Metabolomic Profiling of Nicotiana Spp. Nectars Indicate That Pollinator Feeding Preference Is a Stronger Determinant Than Plant Phylogenetics in Shaping Nectar Diversity
Abstract
1. Introduction
2. Results
2.1. Nicotiana Floral Morphology and Pollination Syndromes
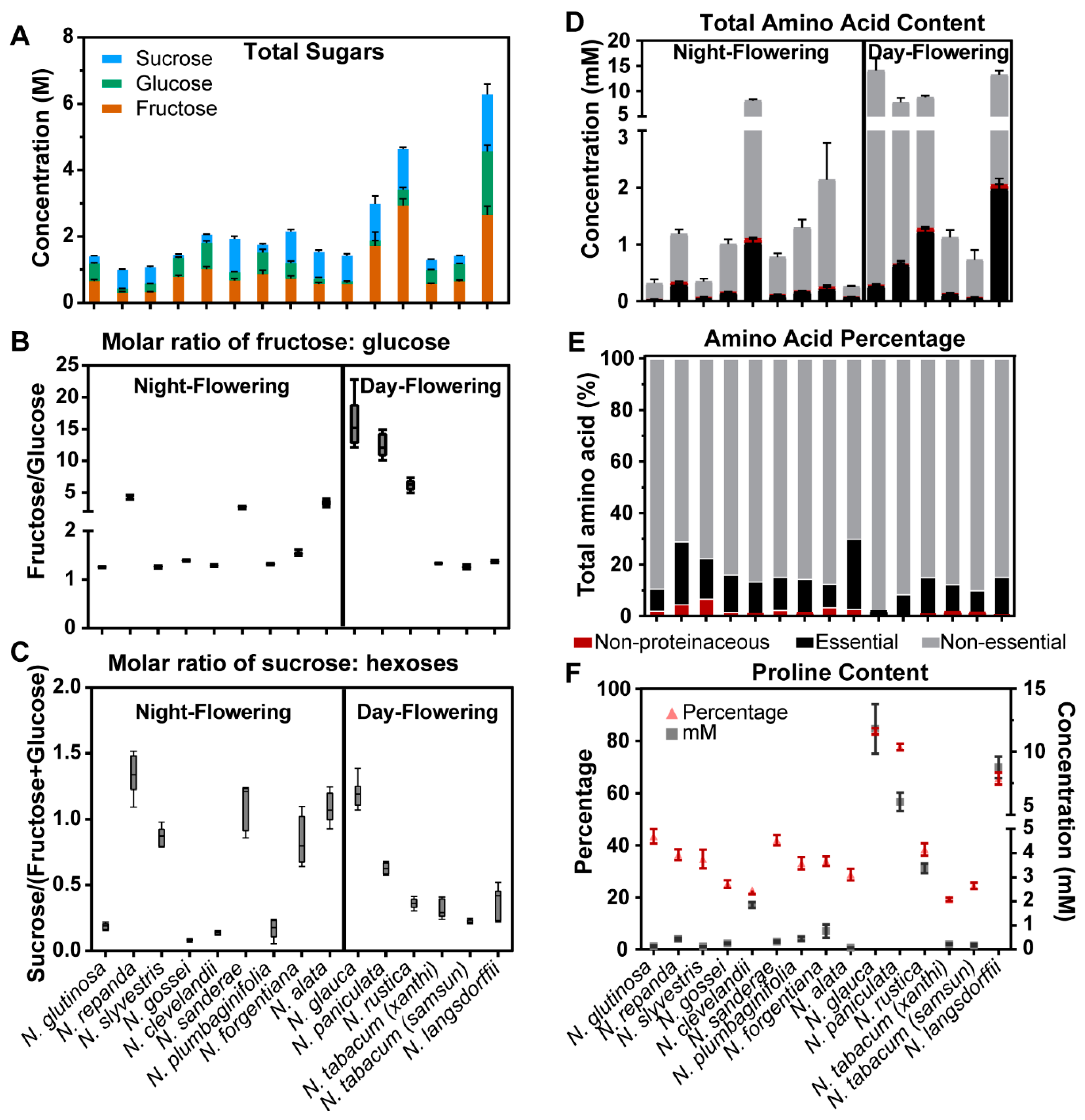
2.2. Nectar Sugars and Amino Acids of Nicotiana Species
2.3. Nectar Composition of Nicotiana Sections
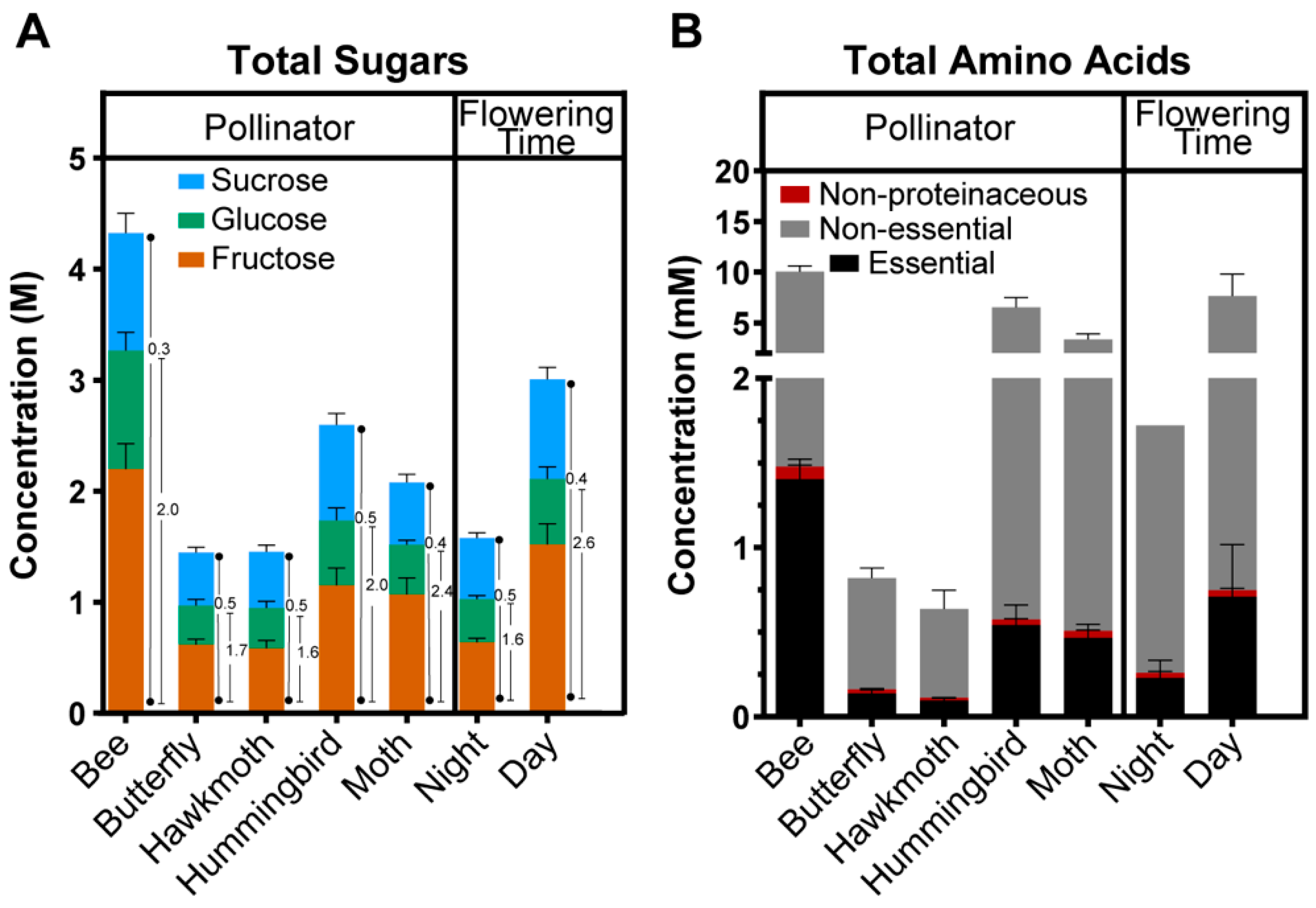
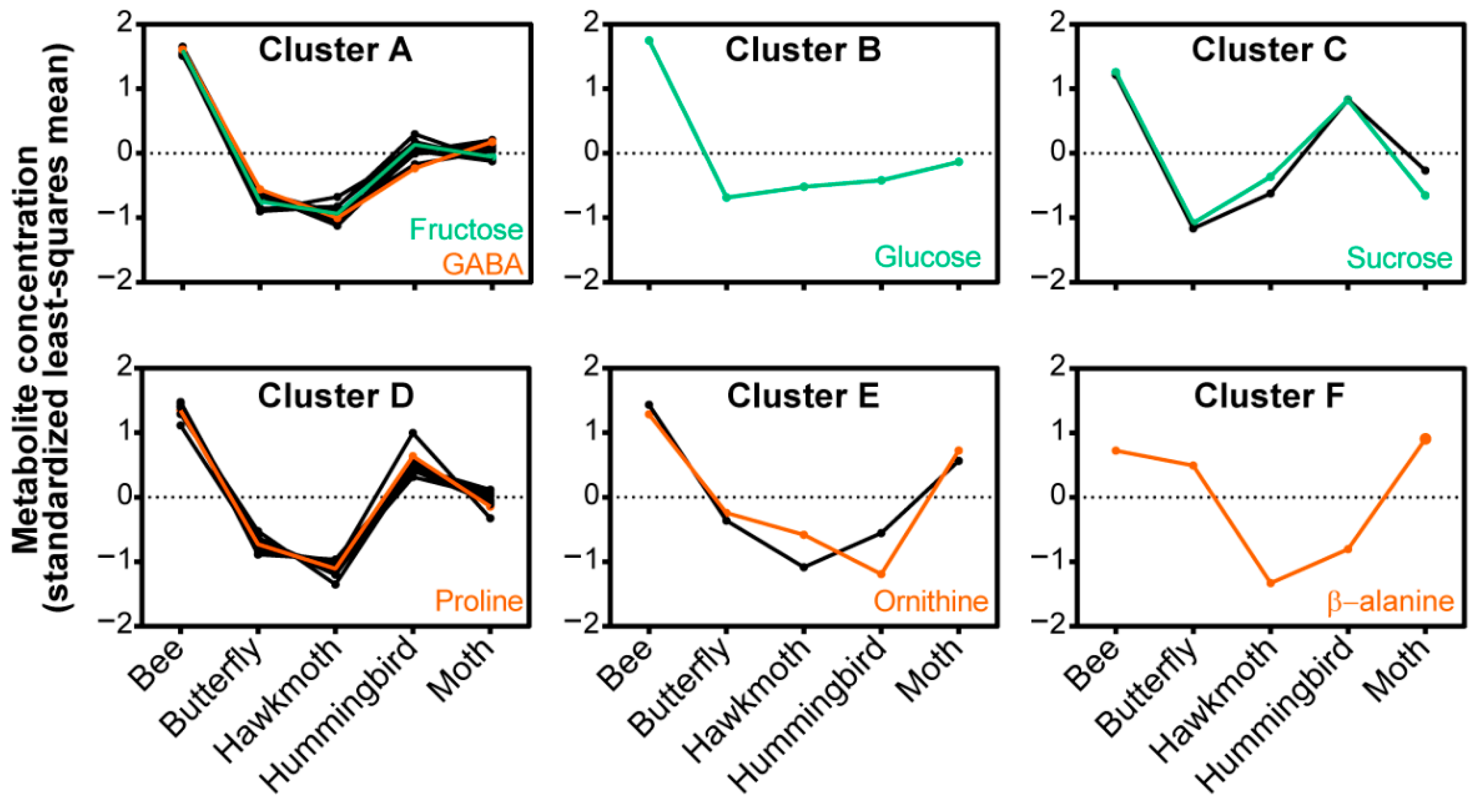
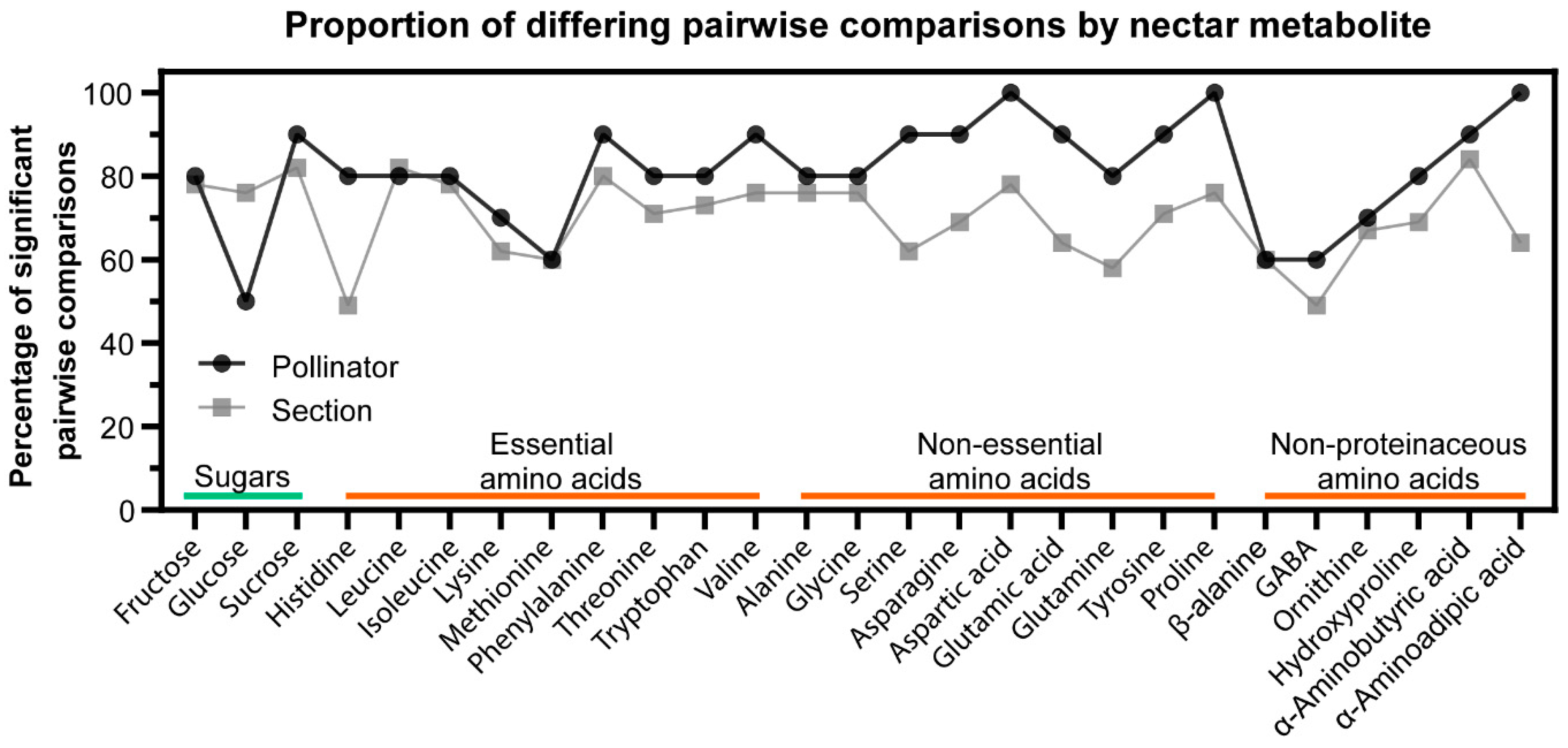
2.4. Relationship between Nectar Composition and Pollinators
3. Discussion
3.1. Nicotiana Nectar Displays Compositional Differences Based on Flower Timing
3.2. Plant Phylogeny and Pollinator Type Both Contribute to Nectar Composition
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Nectar Sample Collection
4.3. Amino Acid Analysis
4.4. Carbohydrate Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48. [Google Scholar] [CrossRef]
- Torres, C.; Galetto, L. Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biol. 2002, 4, 360–366. [Google Scholar] [CrossRef]
- Kaczorowski, R.L.; Gardener, M.C.; Holtsford, T.P. