Characteristic of Metabolic Status in Heart Failure and Its Impact in Outcome Perspective
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Laboratory Findings
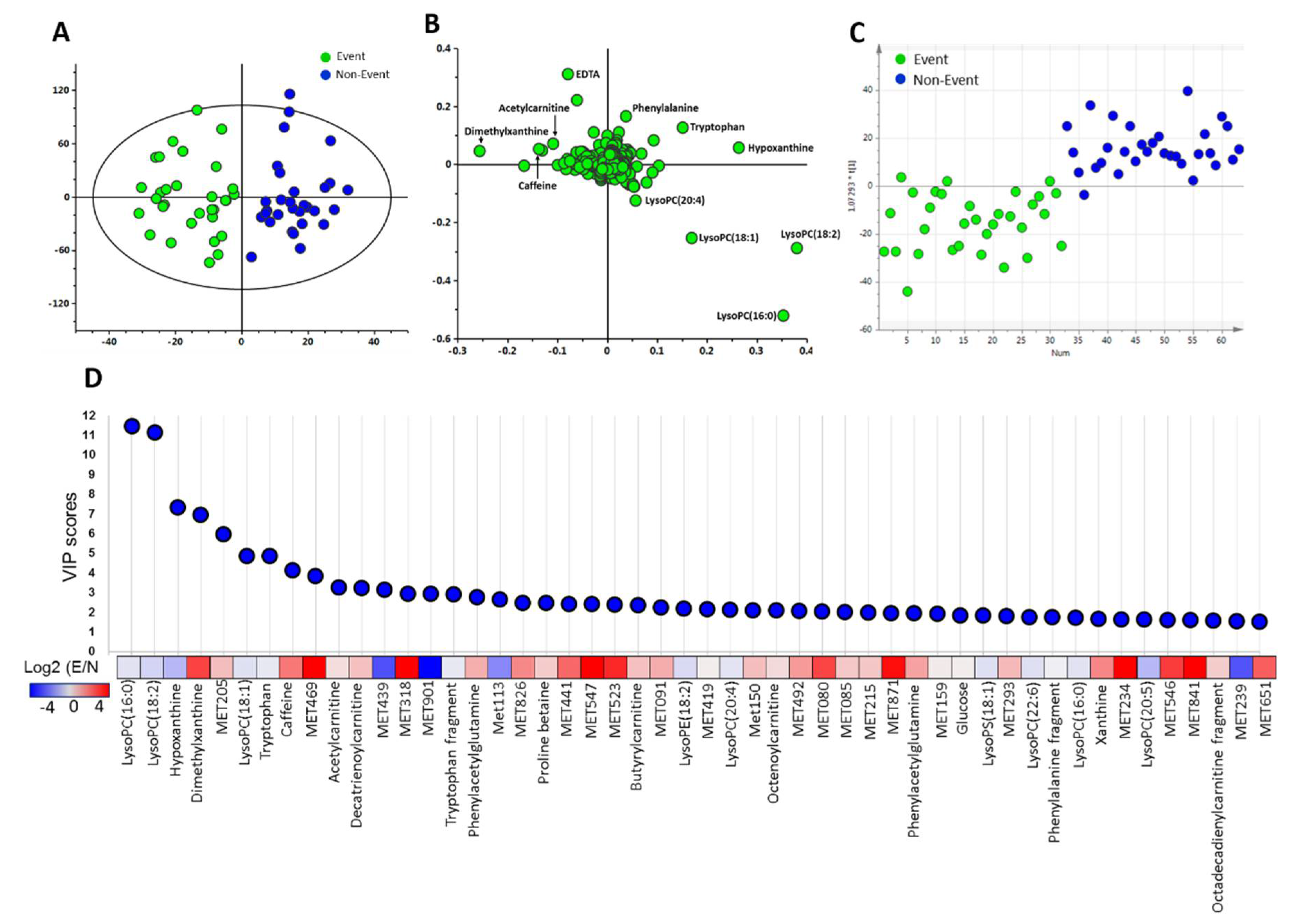
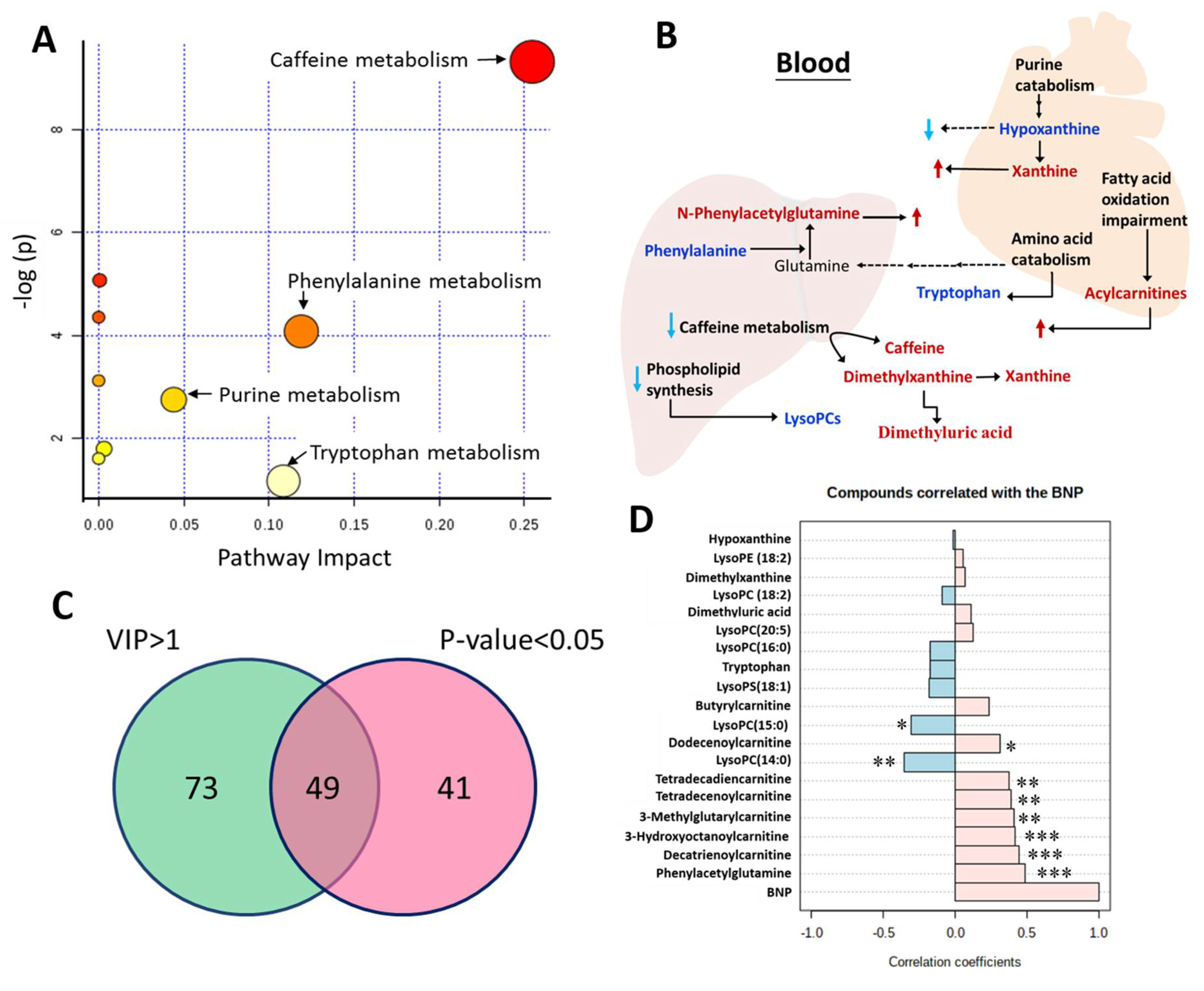
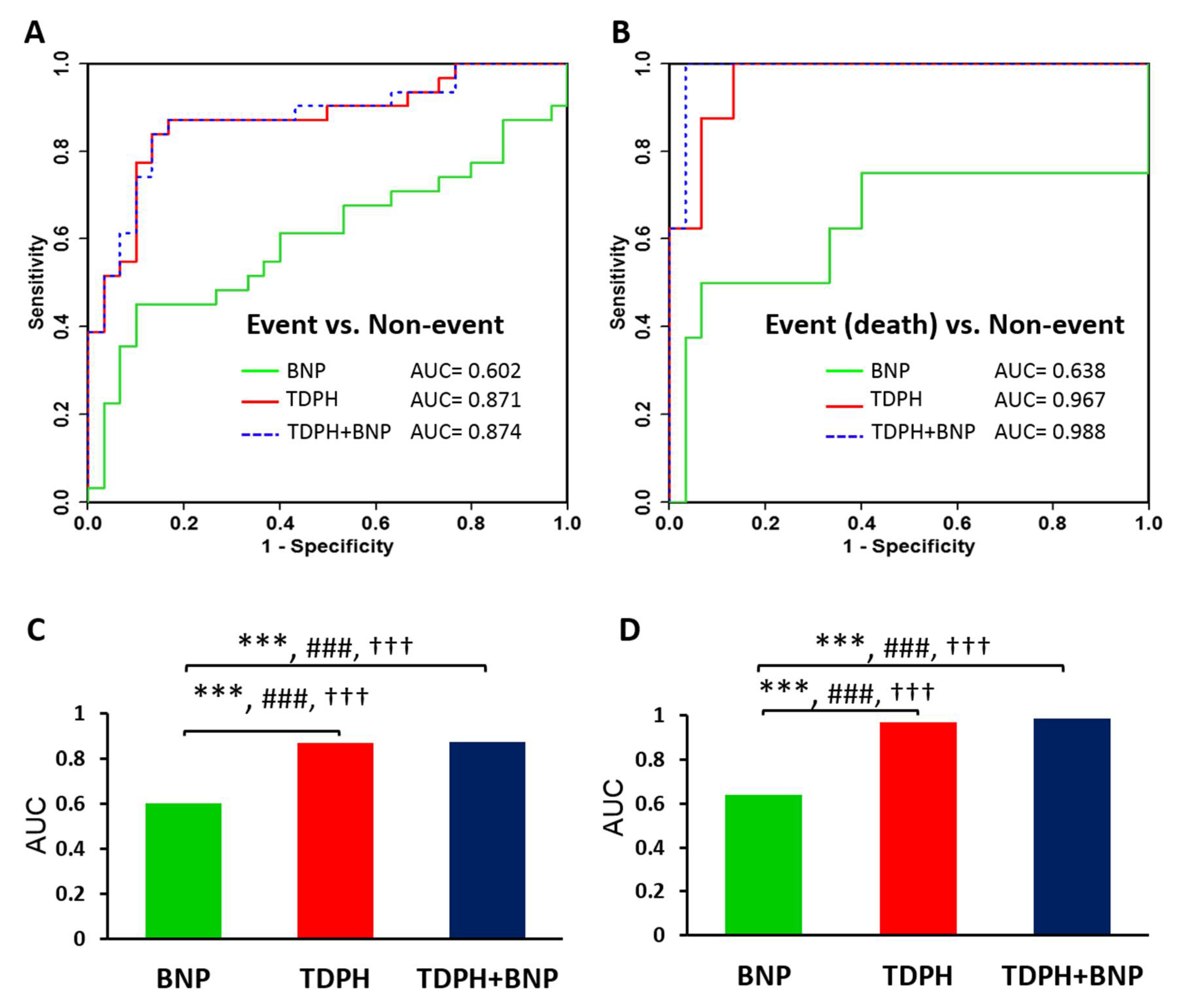
2.2. Discriminative Ability of Potential Metabolite Biomarkers for Events in HF
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Blood Samples Collection
4.3. Metabolomics Analysis by UPLC-TOFMS
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sirich, T.L.; Aronov, P.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 2013, 84, 585–590. [Google Scholar] [CrossRef]
- Jhund, P.S.; MacIntyre, K.; Simpson, C.R.; Lewsey, J.D.; Stewart, S.; Redpath, A.; Chalmers, J.W.; Capewell, S.; McMurray, J.J. Long-Term Trends in First Hospitalization for Heart Failure and Subsequent Survival Between 1986 and 2003: A population study of 5.1 million people. Circulation 2009, 119, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Ammirati, E.; Guida, P.; Passantino, A.; Raimondo, R.; Guida, V.; Braga, S.S.; Pedretti, R.F.; Lagioia, R.; Frigerio, M.; et al. Clinical utility of N-terminal pro-B-type natriuretic peptide for risk stratification of patients with acute decompensated heart failure. Derivation and validation of the ADHF/NT-proBNP risk score. Int. J. Cardiol. 2013, 168, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Salah, K.; Kok, W.E.; Eurlings, L.W.; Bettencourt, P.; Pimenta, J.M.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Bettari, L.; Damman, P.; et al. Faculty Opinions recommendation of A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: A European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014, 100, 115–125. [Google Scholar] [PubMed]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Stienen, S.; Salah, K.; Moons, A.H.; Bakx, A.L.; Van Pol, P.; Kortz, R.A.M.; Ferreira, J.P.; Marques, I.; Schroeder-Tanka, J.M.; Keijer, J.T.; et al. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure: PRIMA II Randomized Controlled Trial (Can NT-ProBNP-Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?). Circulation 2018, 137, 1671–1683. [Google Scholar] [PubMed]
