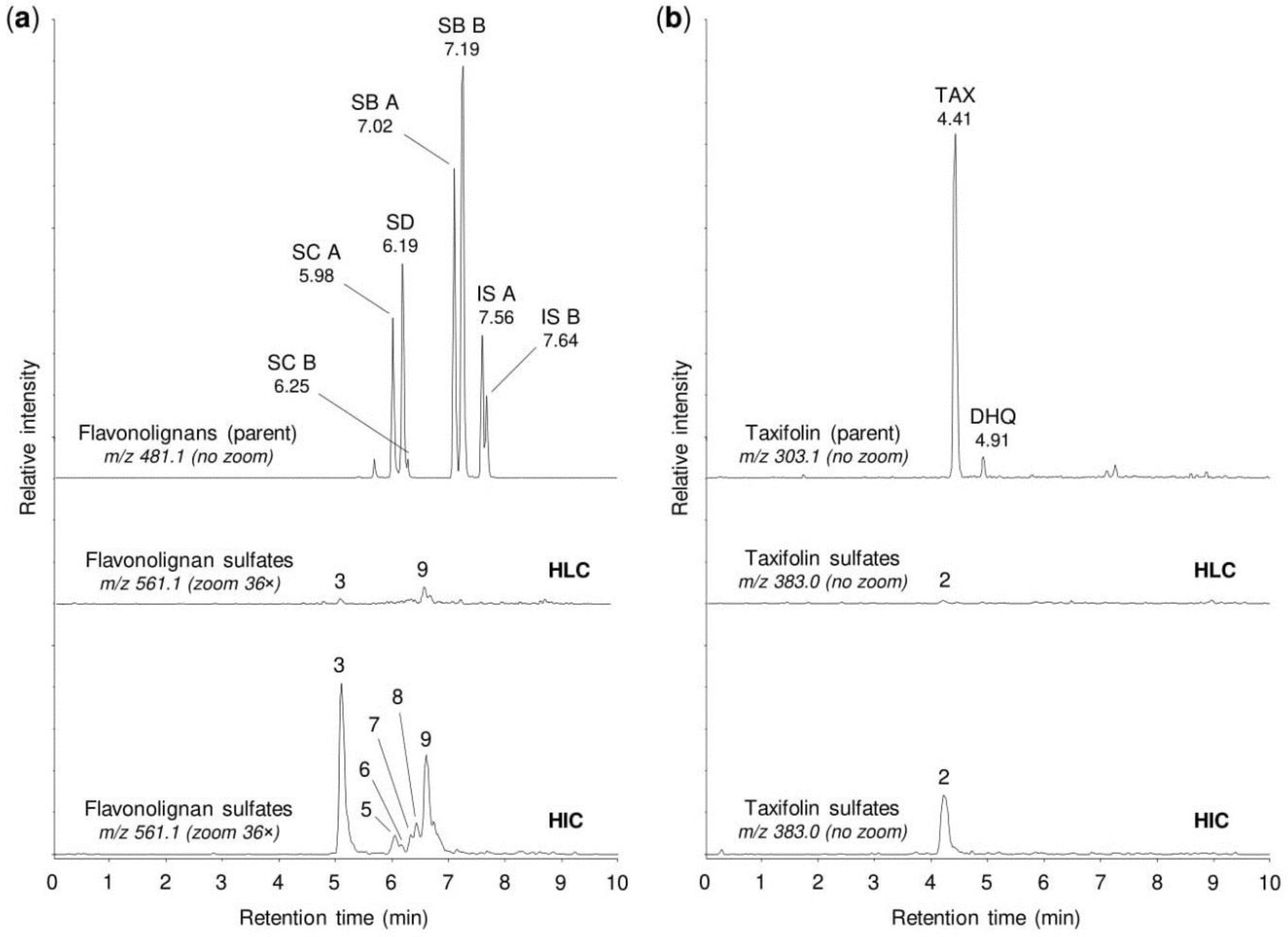
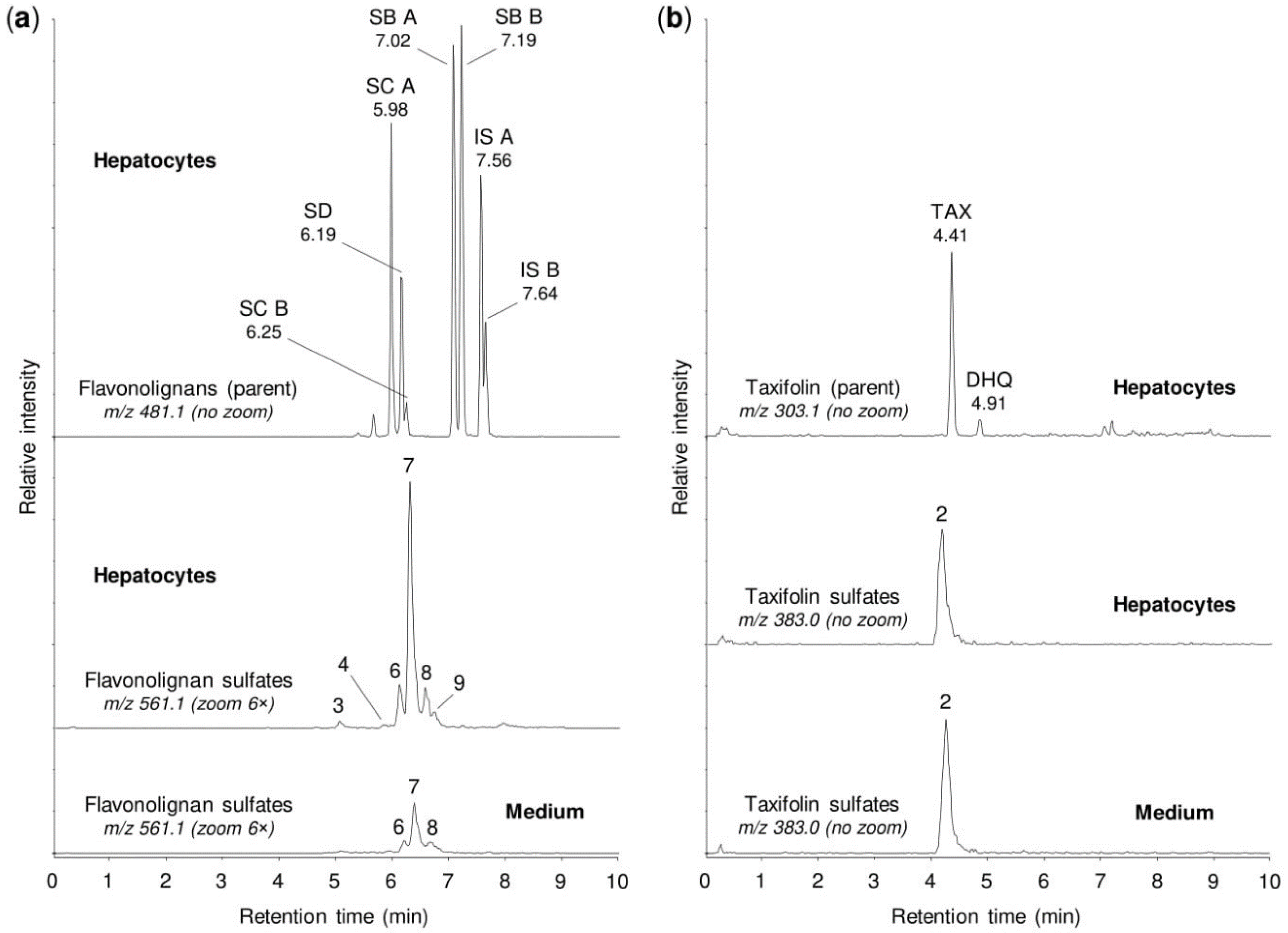
2.4. Sulfation of Silymarin Mixture by Human Tissue Cytosols and Human Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Tested Compounds
4.2. Biotransformation in Human Hepatocytes
4.3. Sulfation by Human Tissue Cytosols and Human Sulfotransferases
4.4. UHPLC-MS Analysis of Biotransformation Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diukendjieva, A.; Al Sharif, M.; Alov, P.; Pencheva, T.; Tsakovska, I.; Pajeva, I. ADME/Tox properties and biochemical interactions of silybin congeners: In silico study. Nat. Prod. Commun. 2017, 12, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holeckova, V.; Petraskova, L.; Biedermann, D.; Valentova, K.; Buchta, M.; Kren, V. The silymarin composition... and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Diukendjieva, A.; Alov, P.; Tsakovska, I.; Pencheva, T.; Richarz, A.; Kren, V.; Cronin, M.T.D.; Pajeva, I. In vitro and in silico studies of the membrane permeability of natural flavonoids from Silybum marianum (L.) Gaertn. and their derivatives. Phytomedicine 2019, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Valentova, K.; Havlik, J.; Kosina, P.; Papouskova, B.; Jaimes, J.D.; Kanova, K.; Petraskova, L.; Ulrichova, J.; Kren, V. Biotransformation of silymarin flavonolignans by human fecal microbiota. Metabolites 2020, 10, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.H.; Zhang, Y.T.; Chen, X.M.; Li, L.L.; Cai, S.Q. Biotransformation on the flavonolignan constituents of Silybi Fructus by an intestinal bacterial strain Eubacterium limosum ZL-II. Fitoterapia 2014, 92, 61–71. [Google Scholar] [CrossRef]
- Kren, V.; Marhol, P.; Purchartova, K.; Gabrielova, E.; Modriansky, M. Biotransformation of silybin and its congeners. Curr. Drug Metab. 2013, 14, 1009–1021. [Google Scholar] [CrossRef]
