Differential Metabolomics Profiles Identified by CE-TOFMS between High and Low Intramuscular Fat Amount in Fattening Pigs
Abstract
1. Introduction
2. Results
2.1. Phenotypic Characteristics
2.2. Screening of Differential Metabolomics Profiles with CE-TOFMS
2.3. Integrated Analysis of Metabolomics Data Using Absolute Quantification
2.4. Comparison of Amino Acids and Related Metabolites
2.5. Amino Acid Content Analysis in the Muscle Tissue of Pig Carcass
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Semi-Quantitative Metabolomics (Basic Scan) by Capillary Electrophoresis-Time of Flight Mass Spectrometry (CE-TOFMS)
4.3. Absolute Quantification of 110 Target Metabolites for CE-TOFMS Analysis
4.4. Measurement of Free Amino Acids, Peptides, and Carcass Traits in Muscle Tissues
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Apple, J.K. Nutritional effects on pork quality in swine production. In National Swine Nutrition Guide; Factsheet No. PIG 12-02-02; U.S. Pork Center of Excellence: Clive, IA, USA, 2015. [Google Scholar]
- Schwab, C.R.; Baas, T.J.; Stalder, K.J. Results from six generations of selection for intramuscular fat in Duroc swine using real-time ultrasound. Ⅱ. Genetic parameters and trends. J. Anim. Sci. 2010, 88, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; de Pedro, E.; Núñez, N.; Silió, L.; García-Casco, J.; Rodríguez, C. Genetic parameters for meat and fat quality and carcass composition traits in Iberian pigs. Meat Sci. 2003, 64, 405–410. [Google Scholar] [CrossRef]
- Won, S.; Jung, J.; Park, E.; Kim, H. Identification of genes related to intramuscular fat content of pigs using genome-wide association study. Asian Aust. J. Anim. Sci. 2018, 31, 157–162. [Google Scholar] [CrossRef]
- Davoli, R.; Catillo, G.; Serra, A.; Zappaterra, M.; Zambonelli, P.; Meo Zilio, D.; Steri, R.; Mele, M.; Buttazzoni, L.; Russo, V. Genetic parameters of backfat fatty acids and carcass traits in Large White pigs. Animal 2019, 13, 924–932. [Google Scholar] [CrossRef]
- Hovenier, R.; Kanis, E.; van Asseldonk, T.H.; Westerink, N.G. Breeding for pig meat quality in halothane negative populations—A review. Pig News Inf. 1993, 14, 17N–25N. [Google Scholar]
- Sellier, P. Genetics of meat and carcass traits. In Genetics of the Pig; Rothchild, M.F., Ruvinsky, A., Eds.; CAB International: Wallingford, Oxon, UK, 1998; pp. 463–510. [Google Scholar]
- Newcom, D.W.; Baas, T.J.; Schwab, C.R.; Stalder, K.J. Genetic and phenotypic relationships between individual subcutaneous backfat layers and percentage of longissimus intramuscular fat in Duroc swine. J. Anim. Sci. 2005, 83, 316–323. [Google Scholar] [CrossRef]
- Suzuki, K.; Irie, M.; Kadowaki, H.; Shibata, T.; Kumagai, M.; Nishida, A. Genetic parameter estimates of meat quality traits in Duroc pigs selected for average daily gain, longissimus muscle area, backfat thickness, and intramuscular fat content. J. Anim. Sci. 2005, 83, 2058–2065. [Google Scholar] [CrossRef]
- Solanes, F.X.; Reixach, J.; Tor, M.; Tibau, J.; Estany, J. Genetic correlations and expected response for intramuscular fat content in a Duroc pig line. Livest. Prod. Sci. 2009, 123, 63–69. [Google Scholar] [CrossRef]
- Cesar, A.S.M.; Regitano, L.C.A.; Koltes, J.E.; Fritz-Waters, E.R.; Lanna, D.P.D.; Gasparin, G.; Mourao, G.B.; Oliveira, P.S.N.; Reecy, J.M.; Coutinho, L.L. Putative Regulatory Factors Associated with Intramuscular Fat Content. PLoS ONE 2015, 10, e0128350. [Google Scholar] [CrossRef]
- Da Costa, N.; McGillivray, C.; Bai, Q.; Wood, J.D.; Evans, G.; Chang, K.C. Restriction of dietary energy and protein induces molecular changes in young porcine skeletal muscles. J. Nutr. 2004, 134, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, M. Promotion of intramuscular fat accumulation in porcine muscle by nutritional regulation. Anim. Sci. J. 2011, 82, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Oe, M.; Nakajima, I.; Ojima, K.; Chikuni, K. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles. Meat Sci. 2014, 98, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and meat metabolomics in domestic animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Fontanesi, L. Metabolomics and livestock genomics: Insights into a phenotyping frontier andits applications in animal breeding. Anim. Front. 2016, 6, 73–79. [Google Scholar] [CrossRef]