- Sabatine, M.S.; Liu, E.; Morrow, D.A.; Heller, E.; McCarroll, R.; Wiegand, R.; Berriz, G.F.; Roth, F.P.; Gerszten, R.E. Metabolomic Identification of Novel Biomarkers of Myocardial Ischemia. Circulation 2005, 112, 3868–3875. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Grassano, A.; Thambisetty, M.; Lovestone, S.; Legido-Quigley, C. A proposed metabolic strategy for monitoring disease progression in Alzheimer’s disease. Electrophoresis 2009, 30, 1235–1239. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Hare, J.M.; Mangal, B.; Brown, J.; Fisher, C., Jr.; Freudenberger, R.; Colucci, W.S.; Mann, D.L.; Liu, P.; Givertz, M.M.; Schwarz, R.P. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 2008, 51, 2301–2309. [Google Scholar] [CrossRef]
- Doehner, W.; Frenneaux, M.; Anker, S.D. Metabolic impairment in heart failure: The myocardial and systemic perspective. J. Am. Coll. Cardiol. 2014, 64, 1388–1400. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Upadhya, B.; Vasu, S. What the dead can teach the living: Systemic nature of heart failure with preserved ejection fraction. Circulation 2015, 131, 522–524. [Google Scholar] [CrossRef]
- Hunter, W.; Kelly, J.P.; McGarrah, R.W., 3rd; Kraus, W.E.; Shah, S.H. Metabolic Dysfunction in Heart Failure: Diagnostic, Prognostic, and Pathophysiologic Insights from Metabolomic Profiling. Curr. Heart Fail. Rep. 2016, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Wang, C.-H.; Shiao, M.-S.; Liu, M.-H.; Huang, Y.-Y.; Huang, C.-Y.; Mao, C.-T.; Lin, J.-F.; Ho, H.Y.; Yang, N.-I. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2012, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Quan, G.; Jiang, X.; Yang, Y.; Ding, X.; Zhang, D.; Wang, X.; Hardwidge, P.R.; Ren, W.; Zhu, G. Effects of Metabolites Derived From Gut Microbiota and Hosts on Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Suttner, S.W.; Boldt, J. Natriuretic peptide system: Physiology and clinical utility. Curr. Opin. Crit. Care 2004, 10, 336–341. [Google Scholar] [CrossRef]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lee, D.H.; Kim, J.K.; Park, M.