- Rowland, A.; Miners, J.O.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef]
- Vrba, J.; Papouskova, B.; Roubalova, L.; Zatloukalova, M.; Biedermann, D.; Kren, V.; Valentova, K.; Ulrichova, J.; Vacek, J. Metabolism of flavonolignans in human hepatocytes. J. Pharm. Biomed. Anal. 2018, 152, 94–101. [Google Scholar] [CrossRef]
- Vrba, J.; Papouskova, B.; Lnenickova, K.; Kosina, P.; Kren, V.; Ulrichova, J. Identification of UDP-glucuronosyltransferases involved in the metabolism of silymarin flavonolignans. J. Pharm. Biomed. Anal. 2020, 178, 112972. [Google Scholar] [CrossRef] [PubMed]
- Calani, L.; Brighenti, F.; Bruni, R.; Del Rio, D. Absorption and metabolism of milk thistle flavanolignans in humans. Phytomedicine 2012, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Dumas, T.E.; Schrieber, S.J.; Hawke, R.L.; Fried, M.W.; Smith, P.C. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab. Dispos. 2008, 36, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Papouskova, B.; Kosina, P.; Vrba, J.; Kren, V.; Ulrichova, J. Biotransformation of flavonols and taxifolin in hepatocyte in vitro systems as determined by liquid chromatography with various stationary phases and electrospray ionization-quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2012, 899, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Kren, V.; Vacek, J.; Papouskova, B.; Ulrichova, J. Quercetin, quercetin glycosides and taxifolin differ in their ability to induce AhR activation and CYP1A1 expression in HepG2 cells. Phytother. Res. 2012, 26, 1746–1752. [Google Scholar] [CrossRef]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 taxifolin metabolites and their distribution in rats using HPLC-ESI-IT-TOF-MSn. Molecules 2016, 21, 1209. [Google Scholar] [CrossRef]