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. Am. J. Bot. 2005, 92, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.; Baldwin, I.T. Making sense of nectar scents: The effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J. 2007, 49, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Afik, O.; Delaplane, K.S.; Shafir, S.; Moo-Valle, H.; Quezada-Euán, J.J.G. Nectar minerals as regulators of flower visitation in stingless bees and nectar hoarding wasps. J. Chem. Ecol. 2014, 40, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Weigend, M. Nectar, floral morphology and pollination syndrome in Loasaceae subfam. Loasoideae (Cornales). Ann. Bot. 2006, 98, 503–514. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. A brief historical review of chemistry of floral nectar. In The Biology of Nectaries; Columbia University Press: New York, NY, USA, 1983; pp. 126–152. [Google Scholar]
- Hendriksma, H.P.; Oxman, K.L.; Shafir, S. Amino acid and carbohydrate tradeoffs by honey bee nectar foragers and their implications for plant–pollinator interactions. J. Insect Physiol. 2014, 69, 56–64. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The taste of nectar: A neglected area of pollination ecology. Oikos 2002, 98, 552–557. [Google Scholar] [CrossRef]
- Richardson, L.L.; Adler, L.S.; Leonard, A.S.; Andicoechea, J.; Regan, K.H.; Anthony, W.E.; Manson, J.S.; Irwin, R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 2015, 282, 20142471. [Google Scholar] [CrossRef]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Review: Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Springer: Dordrecht, The Netherland, 2007; pp. 215–264. [Google Scholar]
- Silva, F.A.; Guirgis, A.; Thornburg, R. Nectar analysis throughout the genus Nicotiana suggests conserved mechanisms of nectar production and biochemical action. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, K.; Lohaus, G. Nectar sugars and amino acids in day- and night-flowering Nicotiana species are more strongly shaped by pollinators’ preferences than organic acids and inorganic ions. PLoS ONE 2017, 12, e0176865. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Gilet, T.; Bush, J.W.M. Optimal concentrations in nectar feeding. Proc. Natl. Acad. Sci. USA 2011, 108, 16618–16621. [Google Scholar] [CrossRef] [PubMed]
- Wykes, G.R. The preferences of honeybees for solutions of various sugars which occur in nectar. J. Exp. Biol. 1952, 29, 511–519. [Google Scholar]
- Pyke, G.H.; Waser, N.M. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica 1981, 13, 260–270. [Google Scholar] [CrossRef]
- Johnson, S.D.; Pauw, A.; Midgley, J. Rodent pollination in the African lily Massonia depressa (Hyacinthaceae). Am. J. Bot. 2001, 88, 1768–1773. [Google Scholar] [CrossRef]