-J.; Yan, J.-J.; Song, D.-K.; Vaziri, N.D.; Noh, J.-W. Lysophosphatidylcholine, Oxidized Low-Density Lipoprotein and Cardiovascular Disease in Korean Hemodialysis Patients: Analysis at 5 Years of Follow-up. J. Korean Med Sci. 2013, 28, 268–273. [Google Scholar] [CrossRef]
- Zhao, X.; Fritsche, J.; Wang, J.; Chen, J.; Rittig, K.; Schmitt-Kopplin, P.; Fritsche, A.; Häring, H.-U.; Schleicher, E.D.; Xu, G.; et al. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics 2010, 6, 362–374. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma Lysophosphatidylcholine Levels Are Reduced in Obesity and Type 2 Diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Gillett, M.P.; Obineche, E.N.; El-Rokhaimi, M.; Lakhani, M.S.; Abdulle, A.; Sulaiman, M. Lecithin: Cholesterol acyltransfer, dyslipoproteinaemia and membrane lipids in uraemia. J. Nephrol. 2002, 14, 472–480. [Google Scholar]
- Shah, S.H.; Sun, J.-L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012, 163, 844–850.e1. [Google Scholar] [CrossRef] [PubMed]
- Zammit, V.A.; Ramsay, R.R.; Bonomini, M.; Arduini, A. Carnitine, mitochondrial function and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.G.; Carpenter, K.; Hammond, J.; Christodoulou, J.; Wilcken, B. Acylcarnitine profiles in fibroblasts from patients with respiratory chain defects can resemble those from patients with mitochondrial fatty acid beta-oxidation disorders. Metab.-Clin. Exp. 2002, 51, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Kamerling, J.; Brouwer, M.; Ketting, D.; Wadman, S. Gas chromatography of urinary N-phenylacetylglutamine. J. Chromatogr. B: Biomed. Sci. Appl. 1979, 164, 217–221. [Google Scholar] [CrossRef]
- Mokhtarani, M.; Diaz, G.; Rhead, W.; Lichter-Konecki, U.; Bartley, J.; Feigenbaum, A.; Longo, N.; Berquist, W.; Berry, S.A.; Gallagher, R.; et al. Urinary phenylacetylglutamine as dosing biomarker for patients with urea cycle disorders. Mol. Genet. Metab. 2012, 107, 308–314. [Google Scholar] [CrossRef]