- Jancova, P.; Siller, M.; Anzenbacherova, E.; Kren, V.; Anzenbacher, P.; Simanek, V. Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica 2011, 41, 743–751. [Google Scholar] [CrossRef]
- Xie, Y.; Miranda, S.R.; Hoskins, J.M.; Hawke, R.L. Role of UDP-glucuronosyltransferase 1A1 in the metabolism and pharmacokinetics of silymarin flavonolignans in patients with HCV and NAFLD. Molecules 2017, 22, 142. [Google Scholar] [CrossRef]
- Petraskova, L.; Kanova, K.; Biedermann, D.; Kren, V.; Valentova, K. Simple and rapid HPLC separation and quantification of flavonoid, flavonolignans, and 2,3-dehydroflavonolignans in silymarin. Foods 2020, 9, 116. [Google Scholar] [CrossRef]
- Lindsay, J.; Wang, L.L.; Li, Y.; Zhou, S.F. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr. Drug Metab. 2008, 9, 99–105. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Noleto-Dias, C.; Harflett, C.; Beale, M.H.; Ward, J.L. Sulfated flavanones and dihydroflavonols from willow. Phytochem. Lett. 2020, 35, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gazak, R.; Kren, V.; Agarwal, R. Anti-cancer efficacy of silybin derivatives—A structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef] [PubMed]
- Marhol, P.; Hartog, A.F.; van der Horst, M.A.; Wever, R.; Purchartova, K.; Fuksova, K.; Kuzma, M.; Cvacka, J.; Kren, V. Preparation of silybin and isosilybin sulfates by sulfotransferase from Desulfitobacterium hafniense. J. Mol. Catal. B-Enzym. 2013, 89, 24–27. [Google Scholar] [CrossRef]
- Roubalova, L.; Purchartova, K.; Papouskova, B.; Vacek, J.; Kren, V.; Ulrichova, J.; Vrba, J. Sulfation modulates the cell uptake, antiradical activity and biological effects of flavonoids in vitro: An examination of quercetin, isoquercitrin and taxifolin. Bioorg. Med. Chem. 2015, 23, 5402–5409. [Google Scholar] [CrossRef]