- Suravajhala, P.; Kogelman, L.J.A.; Kadarmideen, H.N. Multi-omic data integration and analysis using systems genomics approaches: Methods and applications in animal production, health and welfare. Genet. Sel. Evol. 2016, 48, 38. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, X.; Zhu, Z.; Jiao, N.; Qiu, K.; Yin, J. Surplus dietary isoleucine intake enhanced monounsaturated fatty acid synthesis and fat accumulation in skeletal muscle of finishing pigs. J. Anim. Sci. Biotech. 2018, 9, 88. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, L.; Qiao, S.; Mao, X.; Zeng, X. Effects of dietary leucine supplementation in low crude protein diets on performance, nitrogen balance, whole-body protein turnover, carcass characteristics and meat quality of finishing pigs. Anim. Sci. J. 2016, 87, 911–920. [Google Scholar] [CrossRef]
- Zheng, L.; Wei, H.; He, P.; Zhao, S.; Xiang, Q.; Pang, J.; Peng, J. Effects of supplementation of branched-chain amino acids to reduced-protein diet on skeletal muscle protein synthesis and degradation in the fed and fasted states in a piglet model. Nutrients 2017, 9, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotech. 2018, 9, 60. [Google Scholar] [CrossRef]
- Ishii, K.; Arata, S.; Ohnishi, C. Estimates of genetic parameters for meat quality and carcass traits in Duroc pigs. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 8 February 2018. Abstract Number 408. [Google Scholar]
- Smriga, M.; Kameishi, M.; Uneyama, H.; Torii, K. DietaryL-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. J. Nutr. 2002, 132, 3744–3746. [Google Scholar] [CrossRef] [PubMed]
- Srinongkote, S.; Smrige, M.; Toride, Y. Diet supplied with L-lysine and L-arginine during chronic stress of high stock density normalizes growth of broilers. Anim. Sci. J. 2004, 75, 339–343. [Google Scholar] [CrossRef]
- Harris, C.L.; Wang, B.; Deavila, J.M.; Bushboom, J.R.; Maquivar, M.; Parish, S.M.; McCann, B.; Nelson, M.L.; Du, M. Vitamin A administration at birth promotes calf growth and intramuscular fat development in Angus beef cattle. J. Anim. Sci. Biotechnol. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kruk, Z.A.; Bottema, M.J.; Reyes-Veliz, L.; Forder, R.E.A.; Pitchford, W.S.; Bottema, C.D.K. Vitamin A and Marbling Attributes: Intramuscular Fat Hyperplasia Effects in Cattle. Meat Sci. 2018, 137, 139–146. [Google Scholar] [CrossRef]
- Ward, A.K.; McKinnon, J.J.; Hendrick, S.; Buchanan, F.C. The impact of vitamin A restriction and ADH1C genotype on marbling in feedlot steers. J. Anim. Sci. 2015, 90, 2476–2483. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Sugimoto, M.; Goto, H.; Otomo, K.; Ito, M.; Onuma, H.; Suzuki, A.; Sugawara, M.; Abe, S.; Tomita, M.; Soga, T. Metabolomic profiles and sensory attributes of edamame under various storage duration and temperature conditions. J. Agric. Food Chem. 2010, 58, 8418–8425. [Google Scholar] [CrossRef]
- Sakuma, H.; Saito, K.; Kohira, K.; Ohhashi, F.; Shoji, N.; Uemoto, Y. Estimates of genetic parameters for chemical traits of meat quality in Japanese black cattle. Anim. Sci. J. 2017, 88, 203–212. [Google Scholar] [CrossRef]
- Okumura, T.; Saito, K.; Sakuma, H.; Nade, T.; Nakayama, S.; Fujita, K.; Kawamura, T. Intramuscular fat deposition in principal muscles from twenty-four to thirty months of age using identical twins of Japanese Black steers. J. Anim. Sci. 2007, 85, 1902–1907. [Google Scholar] [CrossRef]
- Yamamoto, H. PLS-ROG: Partial least squares with rank order of groups. J. Chemom. 2017, 33, e2883. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 29 May 2020).