- Velasquez-Martinez, M.C.; Santos-Vera, B.; Velez-Hernandez, M.E.; Vazquez-Torres, R.; Jiménez-Rivera, C.A. Alpha-1 Adrenergic Receptors Modulate Glutamate and GABA Neurotransmission onto Ventral Tegmental Dopamine Neurons during Cocaine Sensitization. Int. J. Mol. Sci. 2020, 21, 790. [Google Scholar] [CrossRef]
- Poesen, R.; Claes, K.; Evenepoel, P.; De Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Poe, R.H.; Utell, M.J. Theophylline in asthma and COPD: Changing perspectives and controversies. Geriatrics 1991, 46, 61–65. [Google Scholar]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef]
- Huerta, C.; Lanes, S.F.; Rodríguez, L.A.G. Respiratory Medications and the Risk of Cardiac Arrhythmias. Epidemiology 2005, 16, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Patterson, A.D.; Idle, J.R.; Gonzalez, F.J. Xenobiotic Metabolomics: Major Impact on the Metabolome. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 37–56. [Google Scholar] [CrossRef]
- Aspromonte, N.; Monitillo, F.; Puzzovivo, A.; Valle, R.; Caldarola, P.; Iacoviello, M. Modulation of cardiac cytochrome P450 in patients with heart failure. Expert Opin. Drug Metab. Toxicol. 2014, 10, 327–339. [Google Scholar] [CrossRef]
- Renton, K.W. Cytochrome P450 Regulation and Drug Biotransformation during Inflammation and Infection. Curr. Drug Metab. 2004, 5, 235–243. [Google Scholar] [CrossRef]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of Caffeine on Plasma Renin Activity, Catecholamines and Blood Pressure. Surv. Anesthesiol. 1978, 22, 548–549. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; Dibianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L.; et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef]
- Harzand, A.; Tamariz, L.; Hare, J.M. Uric Acid, Heart Failure Survival, and the Impact of Xanthine Oxidase Inhibition. Congest. Hear. Fail. 2011, 18, 179–182. [Google Scholar] [CrossRef]
- Hare, J.M.; Johnson, R.J. Uric acid predicts clinical outcomes in heart failure: Insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 2003, 107, 1951–1953. [Google Scholar] [CrossRef]