- Hainsworth, F.R.; Wolf, L.L. Nectar characteristics and food selection by hummingbirds. Oecologia 1976, 25, 101–113. [Google Scholar] [CrossRef]
- Stiles, F.G. Taste preferences, color preferences, and flower choice in hummingbirds. Condor 1976, 78, 10–26. [Google Scholar] [CrossRef]
- Inouye, D.W.; Waller, G.D. Responses of honey bees (Apis mellifera) to amino acid solutions mimicking floral nectars. Ecology 1984, 65, 618–625. [Google Scholar] [CrossRef]
- Waller, G.D. Evaluating responses of honey bees to sugar solutions using an artificial-flower feeder. Ann. Entomol. Soc. Am. 1972, 65, 857–862. [Google Scholar] [CrossRef]
- Martínez del Río, C. Sugar preferences in hummingbirds: The influence of subtle chemical differences on food choice. Condor 1990, 92, 1022–1030. [Google Scholar] [CrossRef]
- Goodspeed, T.H. On the evolution of the genus Nicotiana. Proc. Natl. Acad. Sci. USA 1947, 33, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Merxmuller, H.; Buttler, K.P. Nicotiana in der Afrikanischen namib—Ein pflanzengeographisches und phylogenetisches ratsel. Mitt. Bot. Munchen 1975, 12, 91–104. [Google Scholar]
- Knapp, S.; Chase, M.W.; Clarkson, J.J. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 2004, 53, 73–82. [Google Scholar] [CrossRef]