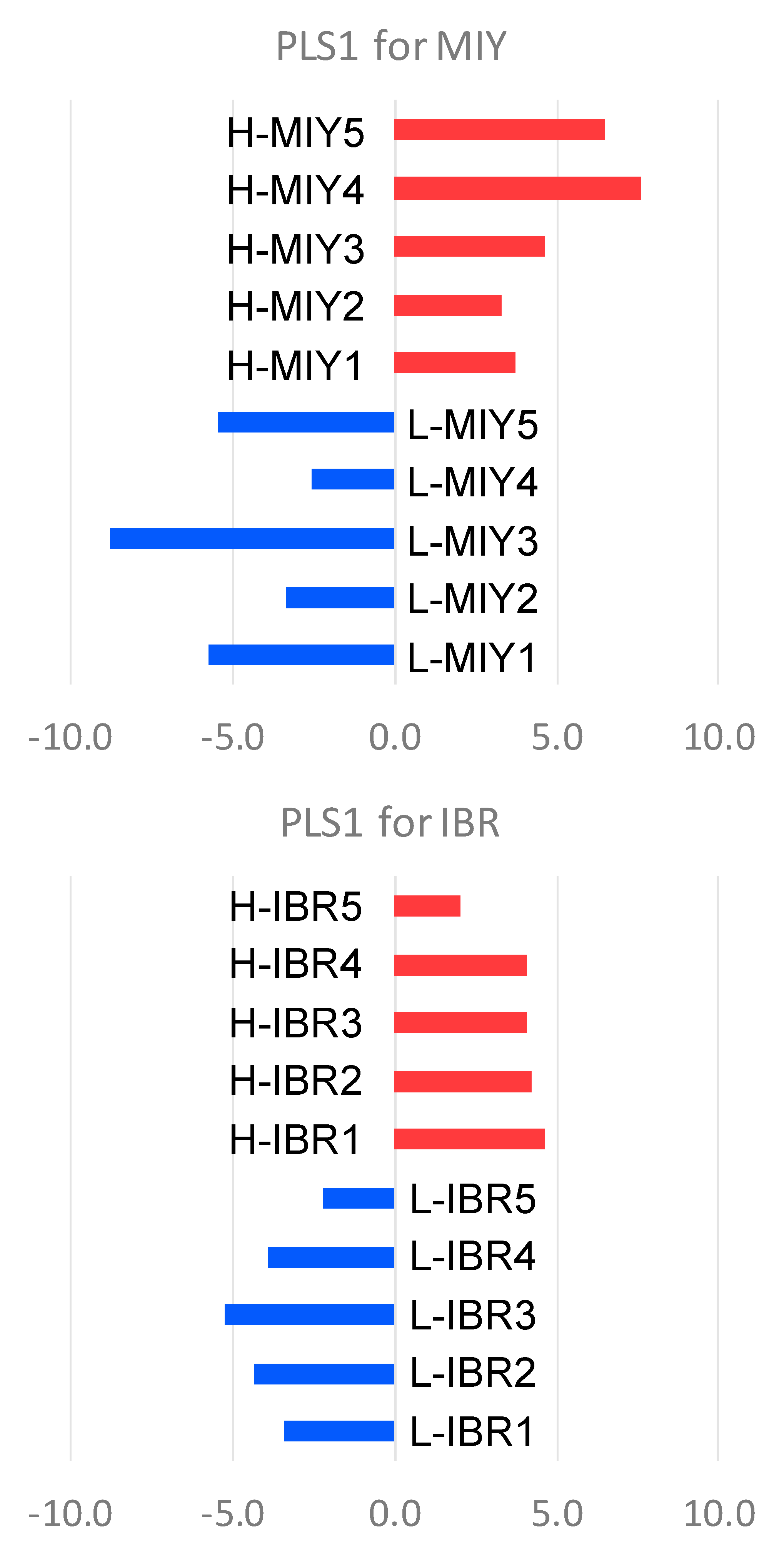

| Growth Performance and Carcass Characteristics | Miyazaki (MIY) Station | Ibaraki (IBR) Station | ||||
|---|---|---|---|---|---|---|
| L-IMF MIY | H-IMF MIY | p *1 | L-IMF IBR | H-IMF IBR | p *1 | |
| IMF (%) | 3.101 ± 0.255 | 8.699 ± 1.441 | p < 0.001 | 2.868 ± 0.550 | 5.447 ± 0.797 | p < 0.001 |
| Days old at slaughter | 149.2 ± 9.99 | 152.2 ± 13.64 | N.S. | 154.4 ± 8.357 | 169.8 ± 6.997 | p < 0.05 |
| Moisture (%) | 73.96 ± 0.339 | 69.98 ± 0.966 | p < 0.001 | 73.81 ± 0.978 | 71.48 ± 0.444 | p < 0.01 |
| Crude protein (%) | 21.82 ± 0.173 | 20.23 ± 0.917 | p < 0.01 | 22.35 ± 0.149 | 22.02 ± 0.350 | N.S. |
| Cooking loss (%) | 24.68 ± 1.548 | 26.52 ± 1.823 | N.S. | 28.04 ± 1.966 | 24.38 ± 2.269 | p < 0.05 |
| WHC (%) *2 | 68.30 ± 5.489 | 72.30 ± 4.511 | N.S. | 68.14 ± 5.824 | 68.94 ± 4.199 | N.S. |
| WBSF (kg) *3 | 2.392 ± 0.338 | 2.048 ± 0.489 | N.S. | 2.963 ± 0.550 | 2.838 ± 0.478 | N.S. |
| Tenderness (kg/cm2) | 38.09 ± 5.586 | 34.81 ± 2.010 | N.S. | 46.45 ± 6.442 | 44.82 ± 6.710 | N.S. |
| Pliability | 1.497 ± 0.0918 | 1.375 ± 0.0609 | N.S. | 1.527 ± 0.0442 | 1.494 ± 0.0679 | N.S. |
| Toughness (kg/cm2 × cm) | 8.163 ± 1.474 | 8.054 ± 1.082 | N.S. | 10.27 ± 1.900 | 10.15 ± 1.888 | N.S. |
| Brittleness | 1.631 ± 0.0750 | 1.662 ± 0.0864 | N.S. | 1.568 ± 0.0880 | 1.632 ± 0.110 | N.S. |
| Average daily gain (g/day) | 1141.3 ± 62.70 | 1035.9 ± 188.8 | N.S. | 1006.7 ± 53.91 | 1008.8 ± 46.73 | N.S. |
| Loin eye area (cm2) | 35.06 ± 1.318 | 31.45 ± 2.059 | p < 0.05 | 35.10 ± 3.369 | 34.29 ± 3.219 | N.S. |