- Doehner, W.; Schoene, N.; Rauchhaus, M.; Leyva-Leon, F.; Pavitt, D.V.; Reaveley, D.A.; Schuler, G.; Coats, A.J.; Anker, S.D.; Hambrecht, R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation 2002, 105, 2619–2624. [Google Scholar] [CrossRef]
- Cappola, T.P.; Kass, D.A.; Nelson, G.S.; Berger, R.D.; Rosas, G.O.; Kobeissi, Z.A.; Marbán, E.; Hare, J.M. Allopurinol Improves Myocardial Efficiency in Patients with Idiopathic Dilated Cardiomyopathy. Circulation 2001, 104, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, H.E.; Plastino, J.A.; Escudero, E.M.; Mangal, B.; Brown, J.; Pérez, N.G. The Effect of Xanthine Oxidase Inhibition upon Ejection Fraction in Heart Failure Patients: La Plata Study. J. Card. Fail. 2006, 12, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; McDonagh, T.A.; Byrne, J.; Blue, L.; Farmer, R.; Morton, J.J.; Dargie, H.J. Titration of vasodilator therapy in chronic heart failure according to plasma brain natriuretic peptide concentration: Randomized comparison of the hemodynamic and neuroendocrine effects of tailored versus empirical therapy. Am. Heart J. 1999, 138, 1126–1132. [Google Scholar] [CrossRef]
- Maisel, A.S.; Clopton, P.; Krishnaswamy, P.; Nowak, R.M.; Mccord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: Results from the Breathing Not Properly (BNP) multinational study. Am. Heart J. 2004, 147, 1078–1084. [Google Scholar] [CrossRef]
| Variable | Non-Event (n = 30) | Event (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 60.3 ± 11.7 | 64.5 ± 11.6 | 0.173 |
| Male (%) | 24 (80.0) | 20 (64.5) | 0.416 |
| EF (%) | 32.2 ± 10.4 | 34.2 ± 13.7 | 0.518 |
| Blood pressure (mm Hg) | |||
| Systolic | 120.2 ± 17.5 | 121.9 ± 16.5 | 0.703 |
| Diastolic | 75.2 ± 13.2 | 70.0 ± 10.9 | 0.094 |
| Heart rate (beats/min) | 76.9 ± 10.1 | 79.5 ± 11.5 | 0.338 |
| Co-morbidity | |||
| Diabetes mellitus (%) | 6 (20.0) | 15 (48.4) | 0.031 |
| Hypertension (%) | 20 (66.7) | 23 (74.2) | 0.582 |
| Atrial fibrillation (%) | 4 (13.3) | 10 (32.3) | 0.127 |
| COPD (%) | 2 (6.7) | 6 (19.4) | 0.255 |