- Valentova, K.; Purchartova, K.; Rydlova, L.; Roubalova, L.; Biedermann, D.; Petraskova, L.; Krenkova, A.; Pelantova, H.; Holeckova-Moravcova, V.; Tesarova, E.; et al. Sulfated metabolites of flavonolignans and 2,3-dehydroflavonolignans: Preparation and properties. Int. J. Mol. Sci. 2018, 19, 2349. [Google Scholar] [CrossRef]
- Purchartova, K.; Engels, L.; Marhol, P.; Sulc, M.; Kuzma, M.; Slamova, K.; Elling, L.; Kren, V. Enzymatic preparation of silybin phase II metabolites: Sulfation using aryl sulfotransferase from rat liver. Appl. Microbiol. Biotechnol. 2013, 97, 10391–10398. [Google Scholar] [CrossRef]
- Purchartova, K.; Valentova, K.; Pelantova, H.; Marhol, P.; Cvacka, J.; Havlicek, L.; Krenkova, A.; Vavrikova, E.; Biedermann, D.; Chambers, C.S.; et al. Prokaryotic and eukaryotic aryl sulfotransferases: Sulfation of quercetin and its derivatives. ChemCatChem 2015, 7, 3152–3162. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Mikell, J.R.; Khan, I.A. Bioconversion of silybin to phase I and II microbial metabolites with retained antioxidant activity. Bioorg. Med. Chem. 2012, 20, 2784–2788. [Google Scholar] [CrossRef]
- Coughtrie, M.W.H. Function and organization of the human cytosolic sulfotransferase (SULT) family. Chem. Biol. Interact. 2016, 259, 2–7. [Google Scholar] [CrossRef]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: The SULT “pie”. Drug Metab. Dispos. 2009, 37, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Modriansky, M.; Ulrichova, J.; Bachleda, P.; Anzenbacher, P.; Anzenbacherova, E.; Walterova, D.; Simanek, V. Human hepatocyte—A model for toxicological studies. Functional and biochemical characterization. Gen. Physiol. Biophys. 2000, 19, 223–235. [Google Scholar] [PubMed]
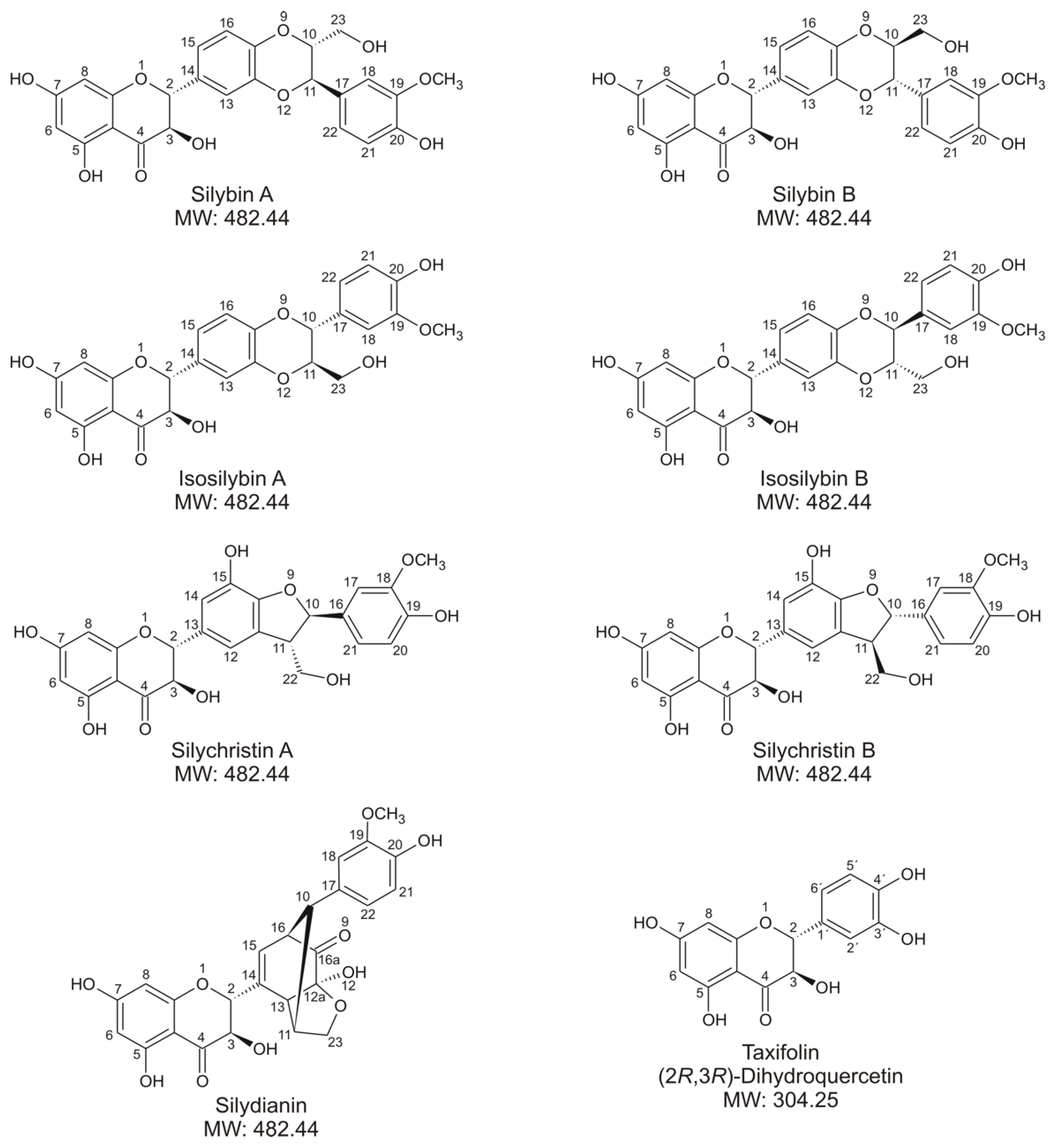