| Back fat thickness (cm) | 2.597 ± 0.547 | 3.376 ± 0.548 | N.S. | 2.044 ± 0.568 | 2.476 ± 0.661 | N.S. |
| Statue height (cm) | 63.16 ± 1.895 | 61.22 ± 0.722 | N.S. | 65.48 ± 1.001 | 64.16 ± 2.330 | N.S. |
| Body length (cm) | 107.6 ± 3.855 | 105.28 ± 2.285 | N.S. | 89.7 ± 39.35 | 106.5 ± 5.040 | N.S. |
| MIY Station | IBR Station | ||||
|---|---|---|---|---|---|
| Metabolites | PLS1 | Metabolites | PLS1 | ||
| R *1 | p | R *1 | p | ||
| Positive loading | |||||
| Leu | 0.731 | 1.6 × 10−2 | Urea | 0.729 | 1.7 × 10−2 |
| O-acetylhomoserine 2-aminoadipic acid | 0.727 | 1.7 × 10−2 | Gluconic acid | 0.654 | 4.0 × 10−2 |
| 1-Methylnicotinamide | 0.711 | 2.1 × 10−2 | 3-hydroxybutyric acid | 0.544 | 1.0 × 10−1 |
| Choline | 0.645 | 4.4 × 10−2 | Isethionic acid | 0.505 | 1.4 × 10−1 |
| Phosphorylcholine | 0.637 | 4.8 × 10−2 | Nicotinamide | 0.494 | 1.5 × 10−1 |
| Ile | 0.609 | 6.1 × 10−2 | Val | 0.493 | 1.5 × 10−1 |
| Creatine | 0.603 | 6.5 × 10−2 | Taurine | 0.488 | 1.5 × 10−1 |
| Val | 0.582 | 7.8 × 10−2 | Mucic acid | 0.476 | 1.6 × 10−1 |
| N,N-dimethylglycine | 0.569 | 8.6 × 10−2 | Homocitrulline | 0.475 | 1.7 × 10−1 |
| Trp | 0.470 | 1.7 × 10−1 | Sarcosine | 0.452 | 1.9 × 10−1 |
| Negative loading | |||||
| Gly | −0.861 | 1.4 × 10−3 | Thr | −0.716 | 2.0 × 10−2 |
| Anserine_divalent | −0.840 | 2.3 × 10−3 | Diethanolamine | −0.705 | 2.3 × 10−2 |
| N-acetylornithine | −0.834 | 2.7 × 10−3 | Thymidine | −0.611 | 6.0 × 10−2 |
| N-acetyllysine | −0.796 | 5.9 × 10−3 | 5-hydroxylysine | −0.589 | 7.3 × 10−2 |
| 5-oxoproline | −0.753 | 1.2 × 10−2 | Asn | −0.566 | 8.8 × 10−2 |