| Ischemic (%) | 19 (63.3) | 16 (51.6) | 0.440 |
| Body mass index (kg/m2) | 24.4 ± 4.9 | 24.8 ± 4.9 | 0.771 |
| Medication | |||
| ACEI or ARB (%) | 28 (93.3) | 24 (77.4) | 0.147 |
| β-Blocker (%) | 27 (90.0) | 21 (67.7) | 0.06 |
| Diuretic (%) | 23 (76.7) | 23 (74.2) | 1.000 |
| Allopurinol | 0 (0) | 6 (19.4) | 0.024 |
| Benzbromarone | 0 (0) | 2 (6.5) | 0.492 |
| Laboratory data | |||
| Serum sodium (mEq/L) | 140.2 ± 2.3 | 137.6 ± 4.7 | 0.008 |
| Hemoglobin (g/dL) | 14.3 ± 1.9 | 13.0 ± 2.4 | 0.028 |
| Total bilirubin (mg/dL) | 1.1 ± 0.6 | 1.3 ± 0.8 | 0.259 |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.5 ± 0.6 | 0.169 |
| eGFR (ml/min/1.73 m2) | 74.3 ± 18.6 | 62.8 ± 37.3 | 0.134 |
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.5 ± 1.1 | 0.024 |
| Cholesterol (mg/dL) | 171.7 ± 44.5 | 173.1 ± 63.1 | 0.919 |
| TG (mg/dL) | 111.6 ± 48.2 | 125.7 ± 67.3 | 0.351 |
| LDL-cholesterol (mg/dL) | 111.1 ± 41.7 | 115.3 ± 60.1 | 0.755 |
| HDL-cholesterol (mg/dL) | 36.4 ± 10.6 | 34.7 ± 15.4 | 0.615 |
| HbA1c (%) | 6.3 ± 1.4 | 6.8 ± 1.7 | 0.234 |
| BNP (pg/mL) | 518.2 ± 620.9 | 891.5 ± 882.6 | 0.063 |
| CRP (mg/L) | 19.3 ± 43.4 | 16.4 ± 25.8 | 0.758 |
| Uric acid (mg/dL) | 7.4 ± 2.1 | 8.1 ± 2.9 | 0.262 |
| Compound Name | Retention Time (min) | m/z | Non-Event (n = 30) | Event (n = 31) | p-Value | VIP Score | q-Value |
|---|---|---|---|---|---|---|---|
| Acylcarnitines | |||||||
| 3-Methylglutarylcarnitine | 0.95 | 290.1592 | 0.8 ± 0.9 | 3.4 ± 5.4 | 0.013 | 1.29 | 0.065 |
| Butyrylcarnitine | 1.01 | 232.1542 | 9.8 ± 5.7 | 16.4 ± 12.7 | 0.011 | 2.08 | 0.064 |
| 3-Hydroxyoctanoylcarnitine | 1.33 | 304.2118 | 5.6 ± 4.8 | 9.1 ± 7.4 | 0.034 | 1.38 | 0.108 |
| Decatrienoylcarnitine | 1.54 | 310.2016 | 17.0 ± 11.9 | 30.9 ± 21.7 | 0.003 | 3.23 | 0.048 |
| Dodecenoylcarnitine | 1.85 | 342.2636 | 3.6 ± 3.3 | 6.7 ± 6.7 | 0.026 | 1.34 | 0.104 |
| Tetradecadiencarnitine | 1.92 | 368.2797 | 3.7 ± 3.6 | 6.3 ± 5.8 | 0.043 | 1.16 | 0.119 |
| Tetradecenoylcarnitine | 2.00 | 370.2951 | 2.7 ± 1.9 | 4.9 ± 4.2 | 0.009 | 1.23 | 0.055 |
| Lysophospholipids | |||||||