| Compound | m/z1 | tR (min) | Semiquantitative Percentage 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLC | HIC | 1A1*1 | 1A1*2 | 1A2 | 1A3 | 1B1 | 1C2 | 1C4 | 1E1 | 2A1 | |||
| Silybin A (parent) | 481.115 | 7.02 | 99.9 | 99.6 | 84.4 | 80.8 | 75.3 | 93.8 | 77.8 | 99.9 | 23.9 | 14.0 | 99.8 |
| Sulfate (1) | 561.069 | 5.67 | 0.0 | 0.1 | 3.7 | 4.3 | 1.3 | 4.3 | 0.6 | 0.1 | 5.0 | 85.4 | 0.1 |
| 20-O-Sulfate (2) | 561.069 | 5.92 | 0.1 | 0.2 | 11.9 | 14.9 | 23.4 | 1.9 | 21.6 | 0.0 | 71.1 | 0.6 | 0.0 |
| Sulfate (3) | 561.069 | 6.08 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Silybin B (parent) | 481.115 | 7.19 | 99.9 | 99.1 | 96.3 | 97.1 | 90.3 | 54.9 | 66.1 | 99.9 | 58.0 | 13.7 | 99.9 |
| Sulfate (1) | 561.069 | 5.84 | 0.0 | 0.5 | 0.2 | 0.1 | 0.1 | 43.2 | 0.0 | 0.1 | 38.6 | 0.5 | 0.0 |
| 20-O-Sulfate (2) | 561.069 | 6.11 | 0.1 | 0.3 | 3.5 | 2.8 | 9.4 | 1.8 | 33.8 | 0.0 | 3.2 | 85.8 | 0.1 |
| Sulfate (3) | 561.069 | 6.43 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 |
| Isosilybin A (parent) | 481.115 | 7.56 | 99.7 | 95.1 | 79.1 | 78.9 | 80.3 | 26.8 | 42.1 | 99.7 | 19.4 | 68.4 | 99.9 |
| Sulfate (1) | 561.069 | 6.23 | 0.1 | 1.4 | 16.3 | 16.6 | 6.0 | 17.1 | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 |
| 20-O-Sulfate (2) | 561.069 | 6.42 | 0.2 | 3.5 | 4.6 | 4.5 | 13.7 | 56.1 | 57.9 | 0.1 | 80.4 | 31.5 | 0.1 |
| Isosilybin B (parent) | 561.069 | 7.64 | 99.7 | 95.9 | 75.7 | 77.9 | 60.3 | 33.7 | 92.0 | 99.9 | 49.6 | 34.3 | 99.9 |
| Sulfate (1) | 561.069 | 6.33 | 0.1 | 1.5 | 13.4 | 12.9 | 36.9 | 31.2 | 3.7 | 0.1 | 37.6 | 9.2 | 0.0 |
| Sulfate (2) | 561.069 | 6.42 | 0.1 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sulfate (3) | 561.069 | 6.57 | 0.1 | 1.9 | 10.9 | 9.2 | 2.8 | 35.1 | 3.6 | 0.0 | 12.8 | 56.5 | 0.1 |
| Silychristin A/B (parent) | 481.115 | 5.98 3, 6.25 3 | 99.9 | 95.7 | 81.9 | 83.6 | 96.0 | 12.4 | 49.6 | 99.6 | 13.4 | 10.0 | 100.0 |