| Carnosine | −0.741 | 1.4 × 10−2 | Arg | −0.533 | 1.1 × 10−1 |
| Creatinine | −0.707 | 2.2 × 10−2 | Lys | −0.521 | 1.2 × 10−1 |
| N5-ethylglutamine | −0.705 | 2.3 × 10−2 | Hydroxyproline | −0.514 | 1.3 × 10−1 |
| cis-Aconitic acid | −0.685 | 2.9 × 10−2 | Met | −0.505 | 1.4 × 10−1 |
| β-Ala | −0.683 | 2.9 × 10−2 | Tyr | −0.459 | 1.8 × 10−1 |
| Metabolites | R *1 | p *2 | MIY Station | IBR Station | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low IMF | High IMF | p *2 | Low IMF | High IMF | p *2 | Low IMF | High IMF | p *2 | |||
| Positively Loading | |||||||||||
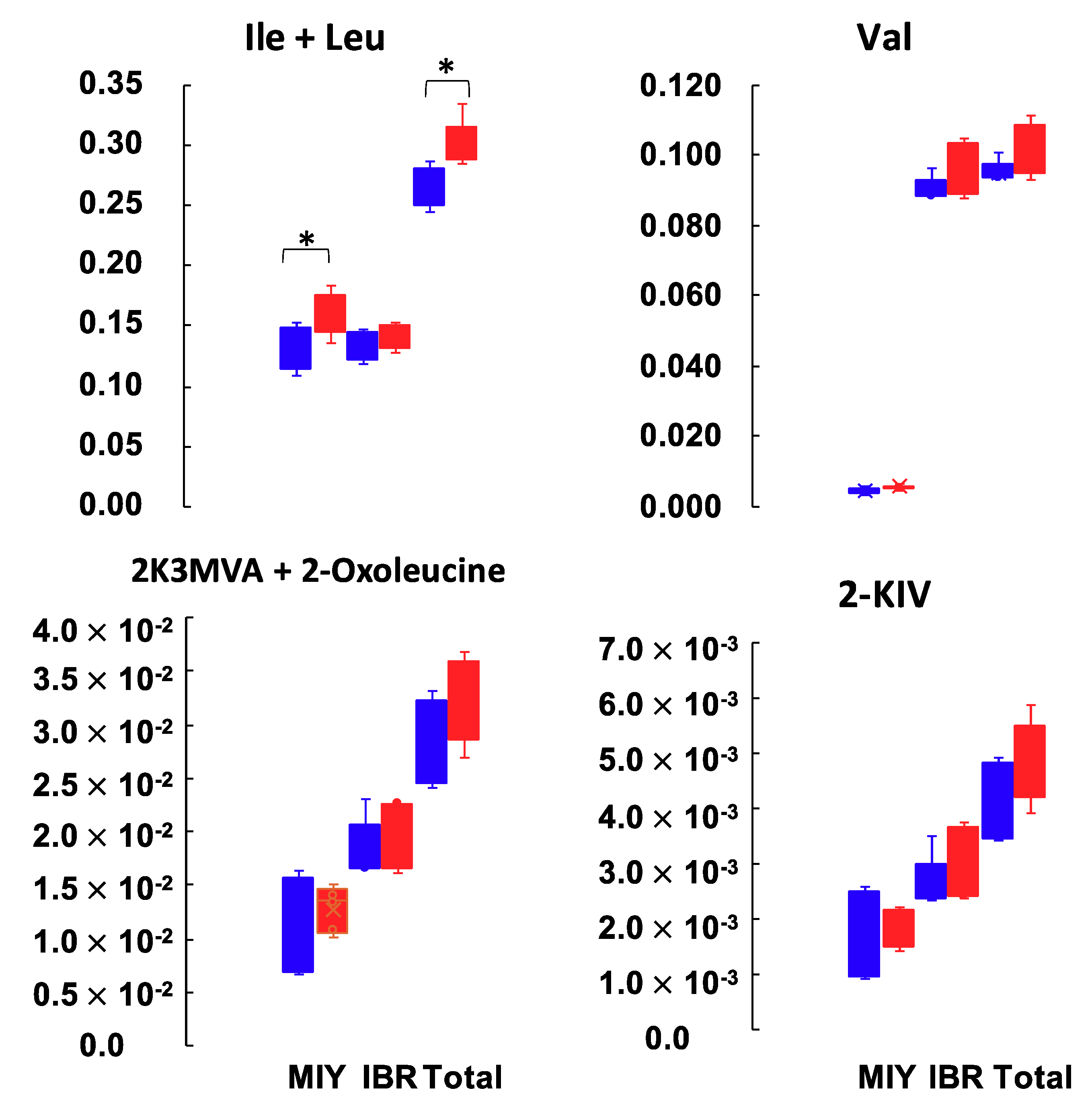
| Leu | 0.610 | *** | 153.33 ± 6.18 | 180.6 ± 6.56 | * | 156.41 ± 6.46 | 165.16 ± 6.13 | N.S. | 154.86 ± 5.81 | 172.88 ± 7.00 | * |
| Ile | 0.577 | *** | 93.43 ± 9.25 | 120.37 ± 8.14 | tnd. | 107.85 ± 4.29 | 110.69 ± 2.94 | N.S. | 100.64 ± 7.65 | 115.53 ± 6.20 | * |
| Val | 0.531 | * | 236.99 ± 19.06 | 284.70 ± 13.93 | tnd. | 268.24 ± 4.32 | 285.19 ± 9.64 | N.S. | 252.62 ± 14.94 | 284.94 ± 11.29 | * |
| Creatine | 0.529 | * | 232.13 ± 34.19 | 307.37 ± 8.54 | tnd. | 302.38 ± 4.64 | 317.32 ± 10.72 | N.S. | 267.25 ± 28.34 | 312.34 ± 9.44 | * |
| Trp | 0.370 | tnd. | 60.06 ± 6.08 | 71.31 ± 4.34 | N.S. | 74.12 ± 2.92 | 72.079 ± 3.35 | N.S. | 67.08 ± 5.59 | 71.69 ± 3.66 | N.S. |
| Negatively Loading | |||||||||||
| Gly | −0.788 | *** | 920.72 ± 48.07 | 666.51 ± 22.31 | ** | 829.52 ± 14.96 | 799.15 ± 43.97 | N.S. | 875.12 ± 39.86 | 732.83 ± 45.36 | *** |
| Creatinine | −0.611 | *** | 102.50 ± 5.45 | 82.42 ± 4.56 | * | 89.64 ± 3.82 | 89.99 ± 3.83 | N.S. | 96.07 ± 5.38 | 86.20 ± 4.35 | tnd. |