| LysoPC(14:0) | 2.28 | 468.3087 | 12.4 ± 6.0 | 9.1 ± 6.7 | 0.049 | 1.29 | 0.122 |
| LysoPC(15:0) | 2.39 | 482.3248 | 9.0 ± 4.1 | 6.9 ± 3.9 | 0.044 | 1.05 | 0.116 |
| LysoPE(18:2) | 2.42 | 478.2926 | 35.5 ± 14.1 | 26.9 ± 17.2 | 0.037 | 2.15 | 0.116 |
| LysoPC(18:2) | 2.43 | 520.3401 | 590 ± 252 | 413 ± 236 | 0.006 | 11.11 | 0.050 |
| LysoPC(20:5) | 2.43 | 542.3244 | 7.3 ± 6.6 | 4.0 ± 4.4 | 0.027 | 1.37 | 0.103 |
| LysoPS(18:1) | 2.49 | 524.2956 | 31.6 ± 11.2 | 25.4 ± 11.9 | 0.040 | 1.81 | 0.115 |
| LysoPC(16:0) | 2.49 | 518.3259 | 33.7 ± 10.2 | 28.1 ± 10.7 | 0.040 | 1.73 | 0.138 |
| Other metabolites | |||||||
| Hypoxanthine | 0.65 | 137.0459 | 174 ± 17 | 93.3 ± 81.4 | 0.023 | 6.93 | 0.102 |
| Dimethyluric acid | 0.96 | 197.0669 | 0.2 ± 0.7 | 3.3 ± 6.7 | 0.014 | 1.41 | 0.070 |
| Dimethylxanthine | 1.05 | 181.0717 | 11.5 ± 24.6 | 87.7 ± 143.9 | 0.007 | 7.30 | 0.045 |
| Tryptophan | 1.06 | 205.0979 | 19.4 ± 4.0 | 16.9 ± 4.9 | 0.027 | 1.21 | 0.100 |
| Phenylacetylglutamine | 1.25 | 265.1184 | 3.1 ± 2.2 | 8.2 ± 8.9 | 0.004 | 1.93 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.-Y.; Wang, C.-H.; Ho, H.-Y.; Lin, J.-F.; Lo, C.-J.; Huang, C.-Y.; Cheng, M.-L. Characteristic of Metabolic Status in Heart Failure and Its Impact in Outcome Perspective. Metabolites 2020, 10, 437. https://doi.org/10.3390/metabo10110437
Tang H-Y, Wang C-H, Ho H-Y, Lin J-F, Lo C-J, Huang C-Y, Cheng M-L. Characteristic of Metabolic Status in Heart Failure and Its Impact in Outcome Perspective. Metabolites. 2020; 10(11):437. https://doi.org/10.3390/metabo10110437
Chicago/Turabian StyleTang, Hsiang-Yu, Chao-Hung Wang, Hung-Yao Ho, Jui-Fen Lin, Chi-Jen Lo, Cheng-Yu Huang, and Mei-Ling Cheng. 2020. "Characteristic of Metabolic Status in Heart Failure and Its Impact in Outcome Perspective" Metabolites 10, no. 11: 437. https://doi.org/10.3390/metabo10110437
APA StyleTang, H.-Y., Wang, C.-H., Ho, H.-Y., Lin, J.-F., Lo, C.-J., Huang, C.-Y., & Cheng, M.-L. (2020). Characteristic of Metabolic Status in Heart Failure and Its Impact in Outcome Perspective. Metabolites, 10(11), 437. https://doi.org/10.3390/metabo10110437