- Clarkson, J.J.; Knapp, S.; Garcia, V.F.; Olmstead, R.G.; Leitch, A.R.; Chase, M.W. Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. Mol. Phylogenet. Evol. 2004, 33, 75–90. [Google Scholar] [CrossRef]
- Anon Tobacco (Nicotiana glutinosa) in the Nicotianas Database. Available online: https://garden.org/plants/view/206624/Tobacco-Nicotiana-glutinosa/ (accessed on 5 February 2019).
- Ollerton, J.; Watts, S.; Connerty, S.; Lock, J.; Parker, L.; Wilson, I.; Sheila, K.; Nattero, J.; Cocucci, A.A.; Izhaki, I.; et al. Pollination ecology of the invasive tree tobacco Nicotiana gluca: Comparisons across native and non-native ranges. J. Pollinat. Ecol. 2012, 9, 85–95. [Google Scholar]
- Ollerton, J.; Alarcon, R.; Waser, N.M.; Price, M.V.; Watts, S.; Cranmer, L.; Hingston, A.; Peter, C.I.; Rotenberry, J. A global test of the pollination syndrome hypothesis. Ann. Bot. 2009, 103, 1471–1480. [Google Scholar] [CrossRef]
- Raguso, R.A.; Levin, R.A.; Foose, S.E.; Holmberg, M.W.; McDade, L.A. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 2003, 63, 265–284. [Google Scholar] [CrossRef]
- Anon Fiddleleaf Tobacco (Nicotiana repanda) in the Nicotianas Database. Available online: https://garden.org/plants/view/83528/Fiddleleaf-tobacco-Nicotiana-repanda/ (accessed on 5 February 2019).