| Hydroxyproline | −0.573 | *** | 81.09 ± 11.06 | 51.67 ± 7.05 | tnd. | 66.47 ± 3.25 | 55.19 ± 5.81 | N.S. | 73.78 ± 8.42 | 53.43 ± 6.15 | * |
| β-Ala | −0.506 | * | 6.362 ± 0.755 | 4.164 ± 0.347 | ** | 6.388 ± 0.323 | 6.522 ± 0.793 | N.S. | 6.375 ± 0.547 | 5.343 ± 0.801 | N.S. |
| Carnosine | −0.443 | tnd. | 10.97 ± 0.590 | 8.239 ± 0.646 | * | 11.52 ± 1.03 | 11.80 ± 1.01 | N.S. | 11.245 ± 0.800 | 10.019 ± 1.157 | N.S. |
| Amino Acid *1 | MIY Station | IBR Station | ||||
|---|---|---|---|---|---|---|
| Low IMF | High IMF | p *2 | Low IMF | High IMF | p *2 | |
| Asp | 9.6 × 10−2 ± 8.1 × 10−3 | 9.5 × 10−2 ± 2.1 × 10−2 | N.S. | 8.1 × 10−2 ± 3.3 × 10−2 | 3.2 × 10−2 ± 1.0 × 10−2 | * |
| Glu | 5.5 × 10−1 ± 1.0 × 10−1 | 6.2 × 10−1 ± 1.2 × 10−1 | N.S. | 6.8 × 10−1 ± 1.5 × 10−1 | 5.7 × 10−1 ± 9.3 × 10−2 | N.S. |
| Asn | 1.6 × 10−1 ± 2.8 × 10−2 | 2.3 × 10−1 ± 5.0 × 10−2 | * | 2.6 × 10−1 ± 6.9 × 10−2 | 2.4 × 10−1 ± 1.7 × 10−2 | N.S. |
| Ser | 4.9 × 10−1 ± 9.1 × 10−2 | 4.8 × 10−1 ± 9.9 × 10−2 | N.S. | 5.5 × 10−1 ± 1.2 × 10−1 | 4.5 × 10−1 ± 5.1 × 10−2 | N.S. |
| Gln | 1.658 ± 0.250 | 1.490 ± 0.144 | N.S. | 1.485 ± 0.438 | 9.9 × 10−1 ± 1.4 × 10−1 | tnd. |
| His | 1.7 × 10−1 ± 2.0 × 10−2 | 1.7 × 10−1 ± 2.6 × 10−2 | N.S. | 1.9 × 10−1 ± 2.3 × 10−2 | 1.5 × 10−1 ± 2.3 × 10−2 | * |
| Gly | 1.62 ± 0.26 | 1.213 ± 0.0966 | * | 1.852 ± 0.498 | 1.789 ± 0.481 | N.S. |
| Thr | 3.4 × 10−1 ± 5.9 × 10−2 | 3.6 × 10−1 ± 6.2 × 10−2 | N.S. | 3.9 × 10−1 ± 7.5 × 10−2 | 3.3 × 10−1 ± 3.1 × 10−2 | N.S. |
| β-Ala | 6.7 × 10−1 ± 2.3 × 10−1 | 4.4 × 10−1 ± 1.0 × 10−1 | N.S. | 5.1 × 10−1 ± 8.3 × 10−2 | 5.6 × 10−1 ± 1.6 × 10−1 | N.S. |