| Sulfate (1) | 561.069 | 4.67 | 0.0 | 0.0 | 2.9 | 3.4 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 |
| Sulfate (2) | 561.069 | 4.97 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 |
| Sulfate (3) | 561.069 | 5.07 | 0.1 | 4.3 | 15.2 | 13.0 | 2.9 | 87.6 | 50.4 | 0.0 | 86.6 | 83.6 | 0.0 |
| Sulfate (4) | 561.069 | 5.25 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.1 | 0.0 |
| Silydianin (parent) | 481.115 | 6.19 | 99.2 | 98.9 | 8.3 | 8.7 | 78.2 | 95.1 | 99.9 | 99.8 | 99.7 | 9.3 | 100.0 |
| Sulfate (1) | 561.069 | 5.10 | 0.8 | 1.1 | 91.7 | 91.3 | 21.8 | 4.9 | 0.1 | 0.2 | 0.3 | 90.7 | 0.0 |
| Taxifolin (parent) | 303.050 | 4.41, 4.91 4 | 99.7 | 85.7 | 83.8 | 87.5 | 41.2 | 0.4 | 33.5 | 99.9 | 0.6 | 59.5 | 100.0 |
| Sulfate (1) | 383.006 | 1.92 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15.1 | 0.0 |
| Sulfate (2) | 383.006 | 2.97 | 0.0 | 0.0 | 0.0 | 0.0 | 5.7 | 0.3 | 0.0 | 0.1 | 0.7 | 16.1 | 0.0 |
| 4′-O-Sulfate (3) | 383.006 | 3.69 | 0.0 | 0.1 | 0.0 | 0.0 | 0.8 | 0.2 | 0.0 | 0.0 | 0.1 | 1.0 | 0.0 |
| Sulfate (4) | 383.006 | 3.97 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 1.8 | 0.0 |
| Sulfate (5) | 383.006 | 4.21 | 0.3 | 14.2 | 16.2 | 12.5 | 52.0 | 99.1 | 66.2 | 0.0 | 98.6 | 4.3 | 0.0 |
| Sulfate (6) | 383.006 | 4.48 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | 0.0 |
| Compound | 1A1*1 | 1A1*2 | 1A2 | 1A3 | 1B1 | 1C2 | 1C4 | 1E1 | 2A1 |
|---|---|---|---|---|---|---|---|---|---|
| Silybin A | 18 | 21 | 28 | 7 | 25 | 0.1 | 76 | 100 | 0.2 |
| Silybin B | 5 | 3 | 11 | 62 | 44 | 0.2 | 53 | 100 | 0.1 |
| Isosilybin A | 24 | 23 | 19 | 81 | 69 | 0.3 | 100 | 35 | 0.1 |
| Isosilybin B | 38 | 34 | 62 | 100 | 12 | 0.2 | 69 | 85 | 0.2 |
| Silychristin A/B | 14 | 12 | 3 | 100 | 47 | 0.3 | 96 | 89 | – |
| Silydianin | 98 | 96 | 18 | 4 | 0.1 | 0.1 | 0.2 | 100 | – |