- Mahr, S. Flowering Tobacco, Nicotiana Sylvestris. Available online: http://wimastergardener.org/article/flowering-tobacco-nicotiana-sylvestris/ (accessed on 6 February 2019).
- Anon Tobacco (Nicotiana gossei) in the Nicotianas Database. Available online: https://garden.org/plants/view/661972/Tobacco-Nicotiana-gossei/ (accessed on 5 February 2019).
- Anon Cleveland’s Tobacco (Nicotiana clevelandii) in the Nicotianas Database. Available online: https://garden.org/plants/view/200829/Clevelands-Tobacco-Nicotiana-clevelandii/ (accessed on 5 February 2019).
- Anon Flowering Tobacco (Nicotiana x sanderae ’Crimson Bedder’) in the Nicotianas Database. Available online: https://garden.org/plants/view/593587/Flowering-Tobacco-Nicotiana-x-sanderae-Crimson-Bedder/ (accessed on 5 February 2019).
- Kornaga, T. Genetic and biochemical characterization of an unstable flower color phenotype in interspecific crosses of Nicotiana sp. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1993. [Google Scholar]
- Ippolito, A. Systematics and floral evolution of Nicotiana. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2000. [Google Scholar]
- Carter, C.; Shafir, S.; Yehonatan, L.; Palmer, R.G.; Thornburg, R. A novel role for proline in plant floral nectars. Naturwissenschaften 2006, 93, 72–79. [Google Scholar] [CrossRef]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Ruhlmann, J.M.; Kram, B.W.; Carter, C.J. CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis. J. Exp. Bot. 2010, 61, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, K.; Lohaus, G. Nectar Sugar Modulation and Cell Wall Invertases in the Nectaries of Day- and Night-Flowering Nicotiana. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; de Vega, C.; Canto, A.; Pozo, M.I. Yeasts in floral nectar: A quantitative survey. Ann. Bot. 2009, 103, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Rattke, J.; Boland, W.; Baulcombe, D.C.; Li, W.X.; Ding, S.W.; Lecellier, C.H.; Parizotto, E.A.; Himber, C.; Voinnet, O.; et al. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science (80-) 2005, 308, 560–563. [Google Scholar] [CrossRef]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Bini, L.; Puglia, M.; Bianchi, L.; Pacini, E. Amino acids and protein profile in floral nectar: Much more than a simple reward. Flora 2012, 207, 475–481. [Google Scholar] [CrossRef]
- Chen, C.C.W.; Welch, K.C. Hummingbirds can fuel expensive hovering flight completely with either exogenous glucose or fructose. Funct. Ecol. 2014, 28, 589–900. [Google Scholar] [CrossRef]
- Witt, T.; Jürgens, A.; Gottsberger, G. Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. J. Evol. Biol. 2013, 26, 2244–2259. [Google Scholar] [CrossRef]
- Petanidou, T.; Goethals, V.; Smets, E. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community—Does flowering time matter? Plant Syst. Evol. 2000, 225, 103–118. [Google Scholar] [CrossRef]
- Felicioli, A.; Sagona, S.; Galloni, M.; Bortolotti, L.; Bogo, G.; Guarnieri, M.; Nepi, M. Effects of non-protein amino acids on survival and locomotion of Osmia bicornis. Insect Mol. Biol. 2018, 27, 556–563. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. Analyzing variability in nectar amino acids: Composition is less variable than concentration. J. Chem. Ecol. 2001, 27, 2545–2558. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.; Ellis, W.N.; Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 2006, 115, 155–169. [Google Scholar] [CrossRef]
- Teulier, L.; Weber, J.-M.; Crevier, J.; Darveau, C.-A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B 2016, 283, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef]