| Arg | 3.6 × 10−1 ± 5.9 × 10−2 | 3.5 × 10−1 ± 6.6 × 10−2 | N.S. | 4.0 × 10−1 ± 8.3 × 10−2 | 2.9 × 10−1 ± 3.5 × 10−2 | tnd. |
| Ala | 2.143 ± 0.325 | 2.329 ± 0.397 | N.S. | 2.643 ± 0.472 | 2.170 ± 0.293 | N.S. |
| Tau | 2.341 ± 0.441 | 2.16 ± 0.289 | N.S. | 2.404 ± 0.772 | 1.829 ± 0.920 | N.S. |
| Car | 26.41 ±1.56 | 20.99 ± 2.35 | ** | 28.95 ± 1.65 | 27.60 ± 2.20 | N.S. |
| Ans | 8.5 × 10−1 ± 6.0 × 10−2 | 6.6 × 10−1 ± 8.4 × 10−2 | ** | 7.1 × 10−1 ± 5.7 × 10−2 | 7.3 × 10−1 ± 1.2 × 10−1 | N.S. |
| Tyr | 2.8 × 10−1 ± 4.7 × 10−2 | 2.7 × 10−1 ± 4.8 × 10−2 | N.S. | 3.0 × 10−1 ± 5.8 × 10−2 | 2.5 × 10−1 ± 2.3 × 10−2 | N.S. |
| Val | 3.9 × 10−1 ± 6.1 × 10−2 | 4.8 × 10−1 ± 5.7 × 10−2 | tnd. | 5.3 × 10−1 ± 8.0 × 10−2 | 4.4 × 10−1 ± 5.6 × 10−2 | N.S. |
| Met | 2.5 × 10−1 ± 4.1 × 10−2 | 2.7 × 10−1 ± 4.6 × 10−2 | N.S. | 3.0 × 10−1 ± 3.4 × 10−2 | 2.3 × 10−1 ± 2.1 × 10−2 | ** |
| Trp | 8.8 × 10−2 ± 7.8 × 10−3 | 9.9 × 10−2 ± 5.9 × 10−3 | tnd. | 1.2 × 10−1 ± 9.8 × 10−3 | 1.0 × 10−1 ± 5.2 × 10−3 | ** |
| Phe | 3.3 × 10−1 ± 4.4 × 10−2 | 3.5 × 10−1 ± 4.1 × 10−2 | N.S. | 3.9 × 10−1 ± 4.0 × 10−2 | 3.4 × 10−1 ± 1.2 × 10−2 | * |
| Ile | 3.1 × 10−1 ± 5.7 × 10−2 | 3.4 × 10−1 ± 5.1 × 10−2 | N.S. | 3.8 × 10−1 ± 4.9 × 10−2 | 3.1 × 10−1 ± 2.5 × 10−2 | * |
| Leu | 7.0 × 10−1 ± 1.2 × 10−1 | 6.3 × 10−1 ± 1.0 × 10−2 | N.S. | 7.3 × 10−1 ± 1.1 × 10−1 | 5.5 × 10−1 ± 6.2 × 10−2 | * |
| Lys | 4.4 × 10−1 ± 8.1 × 10−2 | 4.2 × 10−1 ± 8.1 × 10−2 | N.S. | 4.5 × 10−1 ± 8.1 × 10−2 | 3.5 × 10−1 ± 3.5 × 10−2 | tnd. |
| Hyp | 3.142 ± 0.253 | 2.897 ± 1.630 | N.S. | 2.099 ± 0.198 | 2.054 ± 0.0751 | N.S. |