| Taxifolin | 7 | 5 | 30 | 100 | 38 | 0.1 | 82 | 14 | – |
| Silymarin | 10 | 11 | 44 | 58 | 60 | 0.2 | 55 | 100 | 0.1 |
| Peak No. 1 | tR (min) | m/z | Potential Metabolites 2 |
|---|---|---|---|
| 1 | 1.92 | 383.006 | Taxifolin sulfate (1) |
| 2 | 4.21 | 383.006 | Taxifolin sulfate (5) |
| 3 | 5.10 | 561.069 | Silychristin sulfate (3), silydianin sulfate (1) |
| 4 | 5.84 | 561.069 | Silybin A sulfate (1), silybin B sulfate (1) |
| 5 | 5.92 | 561.069 | Silybin A 20-O-sulfate (2) |
| 6 | 6.11 | 561.069 | Silybin B 20-O-sulfate (2) |
| 7 | 6.33 | 561.069 | Isosilybin A sulfate (1), isosilybin B sulfate (1) |
| 8 | 6.42 | 561.069 | Isosilybin A 20-O-sulfate (2), isosilybin B sulfate (2) |
| 9 | 6.57 | 561.069 | Isosilybin B sulfate (3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrba, J.; Papoušková, B.; Kosina, P.; Lněničková, K.; Valentová, K.; Ulrichová, J. Identification of Human Sulfotransferases Active towards Silymarin Flavonolignans and Taxifolin. Metabolites 2020, 10, 329. https://doi.org/10.3390/metabo10080329
Vrba J, Papoušková B, Kosina P, Lněničková K, Valentová K, Ulrichová J. Identification of Human Sulfotransferases Active towards Silymarin Flavonolignans and Taxifolin. Metabolites. 2020; 10(8):329. https://doi.org/10.3390/metabo10080329
Chicago/Turabian StyleVrba, Jiří, Barbora Papoušková, Pavel Kosina, Kateřina Lněničková, Kateřina Valentová, and Jitka Ulrichová. 2020. "Identification of Human Sulfotransferases Active towards Silymarin Flavonolignans and Taxifolin" Metabolites 10, no. 8: 329. https://doi.org/10.3390/metabo10080329
APA StyleVrba, J., Papoušková, B., Kosina, P., Lněničková, K., Valentová, K., & Ulrichová, J. (2020). Identification of Human Sulfotransferases Active towards Silymarin Flavonolignans and Taxifolin. Metabolites, 10(8), 329. https://doi.org/10.3390/metabo10080329