- Bolten, A.B.; Feinsinger, P. Why do hummingbird flowers secrete dilute nectar? Biotropica 1978, 10, 307–309. [Google Scholar] [CrossRef]
- Zhang, L.; Martin, A.; Perry, M.W.; van der Burg, K.R.L.; Matsuoka, Y.; Monteiro, A.; Reed, R.D. Genetic basis of melanin pigmentation in butterfly wings. Genetics 2017, 205, 1537–1550. [Google Scholar] [CrossRef]
- Carter, C.; Graham, R.A.; Thornburg, R.W. Nectarin I is a novel, soluble germin-like protein expressed in the nectar of Nicotiana sp. Plant Mol. Biol. 1999, 41, 207–216. [Google Scholar] [CrossRef]
- Naqvi, S.M.S.; Harper, A.; Carter, C.; Ren, G.; Guirgis, A.; York, W.S.; Thornburg, R.W. Nectarin IV, a potent endoglucanase inhibitor secreted into the nectar of ornamental tobacco plants. Isolation, cloning, and characterization. Plant Physiol. 2005, 139, 1389–1400. [Google Scholar] [CrossRef]
- Koltunow, A.M.; Truettner, J.; Cox, K.H.; Wallroth, M.; Goldberg, R.B. Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 1990, 2, 1201–1224. [Google Scholar] [CrossRef]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]



| Species | Section | Preferred Pollinators | Flowering Time | Reference |
|---|---|---|---|---|
| N. glutinosa | Undulataea | Moth, butterflies | Night | [28] |
| N. glauca | Noctiflorae | Hummingbird, bird | Day | [14,29] |
| N. paniculata | Paniculatae | Hummingbird | Day | [14,30] |
| N. rustica | Rusticae | Moth, bee | Day | [31] |
| N. repanda | Repandae | Moth, butterflies | Night | [26,32] |
| N. sylvestris | Sylvestres | Hawkmoth | Night | [33] |
| N. gossei | Suaveolentes | Moth, butterflies | Night | [26,34] |
| N. tabacum (Xanthi) | Nicotiana | Hummingbird | Day | [14] |
| N. tabacum (Samsun) | Nicotiana | Hummingbird | Day | [14] |
| N. clevelandii | Polydicliae | Moth, bee, other | Night | [26,35] |
| N. sanderae | Alatae | Moths, butterflies | Night | [36,37] |
| N. plumbaginifolia | Alatae | Hawkmoth, autogamous | Night | [3] |
| N. langsdorffii | Alatae | Hummingbird, bee | Day | [3,38] |
| N. forgentiana | Alatae | Hummingbird | Night | [38] |
| N. alata | Alatae | Hawkmoth | Night | [3] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.A.; Chatt, E.C.; Mahalim, S.-N.; Guirgis, A.; Guo, X.; Nettleton, D.S.; Nikolau, B.J.; Thornburg, R.W. Metabolomic Profiling of Nicotiana Spp. Nectars Indicate That Pollinator Feeding Preference Is a Stronger Determinant Than Plant Phylogenetics in Shaping Nectar Diversity. Metabolites 2020, 10, 214. https://doi.org/10.3390/metabo10050214
Silva FA, Chatt EC, Mahalim S-N, Guirgis A, Guo X, Nettleton DS, Nikolau BJ, Thornburg RW. Metabolomic Profiling of Nicotiana Spp. Nectars Indicate That Pollinator Feeding Preference Is a Stronger Determinant Than Plant Phylogenetics in Shaping Nectar Diversity. Metabolites. 2020; 10(5):214. https://doi.org/10.3390/metabo10050214
Chicago/Turabian StyleSilva, Fredy A., Elizabeth C. Chatt, Siti-Nabilla Mahalim, Adel Guirgis, Xingche Guo, Daniel S. Nettleton, Basil J. Nikolau, and Robert W. Thornburg. 2020. "Metabolomic Profiling of Nicotiana Spp. Nectars Indicate That Pollinator Feeding Preference Is a Stronger Determinant Than Plant Phylogenetics in Shaping Nectar Diversity" Metabolites 10, no. 5: 214. https://doi.org/10.3390/metabo10050214
APA StyleSilva, F. A., Chatt, E. C., Mahalim, S.-N., Guirgis, A., Guo, X., Nettleton, D. S., Nikolau, B. J., & Thornburg, R. W. (2020). Metabolomic Profiling of Nicotiana Spp. Nectars Indicate That Pollinator Feeding Preference Is a Stronger Determinant Than Plant Phylogenetics in Shaping Nectar Diversity. Metabolites, 10(5), 214. https://doi.org/10.3390/metabo10050214