| Pro | 9.6 × 10−1 ± 8.9 × 10−2 | 2.790 ± 3.31 | N.S. | 5.4 × 10−1 ± 1.04 × 10−1 | 3.7 × 10−1 ± 1.3 × 10−1 | tnd. |
| FAA *3 | 14.42 ± 0.732 | 15.67 ± 3.60 | N.S. | 15.26 ± 2.76 | 12.40 ± 1.87 | N.S. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, M.; Arakawa, A.; Nishio, M.; Okamura, T.; Ohnishi, C.; Kadowaki, K.; Kohira, K.; Homma, F.; Matsumoto, K.; Ishii, K. Differential Metabolomics Profiles Identified by CE-TOFMS between High and Low Intramuscular Fat Amount in Fattening Pigs. Metabolites 2020, 10, 322. https://doi.org/10.3390/metabo10080322
Taniguchi M, Arakawa A, Nishio M, Okamura T, Ohnishi C, Kadowaki K, Kohira K, Homma F, Matsumoto K, Ishii K. Differential Metabolomics Profiles Identified by CE-TOFMS between High and Low Intramuscular Fat Amount in Fattening Pigs. Metabolites. 2020; 10(8):322. https://doi.org/10.3390/metabo10080322
Chicago/Turabian StyleTaniguchi, Masaaki, Aisaku Arakawa, Motohide Nishio, Toshihiro Okamura, Chika Ohnishi, Kouen Kadowaki, Kimiko Kohira, Fumika Homma, Kazunori Matsumoto, and Kazuo Ishii. 2020. "Differential Metabolomics Profiles Identified by CE-TOFMS between High and Low Intramuscular Fat Amount in Fattening Pigs" Metabolites 10, no. 8: 322. https://doi.org/10.3390/metabo10080322
APA StyleTaniguchi, M., Arakawa, A., Nishio, M., Okamura, T., Ohnishi, C., Kadowaki, K., Kohira, K., Homma, F., Matsumoto, K., & Ishii, K. (2020). Differential Metabolomics Profiles Identified by CE-TOFMS between High and Low Intramuscular Fat Amount in Fattening Pigs. Metabolites, 10(8), 322. https://doi.org/10.3390/metabo10080322






